In situ reconstruction of ZIF-8 loaded on fibrous supports†
Leilei
Song
abc,
Siqi
Li
a and
Tao
Li
 *a
*a
aSchool of Physical Science and Technology, ShanghaiTech University, 201210 Shanghai, People's Republic of China. E-mail: litao1@shanghaitech.edu.cn
bShanghai Advanced Research Institute, Chinese Academy of Sciences, 201203, Shanghai, People's Republic of China
cUniversity of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 18th August 2021
Abstract
A simple solution casting technique was developed to load ZIF-8 particles on three commercially available fibrous supports. Benefiting from the open structure and high surface area of these fibrous networks, ZIF-8 particles can undergo a full cycle of degradation and recrystallization in a vapor-phase with 46% of their porosity recovered in the end.
Metal–organic frameworks (MOFs) have risen to be a central theme in the field of porous materials as well as materials science in general.1–3 Their highly crystalline framework, high surface area and tunable pore chemistry hold promise for various applications such as gas storage, separation, catalysis, drug delivery, sensing, and more.4–15
At present, MOF materials prepared at the lab scale mostly exist as microcrystalline powders. The irregular shape, fine grain size and the lack of mechanical strength of powders make them difficult to be directly used in industrial applications.16–18 Therefore, finding a low-cost process for structuring MOF materials into pellets, sheets or other shapes is a critical step and a timely requirement toward their industrial deployment. On the other hand, MOFs are widely criticized for their lack of chemical stability. When used as sorbent materials, how to handle chemically degraded MOFs, particularly in their shaped form, is a critical aspect that needs to be properly addressed.
Compared to zeolites which are routinely structured into pellets or monoliths through granulation or pelletization,19,20 MOF structuring meets new challenges. Due to the poor mechanical properties of many MOFs, traditional pelletization and extrusion processes that rely on excessive external forces often cause irreversible damage to the crystallinity of MOFs thereby lowering their inherent porosity.18,21,22 Alternatively, MOFs can be mixed with polymer binders to form MOF–polymer composites of specific shapes.23–28 However, random mixing of polymers and MOF particles often leads to pore blocking thereby rendering a part of the MOF pores inaccessible by guest molecules.26,29,30 In addition, several groups have also shown that MOFs can be grown onto pre-shaped polymer substrates.31–34 However, high MOF loading is difficult to achieve through this method. Meanwhile, the multistep process also increases cost and complicates the production.
Fibrous materials are excellent supports for powders due to several reasons. First, there is a rich library of fibrous materials ranging from natural to synthetic fibres to meet the needs of various applications. Meanwhile, fibres with adjustable thickness and porosity can be easily obtained through technological means such as weaving, melt blowing, electrospinning, etc.35–37 The intertwined network of fibres can spatially separate and compartmentalize porous particles allowing more efficient guest diffusion. In addition, the excellent mechanical properties of fibres allow maximum processability which can compensate the rigidity of powdery materials when made into composites. Based on these attributes, we hypothesize that the high surface area of the fibres can also make them an excellent host for chemically degraded MOFs. The open fibrous network will further allow vapor-phase reconstruction of the MOFs back to their crystalline state. There have been quite a few reports about using fibres as MOF supports which either grow the MOF directly on the fibre or electrospin the MOF/polymer mixture into fibres.38–40 These methods, although promising, are difficult to be used in large scale manufacturing.
Here, we report a facile method to construct MOF–fibre composites. We selected two natural fibrous materials, cotton fabric and filter paper, and one mineral fibre, rockwool, as supporting matrices for zeolitic imidazolate framework-8 (ZIF-8) (Fig. 1). ZIF-8 is an iconic MOF that has showed tremendous potential as a fuel cell membrane and gas separation membrane.41–45 Utilizing the hydrophilicity and capillary action of these fibres, ZIF-8 can be easily loaded onto the support through a simple solution casting step thereby achieving 29–40 wt% loading capacity (Table S1†). The loaded ZIF-8 particles adhered to the surfaces of the fibres and fully retained their sorption properties. More impressively, after degradation of ZIF-8 using acid vapor, the metal ion and ligand precursors were evenly coated on the fibre surfaces which can later be recrystallized back to its crystalline form through an in situ vapor-assisted reconstruction step resulting in a recovery of half of its original N2 adsorption capacity.
