Zn(ii) and Cd(ii) coordination polymers with a new angular bis-pyridyl-bis-amide: synthesis, structures and sensing properties†
Abstract
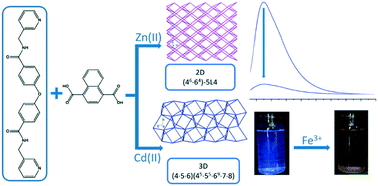
Zn(II) and Cd(II) coordination polymers constructed from a new angular bis-pyridyl-bis-amide ligand and naphthalene-1,4-dicarboxylic acid (1,4-H2NDC), {[Zn2(L)(1,4-NDC)2]·2H2O} [L = 4,4′-oxybis(N-(pyridin-3-ylmethyl)benzamide)], 1, and {[Cd2(L)(1,4-NDC)2]·EtOH}, 2, have been solvothermally synthesized and structurally characterized by using single-crystal X-ray diffraction. While complex 1 displays a 2D double layer with the rare (46·64)-5 L4 topology, complex 2 shows a 3D framework with a new (4·5·6)(45·55·69·7·8) topology. Both complexes 1 and 2 display luminescence properties and 2 exhibits a high sensitivity toward the detection of Fe3+ ions with a Ksv value of 1.9 × 104 M−1. The quenching effect can most probably be ascribed to the interactions between the metal ions and the amide carbonyl oxygen atoms.
- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...