Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of temperature on the transformation of amorphous calcium magnesium carbonate with near-dolomite stoichiometry into high Mg-calcite†
Bettina
Purgstaller
 *a,
Vasileios
Mavromatis
b,
Katja E.
Goetschl
a,
Florian R.
Steindl
a and
Martin
Dietzel
a
*a,
Vasileios
Mavromatis
b,
Katja E.
Goetschl
a,
Florian R.
Steindl
a and
Martin
Dietzel
a
aInstitute of Applied Geosciences, Graz University of Technology, Rechbauerstraße 12, 8010 Graz, Austria. E-mail: bettina.purgstaller@tugraz.at
bGeosciences Environment Toulouse (GET), Observatoire Midi-Pyrénées, Université de Toulouse, CNRS, IRD, UPS, 14 Avenue Edouard Belin, 31400 Toulouse, France
First published on 8th February 2021
Abstract
High Mg-calcite (HMC) is thermodynamically unstable under ambient conditions, yet it has been found in many biogenic and abiotic depositional settings with Mg contents up to 50 mol%. The elevated Mg content of HMC was frequently attributed to an amorphous calcium magnesium carbonate (ACMC) precursor, but the effects of transformation conditions on the Mg content of HMC are still debated and far from being quantified. Therefore, the transformation of ACMC with near-dolomite stoichiometry (∼48 mol% Mg) into HMC has been studied in a MgCl2–NaHCO3 buffered solution at moderate pH (7.6) and temperatures from 10 to 80 °C. The obtained chemical data show that the apparent solubility of ACMC (KACMC) is lower at higher temperature and the relative increase of KACMC as a function of the Mg content is similar between 10 and 80 °C which can be assessed by the equation: log(KACMC) = 0.01629 * [Mg]ACMC − 0.0001096 T2 + 0.0545 T −12.919, where T is reported in Kelvin and [Mg]ACMC refers to the amount of Mg in ACMC in mol%. The Mg content of the final HMC increases from 5 to 40 mol% with increasing temperature, thus is significantly lower compared to the Mg content of the ACMC precursor. Our findings argue for a dissolution and re-precipitation process during ACMC transformation, where at higher temperatures the Mg incorporation into HMC is enhanced due to (i) the reduced solvation energy barrier of aqueous Mg2+ and (ii) the high prevailing molar Mg2+/Ca2+ ratio of the solution after its reaction with the ACMC. Notably, the Mg2+/Ca2+ ratio of the latter solution is controlled by the temperature-dependent solubility of ACMC. Finally, the findings indicate that the nanocrystalline HMCs formed via transformation of ACMC are physically defective from rapid crystal growth and have higher solubilities compared to well-crystallized Mg-bearing calcites.
1. Introduction
Calcite is the most abundant calcium carbonate (CaCO3) mineral in marine and terrestrial environments.1,2 In the crystal structure of calcite, Ca2+ can be substituted by other divalent cations present in natural waters. Predominant in these replacement reactions is the substitution of Ca2+ by Mg2+.3–5 In the literature, calcite containing >4 mol% Mg is usually referred to as high Mg-calcite (HMC; Ca1−xMgxCO3, x > 0.04), whereas calcite with lower Mg content is referred to as low Mg-calcite (LMC; Ca1−xMgxCO3, x < 0.04).5,6 Abiogenic HMCs with <20 mol% Mg form marine cements consolidating sedimentary materials, e.g. in shallow tropical carbonate factories,3 in zones affected by anaerobic oxidation of methane7 and in coastal sabkha settings.8 Biogenic HMCs with up to 25 mol% Mg are a common building material of e.g. skeletons of coralline algae, foraminifers and echinoderms.3,9–12 The Mg content can be even higher (40–45 mol%) in structural elements of sea urchin teeth.13,14 A few examples of HMC with up to nearly 50 mol% Mg have been reported in natural depositional settings which are usually rich in microbial activity such as in algal sediments from a hypersaline lake (salt pond) of San Salvador Island, Bahamas,15 in microbial mats of the Lagoa Vermelha, Brazil,16 in microbial films of the Seroe Domi Formation of Curacao, The Netherlands17 and in sediments of the shallow, alkaline Lake Neusiedl, Austria.18 Note here that HMC phases with near-dolomite stoichiometry, but lacking cation ordering, are also referred to as very high Mg-calcite (VHMC) or protodolomite.5Despite several decades of research, the formation of HMC and VHMC in natural systems is a poorly understood process because (i) the (V)HMC structure is destabilized by the uptake of Mg2+ (ref. 19) and (ii) the inorganic precipitation of HMC with >20 mol% Mg in the laboratory under ambient conditions remains an enigma as high aqueous Mg2+/Ca2+ ratios inhibit the nucleation and growth of HMC from solution and favour the precipitation of aragonite (CaCO3).20–23 The inhibiting effect of aqueous Mg2+ on the formation of calcite originates from the strong affinity of the Mg2+ ion for the aqueous solution which is reflected by its high hydration Gibbs free energy24 and low rate of exchange of water molecules in its hydration sphere.25,26 The dehydration of the hydrophilic Mg2+ ion at the lattice growth site is therefore one of the most rate-limiting steps during the formation of (V)HMC.
Experimental studies on mineral formation, however, have shown that besides direct precipitation from solution (classical formation pathway), carbonate minerals can be formed via the transformation of amorphous precursor phases.27,28 Evidence for this alternative formation pathway also comes from calcifying organisms, where calcite formation often proceeds through an amorphous calcium carbonate (ACC) precursor phase.29,30 In this context, it has been suggested that Mg-containing ACC is a necessary prerequisite for the formation of HMC10 and VHMC.31 In most studies, the formation of amorphous calcium magnesium carbonate (ACMC) was induced by mixing a Na2CO3 solution (pH > 11) with (Ca, Mg)Cl2 solutions (pH = 6–7) at room temperature.10,31–35 The chemical composition of the initial solution for ACMC synthesis (e.g. Mg/Ca ratio, pH) has been suggested to control the amount of Mg incorporated into the ACMC and the final HMC.36–38 Specifically, the formation of VHMC via ACMC has been achieved from synthesis in aqueous solutions with high Mg/Ca ratios at high pH (∼ 10), high carbonate-to-calcium ratios (>2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or by thermal treatment (>40 °C).31,36,38–40 However, in most studies, the chemical compositions of the reactive solutions and solids during the formation of ACMC and its transformation into Mg-calcite were not concerned or even not documented. In particular, the effect of temperature on the Mg content of HMC formed via the amorphous pathway in aqueous solution has not been investigated in earlier studies. In order to better understand the fate of Mg during the transformation of ACMC into HMC, we investigated the transformation behaviour of synthetic ACMC with near-dolomite stoichiometry (48 mol% Mg) in solution at moderate pH (7.6) and reaction temperatures ranging from 10 to 80 ± 1 °C. The aim of the present study is to provide a more detailed understanding of the interplay between the chemical composition of (i) ACMC, (ii) the corresponding reactive solution and (iii) (V)HMC formed via ACMC in the apparent aqueous environment. Moreover, the obtained chemical data were used to assess the solubility of ACMC and HMC.