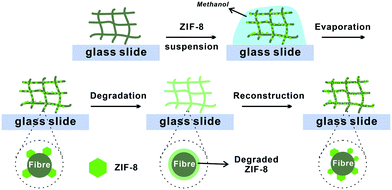 | ||
| Fig. 1 Schematic illustration of the degradation–reconstruction process of ZIF-8 loaded on various fibres. | ||
To begin with, we selected a widely popular MOF, ZIF-8,41 as a model system for this study. Uniform ZIF-8 particles with sizes of 900 ± 50 nm and 15 ± 5 μm were synthesized according to the reported method (Fig. S1†).46 The powder X-ray diffraction pattern showed highly matched diffraction peaks between the as-synthesized ZIF-8 and the simulated pattern suggesting good crystallinity (Fig. S2†). For the support, two natural fibrous materials, cotton fabric and filter paper, and one mineral fibre, rockwool (commonly used as a growth medium in hydroponic farming), were selected for this study.
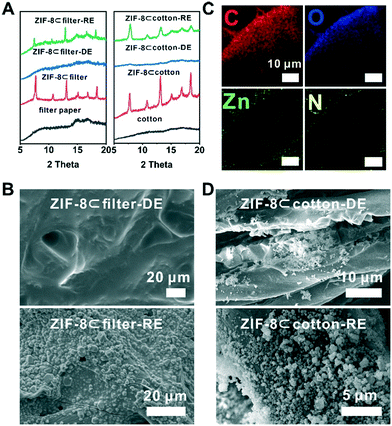
These fibres are all hydrophilic and water absorbent due to the abundance of hydroxyl groups. The hydrophilicity of the fibrous-support creates capillary action and helps ZIF-8 particles evenly disperse in the support. Moreover, the polar functional groups presented on the fiber can serve as the nucleation centres during the latter ZIF-8 reconstruction step. These fabrics were cut into samples of 2 × 2 cm and spread onto a glass slide. The scanning electron microscopy (SEM) images showed that the diameters of the fibres for cotton, filter paper and rockwool are mostly within the range of 5–20 μm (Fig. 2A, C and S4A†). The porosities of these three fibrous materials are estimated to be 4.4, 2.3 and 12.5 cc g−1 (see the ESI†). To load ZIF-8 particles onto these supports, a prescribed amount of 120 mg ml−1 ZIF-8 suspension in methanol was drop casted onto the fibre and was allowed to evaporate naturally at room temperature. This process was repeated 2–3 times to obtain the final composites, namely ZIF-8⊂cotton, ZIF-8⊂filter, and ZIF-8⊂rockwool. In principle, the MOF loading capacity is only limited by the porosity of the fibrous support. The SEM images clearly showed that ZIF-8 was occupying the gaps in between fibres in all three samples (Fig. 2B, D and S4B†). The PXRD patterns revealed that these composites exhibited identical diffraction peaks as those of the as-synthesized ZIF-8 suggesting that the crystallinity was well preserved in their respective composites (Fig. 3A and S5†).
 | ||
| Fig. 2 SEM images of the (A) filter paper, (B) ZIF-8⊂filter, (C) cotton fabric, and (D) ZIF-8⊂cotton (the inset is a close-up image of the ZIF-8 particle on the fibre surface). | ||
Next, to demonstrate the benefit of the fibre supported MOF, we turned to explore the degradation–reconstruction process of ZIF-8 on the fibres. A previous study by Majano et al. has shown that certain MOFs can be recrystallized through solvent vapor treatment after degradation.47 However, in most cases, the morphology of the MOF particles was changed. The presence of fibrous supports spatially separates MOF particles and therefore may lead to a higher recrystallization rate. To demonstrate this hypothesis, first, a piece of a ZIF-8⊂cotton sample was placed in a container exposed to acetic acid vapor at room temperature for 1 h. During this process, ZIF-8 particles gradually degraded into Zn2+ ions and 2-methylimidazole (2-MIM). We denote this sample as ZIF-8⊂cotton-DE. Similarly, ZIF-8⊂rockwool-DE and ZIF-8⊂filter-DE were also prepared using the same protocol. The PXRD patterns of ZIF-8⊂cotton-DE, ZIF-8⊂rockwool-DE and ZIF-8⊂filter-DE showed that the characteristic diffraction peaks of ZIF-8 disappeared after acid treatment indicating complete degradation of ZIF-8 (Fig. 3A and S5†). SEM images also confirmed that the fibres were visibly coated by a layer of ZIF-8 precursors (Fig. 3B, D and S6A†). Taking ZIF-8⊂filter-DE as an example, the energy dispersion X-ray spectroscopy (EDS) mapping showed that the two characteristic elements, Zn and N, from ZIF-8 were distributed mainly on the surface of the filter paper fibre (Fig. 3C). This evidence confirms that the decomposition products of ZIF-8 were retained on the surfaces of the fibres.
