Proflavine and zinc chloride “team chemistry”: combining antibacterial agents via solid-state interaction†
Abstract
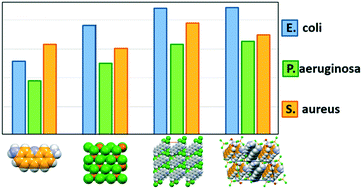
Co-crystallization of the antibacterial agent proflavine (PF) with the inorganic salt ZnCl2 by mechanochemical and solution methods results in the formation of novel compounds ZnCl3(HPF) (1) and [HPF]2[ZnCl4]·H2O (2), both containing the proflavinium cation (HPF)+. Both compounds show a 50–125% enhanced antimicrobial activity with respect to a reference standard of AgNO3, and a 25–50% enhancement to the behaviour of the separate components against pathogen indicator strains of Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. In terms of crystal structure, both compounds ZnCl3(HPF) and [HPF]2[ZnCl4]·H2O are characterized by extensive π-stacking interactions between the proflavine moieties. The same interaction is predominant in the previously unknown crystal structures of neutral proflavine (PF), as well as in that of its dihydrated monochloride salt, [HPF]Cl·2H2O, which are also described in this paper.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...