1) or by thermal treatment (>40 °C).31,36,38–40 However, in most studies, the chemical compositions of the reactive solutions and solids during the formation of ACMC and its transformation into Mg-calcite were not concerned or even not documented. In particular, the effect of temperature on the Mg content of HMC formed via the amorphous pathway in aqueous solution has not been investigated in earlier studies. In order to better understand the fate of Mg during the transformation of ACMC into HMC, we investigated the transformation behaviour of synthetic ACMC with near-dolomite stoichiometry (48 mol% Mg) in solution at moderate pH (7.6) and reaction temperatures ranging from 10 to 80 ± 1 °C. The aim of the present study is to provide a more detailed understanding of the interplay between the chemical composition of (i) ACMC, (ii) the corresponding reactive solution and (iii) (V)HMC formed via ACMC in the apparent aqueous environment. Moreover, the obtained chemical data were used to assess the solubility of ACMC and HMC.
2. Methods
2.1 Synthesis of amorphous calcium magnesium carbonate
The synthesis of ACMC material with 47.9 ± 1.5 mol% Mg was carried out by a previously developed protocol.33,41 Briefly, a 0.25 M (Ca, Mg)Cl2 solution (Mg/Ca ratio = 2.1) and a 0.25 M Na2CO3 solution were prepared using CaCl2·2H2O, MgCl2·6H2O, and Na2CO3 chemicals (p.a. Roth) and ultrapure water (Millipore Integral 3; 18.2 MΩ cm−1). The two stock solutions were cooled in a fridge for 4 hours (T = 10 ± 2 °C). The precipitation of ACMC was induced by pouring 100 mL of the (Ca, Mg)Cl2 solution into a beaker containing 100 mL of the Na2CO3 solution. The ACMC was separated from solution by a 0.2 μm cellulose filter using a suction filtration unit and was subsequently freeze-dried for 12 hours (Virtis Benchtop 3 L). The synthesis protocol was repeated several times until about 20 g of ACMC were produced. The obtained ACMC material was analysed by X-ray diffraction (Fig. S1A†) and was stored in a desiccator with silica gel.2.2 Experimental setup for ACMC transformation into HMC
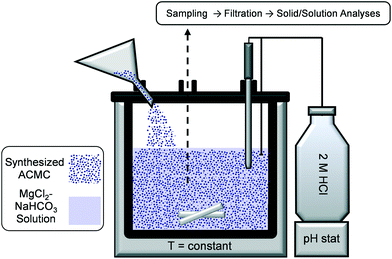
Transformation of ACMC in solution was performed at 10, 20, 40, 60 and 80 °C (see Table 1). Initially, 50 mM NaHCO3 solutions were prepared in 150 mL gas-tight glass bottles which were placed either in a cooling chamber (10 ± 2 °C), a temperature-controlled room (20 ± 2 °C) or in ovens (40 ± 2 °C, 60 ± 2 °C and 80 ± 2 °C) over a period of 24 to 60 h. 52 mL of the pre-cooled/heated NaHCO3 solution was added into a 150 mL borosilicate glass reactor containing 6.099 g MgCl2·6H2O to obtain a Mg concentration of 30 mM. 2 mL of the NaHCO3–MgCl2 solution was collected with a pipette to measure its initial Mg and alkalinity concentration (Table 2). The MgCl2–NaHCO3 solutions exhibit an initial pH value of 8.02 ± 0.15 (Table 2). The reactor was placed in an Easy Max System (Max™ 102; Mettler Toledo) in order to control the stirring of the solution at 200 rpm and its temperature at 10.0, 20.0, 40.0, 60.0 or 80.0 ± 0.1 °C (Fig. 1). Subsequently, 1.5 g of the synthesized ACMC was introduced into the buffered NaHCO3–MgCl2 solution (Fig. 1). After a short homogenization period between the ACMC material and the solution, a homogeneous sub-sample of the suspension (2 mL) was collected with a pipette (t = 0.3 min). Subsequently, the pH of the experimental solution was adjusted to 7.58 ± 0.04 (within ≤ 3 min) by computer-controlled titration of a 2 M HCl solution (Schott; TitroLine alpha plus) (Fig. S2†). Note here that the pH of the solution was adjusted to 7.6 to prevent the precipitation of hydrous Ca- or Mg- carbonate phases, such as monohydrocalcite and nesquehonite.36,42,43 Further homogeneous sub-samples of the experimental suspension (2 mL) were collected with a pipette at 5 min, 11 min, 21 min, 32 min and 61 min. After 61 min of reaction time, the experimental solutions were transferred into 100 mL gastight bottles which were again placed in the fridge (10 ± 2 °C), temperature-controlled room (20 ± 2 °C) and ovens (40 ± 2 °C, 60 ± 2 °C and 80 ± 2 °C) over a period of 2 months. Additional homogeneous sub-samples were collected after 1 day, 1 week and 2 months of reaction time. The solids were separated from the solutions by a 0.2 μm cellulose acetate filter using a suction filtration unit and washed with ethanol. Subsequently, the solids were dried overnight in an oven at 40 °C and stored in a desiccator with silica gel.| Experiment | Time | Composition | HMC | ARG | HMG | [Mg]XRD of HMC | d 104 | FWHMd104 |