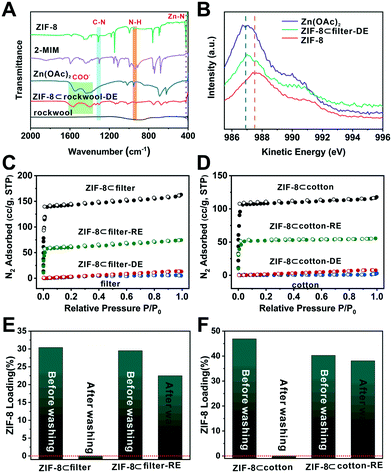
To better understand the chemistry behind the degradation process, Fourier-transform infrared (FTIR) spectroscopy was used to analyze these samples. Zn(OAc)2 showed a series of COO− bands at 1400 and 1550–1650 cm−1 (Fig. 4A). For 2-methylimidazole, the absorption peaks were located at 1303 and 945 cm−1 which correlate to the C–N stretching and N–H bending, respectively. Moreover, the peak at 421 cm−1 can be ascribed to the Zn–N stretching.48 Compared to these standard samples, the N–H bending and Zn–N stretching peaks disappeared in the IR spectra of ZIF-8⊂rockwool-DE. Meanwhile, the COO− signal reappeared. This suggests that Zn2+ primarily occurred in the form of Zn(OAc)2 after degradation. Meanwhile, 2-MIM was likely to be protonated. We further used XPS and Auger spectroscopy to analyze Zn(OAc)2, ZIF-8 and ZIF-8⊂rockwool-DE to further probe the chemical nature of the degraded ZIF-8. Since Zn can only exist as Zn2+ here, the Zn2+ peak in the Zn LMM Auger spectrum of ZIF-8⊂rockwool-DE deviated from that of ZIF-8 and shifted towards that of Zn(OAc)2. This indicates that ZIF-8 was degraded into Zn(OAc)2 and 2-methylimidazole (Fig. 4B). We further proceeded to analyse the Zn2+ chemical environment using X-ray photoelectric spectroscopy (XPS) and Auger spectroscopy. The Zn LMM Auger peaks in Zn(OAc)2 and ZIF-8 were located at 986.9 eV and 987.5 eV. In contrast, the Zn peak in ZIF-8⊂filter-DE, however, manifests a linear combination of those of Zn(OAc)2 and ZIF-8 with Zn(OAc)2 as the dominant phase (Fig. 4B). This again confirmed the near complete degradation of ZIF-8 to Zn(OAc)2 after HOAc vapor treatment.
In situ vapor-assistant reconstruction was then carried out aiming to recrystallize Zn2+ and 2-MIM back into ZIF-8. Specifically, ZIF-8⊂cotton-DE, ZIF-8⊂filter-DE, and ZIF-8⊂rockwool-DE samples were again placed in a sealed chamber along with a small amount of methanol (Fig. S7†). The chamber was heated to 70 °C for 24 h to obtain reconstructed samples which are denoted as ZIF-8⊂cotton-RE, ZIF-8⊂filter-RE, and ZIF-8⊂rockwool-RE, respectively. The PXRD patterns of the three reconstructed samples all showed characteristic peaks of ZIF-8 suggesting the successful recovery of ZIF-8 (Fig. 3A and S4†). The SEM images revealed that crystalline particles with a ZIF-8-like morphology reappeared on the surfaces of fibres in all three cases (Fig. 3B, D and S5B†). A N2 adsorption experiment was then carried out to quantitatively evaluate the loss and recovery of ZIF-8 porosity after the degradation and reconstruction process. As shown in Fig. 4C, after acid vapor degradation, the N2 absorption capacity decreased from 164 cc g−1 for ZIF-8⊂filter to 5 cc g−1 (P/Po = 0.95) for ZIF-8⊂filter -DE indicating the complete loss of porosity. After vapor-assisted reconstruction, the N2 uptake capacity of ZIF-8⊂filter -RE returned to 75 cc g−1 which accounts for 46% of the uptake value of ZIF-8⊂cotton (164 cc g−1) (Fig. 4C). Similarly, ZIF-8⊂cotton exhibited a N2 uptake capacity of 118 cc g−1 (77 K, P/Po = 0.95). After acid vapor treatment, ZIF-8⊂cotton-DE exhibited an uptake capacity of only 6 cc g−1. ZIF-8⊂cotton-RE, however, showed a N2 uptake capacity of 55 cc g−1, which accounts for 46% of the N2 adsorption capacity of ZIF-8⊂cotton (Fig. 4D).