|---|---|---|---|---|---|---|---|---|
| a Average FWHM of HMC_I and HMC_II. b Additional phase that could correspond to magnesite. | ||||||||
| min per day | wt% | wt% | wt% | mol% | °2θ | |||
| T_10 °C | 0.3 min | ACMC | — | — | — | — | — | — |
| 5 min | ACMC | — | — | — | — | — | — | |
| 11 min | ACMC | — | — | — | — | — | — | |
| 21 min | ACMC, HMC_I | — | — | — | I: 4.8 | I: 3.0204 | — | |
| 32 min | HMC_I, ACMC | — | — | — | I: 5.1 | I: 3.0194 | — | |
| 61 min | HMC_I, HMC_II | — | — | — | I: 6.2, II: 14.5 | I: 3.0163, II: 2.9921 | — | |
| 1 day | HMC_I, HMC_II, ARG | I: 56, II: 42 | 2 | 0 | I: 6.6, II: 15.4 | I: 3.0153, II: 2.9896 | 0.375a | |
| 1 week | HMC_I, HMC_II, ARG | I: 57, II: 41 | 2 | 0 | I: 7.1, II: 16.3 | I: 3.0136, II: 2.9869 | 0.373a | |
| 2 months | HMC_I, HMC_II, ARG | I: 56, II: 41 | 3 | 0 | I: 6.4, II: 15.8 | I: 3.0157, II: 2.9882 | 0.380a | |
| T_20 °C | 0.3 min | ACMC | — | — | — | — | — | — |
| 5 min | ACMC | — | — | — | — | — | — | |
| 11 min | ACMC, HMC | — | — | — | 11.9 | 2.9996 | — | |
| 21 min | ACMC, HMC | — | — | — | 12.8 | 2.9970 | — | |
| 32 min | HMC, ACMC | — | — | — | 15.2 | 2.9902 | — | |
| 61 min | HMC, ARG | — | — | — | 17.0 | 2.9848 | — | |
| 1 day | HMC, ARG | 99 | 1 | 0 | 18.0 | 2.9820 | 0.459 | |
| 1 week | HMC, ARG | 99 | 1 | 0 | 18.3 | 2.9810 | 0.475 | |
| 2 months | HMC, ARG | 98 | 2 | 0 | 18.8 | 2.9795 | 0.489 | |
| T_40 °C | 0.3 min | ACMC | — | — | — | — | — | — |
| 5 min | ACMC | — | — | — | — | — | — | |
| 11 min | ACMC, HMC | — | — | — | 23.0 | 2.9675 | — | |
| 21 min | HMC, ACMC | — | — | — | 28.4 | 2.9516 | — | |
| 32 min | HMC | — | — | — | 29.9 | 2.9471 | — | |
| 61 min | HMC | — | — | — | 29.7 | 2.9477 | — | |
| 1 day | HMC | 100 | 0 | 0 | 30.3 | 2.9460 | 0.644 | |
| 1 week | HMC, HMG | 94 | 0 | 6 | 29.7 | 2.9479 | 0.596 | |
| 2 months | HMC, HMG | 91 | 0 | 9 | 30.9 | 2.9443 | 0.544 | |
| T_60 °C | 0.3 min | ACMC | — | — | — | — | — | — |
| 5 min | ACMC | — | — | — | — | — | — | |
| 11 min | ACMC, HMC | — | — | — | 34.2 | 2.9346 | — | |
| 21 min | HMC, ACMC | — | — | — | 39.4 | 2.9197 | — | |
| 32 min | HMC | — | — | — | 39.9 | 2.9181 | — | |
| 61 min | HMC | — | — | — | 39.8 | 2.9183 | — | |
| 1 day | HMC | 100 | 0 | 0 | 40.7 | 2.9159 | 0.659 | |
| 1 week | HMC | 100 | 0 | 0 | 40.5 | 2.9163 | 0.550 | |
| 2 months | HMC | 100 | 0 | 0 | 40.6 | 2.9159 | 0.530 | |
| T_80 °C | 0.3 min | ACMC | — | — | — | — | — | — |
| 5 min | ACMC | — | — | — | — | — | — | |
| 11 min | HMC, HMG, ACMC | — | — | — | 39.6 | 2.9189 | — | |
| 21 min | HMC, HMG, ARG | — | — | — | 39.2 | 2.9201 | — | |
| 32 min | HMC, HMG, ARG | — | — | — | 39.5 | 2.9193 | — | |
| 61 min | HMC, HMG, ARG | — | — | — | 39.7 | 2.9187 | — | |
| 1 day | HMC, HMG, ARG | 78 | 7 | 15 | 39.7 | 2.9187 | 0.351 | |
| 1 week | HMC, HMG, ARG | 80 | 7 | 13 | 40.4 | 2.9167 | 0.354 | |
| 2 months | HMC, ARG,b | — | — | — | 41.6 | 2.9132 | 0.362 | |
| Experiment | Time | pH | Alkalinity | [Ca]aq | [Mg]aq | [Mg]solid |
|---|---|---|---|---|---|---|
| min per day | mM | mM | mM | mol% | ||
| T_10 °C | 0 min | 8.15 | 49.7 | 0.0 | 29.0 | 47.9 ± 1.5 |
| 0.3 min | 9.15 | 80.6 | 2.5 | 41.7 | 46.0 ± 1.5 | |
| 5 min | 7.52 | 126.1 | 35.1 | 84.3 | 38.0 ± 1.4 | |
| 11 min | 7.55 | 138.8 | 41.3 | 94.3 | 35.4 ± 1.4 | |
| 21 min | 7.31 | 134.3 | 24.5 | 110.1 | 25.5 ± 1.1 | |
| 32 min | 7.59 | 137.8 | 20.1 | 118.1 | 22.3 ± 1.0 | |
| 61 min | 7.53 | 133.8 | 12.9 | 125.2 | 11.4 ± 0.6 | |
| 1 day | 7.31 | 109.1 | 2.3 | 122.0 | 13.0 ± 0.7 | |
| 1 week | 7.52 | 97.3 | 0.6 | 120.3 | 14.4 ± 0.7 | |
| 2 months | 7.77 | 89.9 | 0.3 | 120.7 | 15.5 ± 0.8 | |
| T_20 °C | 0 min | 8.17 | 48.4 | 0.0 | 29.1 | 47.9 ± 1.5 |
| 0.3 min | 9.21 | 77.5 | 2.1 | 41.4 | 45.6 ± 1.5 | |
| 5 min | 7.62 | 119.4 | 25.1 | 81.8 | 38.0 ± 1.4 | |
| 11 min | 7.53 | 126.1 | 20.8 | 92.8 | 34.7 ± 1.4 | |
| 21 min | 7.59 | 129.5 | 16.7 | 105.6 | 29.5 ± 1.2 | |
| 32 min | 7.59 | 134.2 | 15.7 | 115.1 | 21.9 ± 1.0 | |
| 61 min | 7.53 | 118.3 | 6.9 | 117.7 | 16.6 ± 0.8 | |
| 1 day | 7.49 | 96.2 | 0.9 | 114.2 | 19.0 ± 0.9 | |
| 1 week | 7.80 | 88.2 | 0.3 | 111.6 | 20.8 ± 1.0 | |
| 2 months | 7.92 | 79.7 | 0.2 | 110.4 | 22.2 ± 1.0 | |
| T_40 °C | 0 min | 8.01 | 49.7 | 0.0 | 29.0 | 47.9 ± 1.5 |
| 0.3 min | 8.85 | 70.2 | 1.7 | 38.3 | 47.0 ± 1.5 | |
| 5 min | 7.59 | 102.1 | 14.2 | 75.3 | 39.3 ± 1.4 | |
| 11 min | 7.60 | 108.3 | 10.5 | 85.5 | 35.5 ± 1.4 | |
| 21 min | 7.59 | 110.4 | 7.8 | 97.1 | 30.4 ± 1.3 | |
| 32 min | 7.55 | 99.5 | 3.1 | 99.0 | 28.8 ± 1.2 | |
| 61 min | 7.58 | 86.4 | 1.1 | 97.0 | 29.6 ± 1.2 | |
| 1 day | 7.57 | 60.9 | 0.4 | 85.1 | 33.0 ± 1.3 | |
| 1 week | 8.52 | 12.0 | 0.3 | 65.2 | 40.1 ± 1.4 | |
| 2 months | 8.39 | 8.1 | 0.3 | 64.8 | 41.2 ± 1.5 | |
| T_60 °C | 0 min | 8.00 | 49.6 | 0.0 | 29.2 | 47.9 ± 1.5 |
| 0.3 min | 8.35 | 60.4 | 1.7 | 32.9 | 47.5 ± 1.5 | |
| 5 min | 7.60 | 80.2 | 6.6 | 58.9 | 43.0 ± 1.5 | |
| 11 min | 7.59 | 83.3 | 5.5 | 65.6 | 41.1 ± 1.5 | |
| 21 min | 7.59 | 73.9 | 3.9 | 67.1 | 40.5 ± 1.4 | |
| 32 min | 7.55 | 57.7 | 1.1 | 64.2 | 40.4 ± 1.4 | |
| 61 min | 7.57 | 45.5 | 0.6 | 61.3 | 41.2 ± 1.5 | |
| 1 day | 7.46 | 20.1 | 0.6 | 48.9 | 43.8 ± 1.5 | |
| 1 week | 7.46 | 7.5 | 0.5 | 43.3 | 45.2 ± 1.5 | |
| 2 months | 7.64 | 3.1 | 0.8 | 42.2 | 45.3 ± 1.5 | |
| T_80 °C | 0 min | 7.79 | 48.5 | 0.0 | 29.4 | 47.9 ± 1.5 |
| 0.3 min | 7.82 | 51.3 | 1.6 | 28.1 | 48.3 ± 1.5 | |
| 5 min | 7.59 | 53.7 | 3.2 | 33.1 | 47.6 ± 1.5 | |
| 11 min | 7.49 | 39.7 | 1.4 | 25.0 | 48.8 ± 1.5 | |
| 21 min | 7.41 | 20.7 | 0.3 | 21.8 | 49.0 ± 1.5 | |
| 32 min | 7.42 | 16.7 | 0.3 | 20.4 | 49.3 ± 1.5 | |
| 61 min | 7.57 | 10.7 | 0.3 | 17.3 | 49.5 ± 1.5 | |
| 1 day | 7.87 | 5.6 | 0.3 | 15.7 | 49.8 ± 1.5 | |
| 1 week | 7.87 | 6.3 | 0.3 | 16.0 | 49.6 ± 1.5 | |
| 2 months | 7.54 | 6.7 | 0.9 | 15.3 | 49.8 ± 1.5 |
2.3 Analytical procedures
Thermogravimetric analyses (TGA) of the freeze-dried ACMC was realized using a PerkinElmer STA8000. The ACMC was heated from 25 °C to 800 °C at 10 °C min−1 in the presence of 99.999% N2 atmosphere. The mineralogy of the precipitates collected during the ACMC transformation experiments was determined by a PANalytical X'Pert PRO diffractometer using Co-Kα radiation (40 mA, 40 kV) at a 2θ range from 4 to 85° with a step size of 0.008° 2θ and 40 s count time per step. The mineral phases of the reaction products were quantified using Rietveld refinement (software HighScore Plus, PANalytical) using the crystal structure data given in Table S1.† The estimated analytical uncertainty of the quantification lies within 1 wt%. The refinement values consisted of the specimen displacement, a 7-term polynomial background function and scale factors and lattice parameters for all phases. For major phases, the peak shape was refined using a pseudo-Voigt profile function and peak asymmetry. The preferred orientation of calcite was refined for the (104) plane with the March–Dollase function. If two separate calcite phases were present, all their refined values, except of the scale factor and lattice parameters, were constrained with each other to ensure a stable refinement. The d-value and the left full-width at half-maximum (left FWHM, for asymmetric peaks) of the (104) reflection (hereafter referred to as d104 and FWHMd104, respectively) was obtained from the refinement results. The d104 value of the calcite phases was used to calculate their Mg content ([Mg]XRD) according to Goldsmith et al.44 and this content was then used to adapt the occupancy of Ca and Mg in the structure data used for the refinement. Note that the accuracy of the determination of the d104 value and the FWHMd104 is limited by the step size of the XRD measurement (0.008° 2θ).Selected precipitates were gold-coated and imaged using a scanning electron microscope (SEM, ZEISS DSM 982 Gemini). The total alkalinity of the experimental solutions was measured by a Schott TitroLine alpha plus titrator using a 10 mM HCl solution with a precision of ±2%. The aqueous Ca and Mg concentrations of experimental solutions and solids (digested in 6% HNO3) were determined using inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin Elmer Optima 8300 DV) with an analytical precision of ±3% for Ca and Mg analyses. The Mg content of the bulk solid ([Mg]solid in mol%, Table 2) was calculated according to the equation:
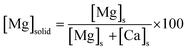 | (1) |
2.4 Geochemical modeling