Lastly, the attrition resistance of these fibre-supported MOF composites was investigated. Specifically, we soaked ZIF-8⊂filter and ZIF-8⊂filter-RE (∼8 × 8 mm in size) in an excess of methanol (∼4 ml) and the samples were agitated on the rotator for 20 min. Then, SEM and TGA were used to characterize and quantify the amount of MOFs retained after washing. The SEM images showed that the washing step removed most of the ZIF-8 particles from ZIF-8⊂filter (Fig. 4E, S8A and S10A†). Interestingly, after 1 cycle of degradation–reconstruction, the ZIF-8 particles became more attrition resistant as can be seen from the SEM images of ZIF-8⊂filter-RE after washing (Fig. S8B†). TGA analysis showed that more than 79% of the ZIF-8 particles were retained after washing. It is clear that the reconstruction step forced the newly formed ZIF-8 particles to firmly attach to the surface of the fibres thereby increasing their attrition resistance. Similar results were also obtained for ZIF-8⊂cotton and ZIF-8⊂cotton-RE (Fig. 4F, S9 and S10B†).
In summary, we demonstrated a simple solution casting approach for the preparation of three ZIF-8-loaded fibrous composites. The ZIF-8 particles confined among the fibres fully retained their crystallinity and sorption properties. We further demonstrated that the fibre loaded ZIF-8 could be recrystallized back to its crystalline state through a vapor-assisted reconstruction step with near half of the N2 uptake recovery rate. These results exemplify that supporting MOFs with fibrous materials is a viable option for structuring MOF materials and to extend the longevity of MOF materials under realistic environments through vapor-assisted reconstruction.
This work was supported by the National Natural Science Foundation of China (Grant No. 22075181). The authors thank the support from the Analytical Instrumentation Center (Grant No. SPSTAIC10112914) and the Centre for High-resolution Electron Microscopy (CħEM) of SPST at ShanghaiTech University under Grant No. EM02161943.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- G. Maurin, C. Serre, A. Cooper and G. Férey, Chem. Soc. Rev., 2017, 46, 3104–3107 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- J.-D. Xiao and H.-L. Jiang, Acc. Chem. Res., 2019, 52, 356–366 CrossRef CAS PubMed.
- M.-X. Wu and Y.-W. Yang, Adv. Mater., 2017, 29, 1606134 CrossRef PubMed.
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- M.-F. Wang, Y. Mi, F.-L. Hu, Z. Niu, X.-H. Yin, Q. Huang, H.-F. Wang and J.-P. Lang, J. Am. Chem. Soc., 2020, 142, 700–704 CrossRef CAS PubMed.
- X.-M. Liu, L.-H. Xie and Y. Wu, Inorg. Chem. Front., 2020, 7, 2840–2866 RSC.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC.
- B. Valizadeh, T. N. Nguyen and K. C. Stylianou, Polyhedron, 2018, 145, 1–15 CrossRef CAS.
- S. Mitchell, N. L. Michels and J. Perez-Ramirez, Chem. Soc. Rev., 2013, 42, 6094–6112 RSC.
- W. Schwieger, A. G. Machoke, T. Weissenberger, A. Inayat, T. Selvam, M. Klumpp and A. Inayat, Chem. Soc. Rev., 2016, 45, 3353–3376 RSC.
- J. Ren, N. M. Musyoka, H. W. Langmi, A. Swartbooi, B. C. North and M. Mathe, Int. J. Hydrogen Energy, 2015, 40, 4617–4622 CrossRef CAS.
- T. Tian, Z. Zeng, D. Vulpe, M. E. Casco, G. Divitini, P. A. Midgley, J. Silvestre-Albero, J.-C. Tan, P. Z. Moghadam and D. Fairen-Jimenez, Nat. Mater., 2018, 17, 174–179 CrossRef CAS PubMed.
- J. Cousin-Saint-Remi, S. Van Der Perre, T. Segato, M.-P. Delplancke, S. Goderis, H. Terryn, G. Baron and J. Denayer, ACS Appl. Mater. Interfaces, 2019, 11, 13694–13703 CrossRef CAS PubMed.
- A. Mallick, G. Mouchaham, P. M. Bhatt, W. Liang, Y. Belmabkhout, K. Adil, A. Jamal and M. Eddaoudi, Ind. Eng. Chem. Res., 2018, 57, 16897–16902 CrossRef CAS.