The aqueous speciation and ion activities of the reactive solutions were calculated using the PHREEQC computer code with the minteq.v4 database. The apparent solubility product (K) for amorphous calcium magnesium carbonate (ACMC, Ca1−xMgxCO3·nH2O) and high Mg-calcite (HMC, Ca1−xMgxCO3) was calculated using the equation| K = (aCa2+)1−x(aMg2+)x(aCO32−) | (2) |
3. Results
3.1 Solid phase characterization
Scanning electron microscope observations of the synthesised ACMC (47.9 ± 1.5 mol% Mg) solid reveal spherical particles with <100 nm in size (Fig. S1B†) similar to synthetic amorphous calcium (magnesium) carbonate reported by other authors.37,41,45 The water content of the ACMC determined by thermal analysis is 0.67 ± 0.01 moles per unit formula (Fig. S1C†), thus, its stoichiometric formula can be written as Ca0.52Mg0.48CO3·0.67H2O. This water content is in good agreement with that of the ACMC (Ca0.47Mg0.53CO3·0.66H2O) precipitated by Purgstaller et al.33X-ray diffraction (XRD) patterns of the solids collected during the ACMC transformation experiments are shown in Fig. 2 at a 2θ range from 33° to 37°, while overview XRD patterns (2θ range = 4–65°) are displayed in Fig. S3.† The XRD patterns indicate the sole occurrence of ACMC for 11 min in experiment T_10 °C (Fig. 2A and S3A†) and for 5 min in experiments performed at ≥20 °C (Fig. 2B–E and S3B–E†). Subsequently, XRD peaks appear due to the transformation of ACMC into high Mg-calcite (HMC) and partly into aragonite (ARG) and hydromagnesite (HMG). XRD analyses reveal that for all experiments at ≤60 °C, the reaction products consist mainly of HMC (≥98 wt%) and traces of ARG (<2 wt%) (Table 1). In experiment T_10 °C two peaks were detected in the XRD pattern corresponding to high Mg-calcites with distinct Mg contents, hereafter mentioned as HMC_I and HMC_II (Table 1 and Fig. 2A). The reflection peaks of HMC_I appear in the XRD pattern at ≥21 min (during ACMC transformation), while those of HMC_II can be detected at ≥61 min of reaction time (Fig. 2A and S3A†). Note that in experiment T_40 °C, minor amounts of HMG (9 wt%) were detected beside HMC and ARG after 2 weeks of reaction time (Table 1, Fig. S3C†). At 80 °C, XRD patterns show the transformation of ACMC into HMC, HMG and ARG which account for 78, 15 and 7 wt%, respectively, after 1 day of reaction time (Fig. S3E;†Table 1). After 2 months of reaction time, the presence of HMG was no longer detected (Fig. S3E†). Instead, a reflection peak at 2.77 Å indicates the presence of new phase (labeled as * in Fig. S3E†), which could correspond to magnesite (MgCO3).2 Indeed, earlier studies reported the formation of magnesite by the transformation of pre-existing hydrated Mg-carbonate phases such as hydrogmagnesite and dypingite at elevated temperatures (e.g. Montes-Hernandez et al.46).
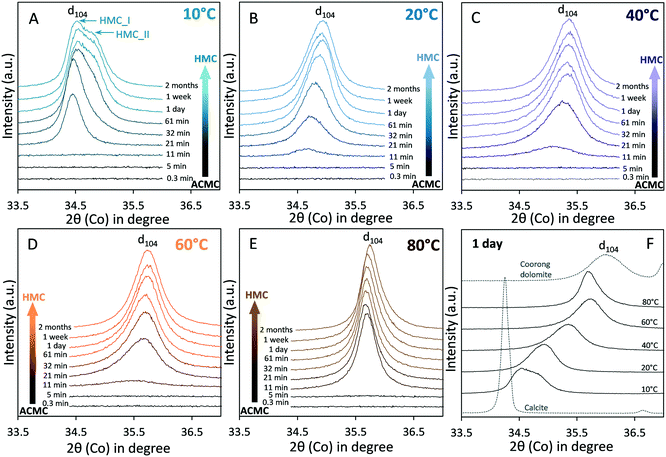 | ||
| Fig. 2 X-ray diffraction pattern showing the evolution of the d104 peak reflection of high Mg-calcite (HMC) formed via transformation of amorphous calcium magnesium carbonate (ACMC) at (A) 10 °C, (B) 20 °C, (C) 40 °C, (D) 60 °C and (E) 80 °C. Xrd pattern from 4° to 65° 2θ are shown in Fig. S3.† Note that the d104-value of the final HMC (F) is generally decreasing at higher temperature (increasing 2θ values) due to elevated Mg content (Goldsmith et al.44). The X-ray diffraction peaks of the HMCs are broader in comparison to the reference calcite (Merck), but are similar to the reference Coorong dolomite (South Australia). | ||
Fig. 2F shows that the XRD reflection peaks of the final HMCs are broader in comparison to the reference calcite (Merck). This observation is confirmed by comparing the FWHMd104 values, which shows that the FWHMd104 is higher for the HMCs (0.351–0.644; Table 1) than for the reference calcite (0.094). The obtained FWHMd104 values of the HMCs however are similar to that determined for the reference Coorong dolomite (FWHMd104 = 0.649; Fig. S4A†). Broad XRD reflection peaks are referred to non-ideal crystal structure phenomena which can be caused by small (nanocrystalline) particle sizes39,40,47 and inhomogeneities in the Ca and Mg composition48 of the crystalline solid. Indeed, the broad XRD peaks obtained for the HMCs and the reference Coorong dolomite (Fig. 2F) can result from their small crystal sizes: SEM images reveal aggregates composed of nanocrystals with <100 and <300 nm in size for the HMCs (Fig. 3D) and the Coorong dolomite (Fig. S4B†), respectively.
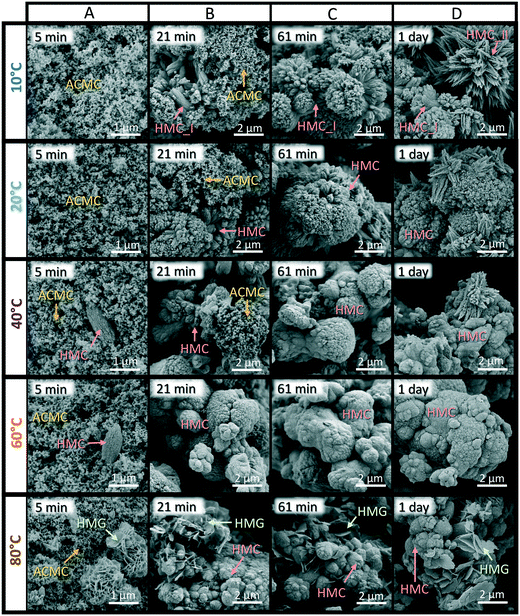 | ||
| Fig. 3 SEM images of solids collected at 5 min (A), 21 min (B), 61 min (C) and 1 day (D) of reaction time in experiments performed from 10 to 80 °C, showing the transformation of amorphous calcium magnesium carbonate (ACMC) into high Mg-calcite (HMC) at ≤60 °C and into HMC and hydromagnesite (HMG) at 80 °C (see Table 1). | ||
To get insights into the evolution of the Mg content of the forming HMC via ACMC, the characteristic d104-peak value of HMC was used to calculate its Mg content ([Mg]XRD) according to Goldsmith et al.44 (Table 1). The Mg content of the HMCs obtained after 1 day of reaction time is higher at elevated temperature as indicated by lower d104-values (corresponds to higher 2θ values in Fig. 2F). Note that a maximum concentration of 40 ± 1 mol% Mg in HMC was obtained in experiments T_60 °C and T_80 °C (Table 1). In experiment T_10 °C, the HMC_I that was detected in the samples collected at ≥21 min exhibits 6 ± 1 mol% Mg, which is ∼9 mol% lower than the Mg content of HMC_II (15 ± 1 mol%) that was detected in the samples obtained at ≥61 min of reaction time (Fig. 2A, Table 1). Rietveld refinement of the reaction products after 1 day shows that the amount of HMC_I (7 mol% Mg) and HMC_II (15 mol% Mg) is 56 and 42 wt%, respectively (Table 1). In experiments performed between 20 °C and 60 °C, the initial HMC phase that was detected beside ACMC (at 11 min in Fig. 2B–E; Table 1) shows a significant lower Mg content than the HMC that was obtained during ACMC transformation (Table 1). This indicates that the Mg uptake in HMC is not uniform throughout the transformation process but ends up in HMC with distinct Mg content. In contrast, in experiment T_80 °C, the HMCs show no significant change in the [Mg]XRD values during the experimental run (Table 1).
Scanning electron microscope images show that in experiments performed at ≤20 °C, the precipitates obtained at 5 min of reaction time consist of amorphous spherical particles (Fig. 3A), similar to the synthesized ACMC standard material (Fig. S1B†). In experiments performed ≥40 °C, traces of HMC or HMG nanocrystals were observed beside the ACMC particles at 5 min of reaction time (Fig. 3A), although XRD results show that the solids obtained at 5 min are solely amorphous (Fig. 2C–E). The HMC (T_40 °C and T_60 °C) consists of spindle-shaped aggregates composed of nanocrystals, whereas the HMG crystals (T_80 °C) show a plate-like morphology. These morphologies are similar to those observed for HMC and HMG obtained at a later stage in the experimental runs (Fig. 3B–D). At a reaction time of 21 min (Fig. 3B), large amounts of spherical ACMC particles were observed in the precipitates obtained in experiments performed at ≤20 °C, whereas at ≥40 °C ACMC was rarely observed in the collected solids. Note here that no ACMC particles were observed in the collected solids at ≥21 min in experiment T_80 °C (Fig. 3B), at ≥32 min in experiments T_40 °C and T_60 °C (not shown) and at ≥61 min in experiments T_10 °C and T_20 °C (Fig. 3C).
SEM images of the final HMC show aggregates composed of spindle-shaped assemblages of nanocrystals at lower temperatures (≤20 °C), whereas at higher temperatures (≥40 °C) the HMC-nanocrystals coalesced into spheroidal aggregates (Fig. 3C and D). These spindle-shaped and spherical morphologies are consistent with those of high Mg-calcites formed via amorphous precursors from previous studies.39,48–51 In experiment T_10 °C, large amounts of star-shaped aggregates composed of linearly arranged nanoparticles were detected beside the spindle-shaped HMC aggregates (Fig. 3D). These star-shaped aggregates most likely correspond to HMC_II that was detected beside HMC_I in the XRD-pattern at ≥61 min in experiment T_10 °C (Fig. 2A). Trace amounts of those aggregates could also be observed in the final solids of experiments T_20 °C and T_40 °C (Fig. 3D). Note here that the aragonite detected in experiments T_10 °C, T_20 °C and T_40 °C (2–7 wt%, Table 1) could not be found in SEM images.
3.2 Chemical evolution of the reactive solutions and solids
The addition of ACMC into the MgCl2–NaHCO3 solutions induced an increase of pH as well as of Ca, Mg and alkalinity concentrations (at 0.3 min in Fig. 4A–C, Table 2) which is attributed to the dissolution of ACMC. At lower temperatures, higher amounts of ACMC likely dissolved as it is indicated by higher Ca, Mg and alkalinity concentrations at 0.3 min of reaction time compared to higher temperature experiments (Fig. 4A–C, Table 2). Moreover, the obtained data from experiments performed at lower temperatures show a preferential release of Mg ions into the solution, resulting in ACMCs with slightly lower Mg contents (e.g. 46 mol% Mg in T_10 °C) compared to original composition of the synthetic ACMC material introduced in the reactors (48 mol% Mg) (Fig. 4D).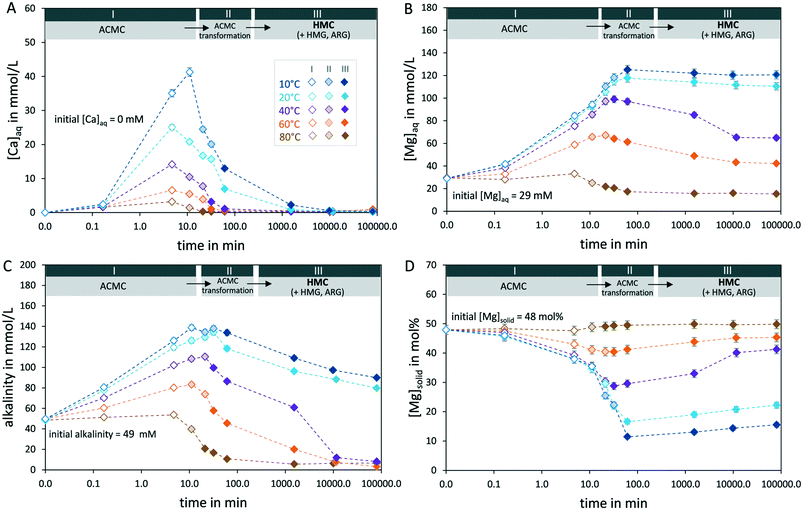 | ||
| Fig. 4 Concentration of calcium (A), magnesium (B), alkalinity (C) of the solution and Mg content of the bulk solid (D) (see eqn 1) during amorphous calcium magnesium carbonate (ACMC) transformation into high Mg-calcite (HMC) and in case partly into hydromagnesite (HMG) and aragonite (ARG) (see Table 1) at 10, 20, 40, 60 and 80 °C. Stage I: ACMC is the sole solid phase (open diamonds); stage II: ACMC transforms to the crystalline phases (shaded diamonds); stage III: solid phase is crystalline (solid diamonds). Note that the pH of the solution was adjusted to 7.58 ± 0.04 within ≤3 min by computer-controlled titration of a 2 M HCl solution (Fig. S2†). | ||
After a short initial homogenization period between the ACMC material and the solution, the pH of the experimental solutions was adjusted to 7.58 ± 0.04 by automatic titration of HCl solution (Fig. S2†). The adjustment of the pH resulted in the further dissolution of ACMC, which causes an increase in alkalinity, Ca and Mg concentrations of the experimental solutions between 0.3 and 5 min (Fig. 4A–C). After about 5 min, the Ca and alkalinity concentrations of the experimental solutions decrease (Fig. 4A and C) due to the transformation of ACMC into crystalline Ca–Mg carbonate phases (Fig. 2). With the exception of experiment T_80 °C, the aqueous Mg concentrations of the solutions increase in the same time frame (Fig. 4B). The net release of Mg into the solution with an uptake of Ca into the solid during the ACMC transformation step results in HMCs with lower Mg contents compared to that of the initial ACMC precursor in experiments performed at ≤60 °C (Fig. 4D). In contrast, in experiment T_80 °C, the Mg content of the bulk solid increases slightly during the ACMC transformation step (Fig. 4D), due to the formation of HMG (Mg5(CO3)4(OH)2·4H2O) beside HMC and ARG (Table 1, Fig. S3†). Note that in experiment T_40 °C, the Mg content of the bulk solid increased between 1 week and 2 months of reaction time, owing to the additional formation of HMG at this stage of the experiment (Fig. 4D).