- H. Zhu, X. Yang, E. D. Cranston and S. Zhu, Adv. Mater., 2016, 28, 7652–7657 CrossRef CAS PubMed.
- J. C. Moreton, M. S. Denny and S. M. Cohen, Chem. Commun., 2016, 52, 14376–14379 RSC.
- B. Valizadeh, T. N. Nguyen, B. Smit and K. C. Stylianou, Adv. Funct. Mater., 2018, 28, 1801596 CrossRef.
- G. W. Peterson, J. J. Mahle, T. M. Tovar and T. H. Epps, Adv. Funct. Mater., 2020, 30, 2005517 CrossRef CAS.
- C. Lu, T. Ben, S. Xu and S. Qiu, Angew. Chem., Int. Ed., 2014, 53, 6454–6458 CrossRef CAS PubMed.
- A. Sabetghadam, X. Liu, M. Benzaqui, E. Gkaniatsou, A. Orsi, M. M. Lozinska, C. Sicard, T. Johnson, N. Steunou, P. A. Wright, C. Serre, J. Gascon and F. Kapteijn, Chem. – Eur. J., 2018, 24, 7949–7956 CrossRef CAS PubMed.
- C. Zhang, Y. Li, H. Wang, S. He, Y. Xu, C. Zhong and T. Li, Chem. Sci., 2018, 9, 5672–5678 RSC.
- L. D. O'Neill, H. Zhang and D. Bradshaw, J. Mater. Chem., 2010, 20, 5720 RSC.
- A. Centrone, Y. Yang, S. Speakman, L. Bromberg, G. C. Rutledge and T. A. Hatton, J. Am. Chem. Soc., 2010, 132, 15687–15691 CrossRef CAS PubMed.
- M. L. Pinto, S. Dias and J. Pires, ACS Appl. Mater. Interfaces, 2013, 5, 2360–2363 CrossRef CAS PubMed.
- K. Bilisik, Text. Res. J., 2012, 82, 725–743 CrossRef CAS.
- F. Zuo, D. H. Tan, Z. Wang, S. Jeung, C. W. Macosko and F. S. Bates, ACS Macro Lett., 2013, 2, 301–305 CrossRef CAS.
- S. Jiang, Y. Chen, G. Duan, C. Mei, A. Greiner and S. Agarwal, Polym. Chem., 2018, 9, 2685–2720 RSC.
- M. A. Bunge, A. B. Davis, K. N. West, C. W. West and T. G. Glover, Ind. Eng. Chem. Res., 2018, 57, 9151–9161 CrossRef CAS.
- D. B. Dwyer, N. Dugan, N. Hoffman, D. J. Cooke, M. G. Hall, T. M. Tovar, W. E. Bernier, J. Decoste, N. L. Pomerantz and W. E. Jones, ACS Appl. Mater. Interfaces, 2018, 10, 34585–34591 CrossRef CAS PubMed.
- Y. Dou, W. Zhang and A. Kaiser, Adv. Sci., 2020, 7, 1902590 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- H. Q. Liang, Y. Guo, Y. Shi, X. Peng, B. Liang and B. Chen, Angew. Chem., 2020, 59, 7732–7737 CrossRef CAS PubMed.
- Y. Guo, Z. Jiang, W. Ying, L. Chen, Y. Liu, X. Wang, Z.-J. Jiang, B. Chen and X. Peng, Adv. Mater., 2018, 30, 1705155 CrossRef PubMed.
- H.-Q. Liang, Y. Guo, X. Peng and B. Chen, J. Mater. Chem. A, 2020, 8, 11399–11405 RSC.
- K. Li, D. H. Olson, J. Seidel, T. J. Emge, H. Gong, H. Zeng and J. Li, J. Am. Chem. Soc., 2009, 131, 10368–10369 CrossRef CAS PubMed.
- C. Zhang, R. P. Lively, K. Zhang, J. R. Johnson, O. Karvan and W. J. Koros, J. Phys. Chem. Lett., 2012, 3, 2130–2134 CrossRef CAS PubMed.
- G. Majano, O. Martin, M. Hammes, S. Smeets, C. Baerlocher and J. Pérez-Ramírez, Adv. Funct. Mater., 2014, 24, 3855–3865 CrossRef CAS.
- Y. Hu, H. Kazemian, S. Rohani, Y. Huang and Y. Song, Chem. Commun., 2011, 47, 12694 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ce00790d |
| This journal is © The Royal Society of Chemistry 2021 |