At higher temperatures, the transformation of ACMC into HMC occurs faster as revealed by XRD and SEM observations (Fig. 2 and 3). This is further supported by the temporal evolution of pH and HCl solution addition derived from titration data (Fig. S2†). In experiments performed at ≤60 °C, our data reveal that additional amounts of HCl solution were automatically added into the experimental solution to keep the pH constant at 7.58 ± 0.04 during the transformation of ACMC into HMC (Fig. S2A–D†). The rise in pH and consequent titration of HCl may be explained by a fast dissolution of ACMC and slow precipitation of HMC during the ACMC transformation step. At higher temperatures, the HCl solution was added into the experimental solution in shorter periods of time (e.g. 17 min in T_60 °C) than at lower temperatures (e.g. 34 min in T_20 °C), indicating that the transformation of ACMC into HMC occurs faster at elevated temperatures. Note that in experiment T_80 °C, where ACMC transformed into HMC, HMG and ARG (Fig. 2E, Table 1), no additional HCl was added into the solution (Fig. S2E†). In the latter case, the pH decreased slightly from 7.59 to 7.41 during the ACMC transformation step.
4. Discussion
4.1 Effect of temperature on the Mg content of HMC formed via ACMC with near-dolomite stoichiometry
Earlier studies showed a strong correlation between the physicochemical parameters of the initial solution used for ACMC synthesis and the amount of Mg incorporated into ACMC and the final HMC.31,37,49,51 In this context, it has been suggested that tuning the amount of Mg in ACMC controls the amount of Mg incorporated into HMC. In contrast, the results of this study show that the Mg content of the HMC is by far not pre-determined by the Mg content of the initial ACMC (48 mol% Mg) that was introduced into the solution (Fig. 5A). In experiments performed at temperatures ≤40 °C, the Mg content of the final HMC is significantly lower (e.g. 18 mol% Mg in experiment T_20 °C) than of the initial ACMC, whereas at higher temperatures (≥60 °C), the final HMCs reach Mg contents comparable to those of VHMC (40 mol%; Fig. 5A). These findings clearly document that the Mg content of the forming calcite is strongly regulated by the reaction temperature prevailing during ACMC transformation. It is generally acknowledged that the transformation mechanism of ACMC into (V)HMC involves (i) dissolution of ACMC and (ii) nucleation and crystal growth of (V)HMC.31,36,40,51 The relationship in Fig. 5A suggests that the Mg content of calcite forming at the ACMC-solution interface is significantly controlled by the temperature-dependent dehydration kinetic of aqueous Mg2+. The incorporation of Mg into anhydrous carbonates is generally postulated to be kinetically limited under low-temperature conditions, due to the strong hydration of Mg2+ compared to Ca2+.20,52 In contrast, higher temperatures reduce the Mg-solvation energy barrier and favour anhydrous Ca–Mg-carbonate formation.53 Thus, the amount of Mg incorporated into calcite formed via ACMC is enhanced at higher reaction temperatures (Fig. 5A).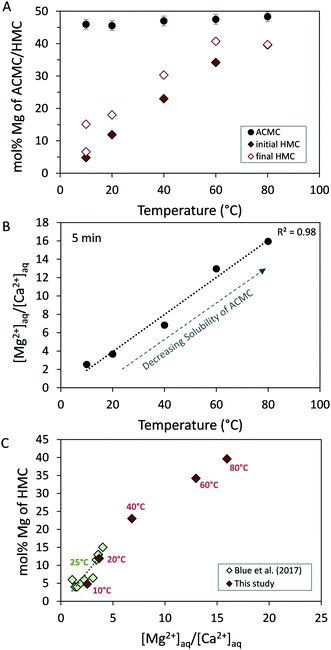 | ||
| Fig. 5 (A) Temperature of the solution versus the Mg content of (i) ACMC obtained at 0.3 min of reaction time ([Mg]solid values in Table 2), (ii) initial HMC obtained at 21 min in experiment T_10 °C and 11 min in experiments performed at ≥20 °C and (iii) final HMC obtained at 1 day of reaction time. Note here that the Mg contents of HMCs were calculated from the d104 values of calcite XRD peaks ([Mg]XRD values in Table 1), whereas the Mg content of ACMC was calculated from solid digestion ([Mg]solid values in Table 2). (B) Temperature of the solution versus the Mg2+/Ca2+ ratio of the solution at 5 min of reaction time (Table S2†). (C) Mg2+/Ca2+ ratio of the solution at 5 min versus the Mg content of (i) initial HMC from this study, of initial HMC from experiments conducted at stirred conditions reported by Blue et al.51 | ||
The obtained results further support previous observations51 that the incorporation of Mg into calcite formed via ACMC is controlled by the Mg2+/Ca2+ ratio of the reactive solution at the time of transformation. Blue et al.51 synthesized ACMCs with different Mg contents under constant pH conditions (pH 8.3–9.1) using a mixed flow reactor and investigated their crystallization pathway in aqueous solution under stirred and unstirred conditions. Their results document a positive correlation between the Mg content of calcite and the Mg2+/Ca2+ ratio of the solution from which the mineral formed. In the experimental setup used in the present study, Mg2+ and CO32− ions were present in the initial solution, whereas Ca2+ ions exclusively originated from the dissolution of the ACMC. At higher temperatures, lower amounts of ACMC dissolved in the NaHCO3–MgCl2 solution as indicated by less Ca2+ ions released into the reactive solution (Fig. 4A). This can be explained by the anticorrelation between amorphous carbonate mineral solubility and temperature (for ACC: Brečević & Nielsen28). Thus, at 5 min of reaction time, the reactive solutions exhibit higher aqueous Mg2+/Ca2+ ratios as a function of increasing temperature (Fig. 5B; Table S2†). Indeed, by comparing the Mg content of the initial HMC with the Mg2+/Ca2+ ratio of the solution after its reaction with the ACMC, we find a good agreement of the results obtained at 25 °C and those reported by Blue et al.51 (Fig. 5C).
During the ongoing transformation of ACMC into HMC, the Mg2+/Ca2+ ratio of the solution increases (Table S2†) due to stronger uptake of Ca2+ compared to Mg2+ from the solution into the solid. This increasing Mg2+/Ca2+ ratio likely results in the formation of HMC with higher Mg contents as a function of reaction time ([Mg]XRD values in Table 1). Hence, the final HMCs exhibit higher Mg contents compared to the initial HMCs (Fig. 5A). This effect is temporally better resolved at low temperatures, due to slower transformation kinetics of ACMC into HMC (Table 1). For example, at 20 °C the Mg content of HMC increases slowly from 12 to 17 mol% between 11 and 61 min of reaction time (Table 1). In contrast, at higher temperatures, the transformation of ACMC into HMC occurs faster and the transition from lower to higher Mg contents for HMCs occurs in shorter time intervals (Table 1).
The positive relationship between aqueous Mg2+/Ca2+ ratios and Mg content of the forming HMC from ACMC transformation (Fig. 5C) indicates that the Mg content into calcite might be enhanced to higher levels by increasing the Mg2+/Ca2+ ratio of the reactive transformation solution. However, experimental studies at 25 °C and moderate pH conditions (8.3–9.1) documented that at higher prevailing Mg2+/Ca2+ ratios (>4: Blue et al.;51 ≥8: Purgstaller et al.42), ACMC transforms into hydrous Ca- or Mg-carbonate phases, i.e. monohydrocalcite and nesquehonite, rather than into calcite, due to the inhibition of the latter.50,51 These observations suggest a maximum limit of about 20 mol% Mg in calcite formed via the amorphous pathway.49–51 It should be emphasized that when ACMC transforms under unstirred conditions, Mg contents of up to 30 mol% Mg for HMC were reported in experimental studies conducted at moderate pH conditions and ambient temperatures.51,54 However the process by which physical mixing influences the formation of distinct crystalline Ca–Mg-carbonates and the Mg content of calcite is still poorly understood and needs further investigations.
4.2 Solubility considerations of ACMC and HMC
At higher reaction temperatures, less ACMC dissolved in the NaHCO3–MgCl2 solutions as it is indicated by the lower concentrations of Ca, Mg and CO3 ions in the reactive solutions at 0.3 min of reaction time (Fig. 4A). In order to assess the effect of temperature on the solubility (K) of ACMC, the activities of Ca2+, Mg2+ and CO32− ions in the reactive solution (Table S3†) and the ACMC stoichiometry (at 0.3 min) were used to calculate KACMC values according to eqn 2 (see also Purgstaller et al.33). The obtained KACMC values for ACMC with 47.0 ± 1.5 mol% Mg (t = 0.3 min) indicate that the solubility of ACMC is lower at higher temperature (Fig. 6A), analogous to the temperature-dependent solubility of ACC determined by Brečević and Nielsen.28 A similar behaviour is also well known for anhydrous carbonate minerals such as calcite55 and magnesite56 which exhibit lower K values at higher temperatures (Fig. 6A). The relationship between the solubility of ACMC (with 47.0 ± 1.5 mol% Mg) and temperature (T in Kelvin) can be described by the polynomial function:| log(KACMC) = −0.0001434 ± 0.0000137 T2 + 0.0760 ± 0.0087 T − 15.537 ± 1.375 (R2 = 0.99) | (3) |
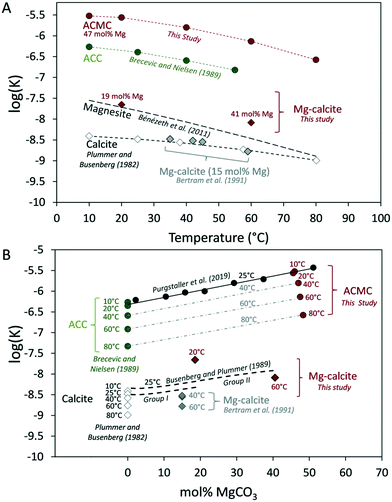 | ||
| Fig. 6 Solublity product (K) of ACC (Brecevic and Nielsen28), ACMC (present study and Purgstaller et al.33), calcite (Plummer and Busenberg55), Mg-calcite (present study, Busenberg and Plummer19 and Bertram et al.57) and magnesite (Bénézeth et al.56) as a function of (A) temperature and (B) Mg mole fraction. The K of ACMC and Mg-calcite was calculated according to eqn 2. | ||
As it can be seen in Fig. 6A the solubility products of ACMC are higher compared to Mg-free ACC at any temperature suggesting that ACMC is more soluble than ACC. This feature can be attributed to the increasing water content and changes in short-range order, as Ca is substituted by Mg in the amorphous structure.33 The relationship between the solubility of ACMC and its Mg content is displayed in Fig. 6B. Note that the slopes of the regression lines between the KACC value of Brečević and Nielsen28 and the KACMC value of this study are similar at 10, 20, 40, 60 and 80 °C (slope = 0.01634 ± 0.00066; Fig. 6B and Table S4†) and are in good agreement with the slope value of the linear relationship between KACMC and the Mg content of ACMC at 25 °C (slope = 0.01602; Mg content of ACMC = 2 to 51 mol%, Table S4†) presented by Purgstaller et al.33 Considering the close agreement between K values obtained in this study and those of Brečević and Nielsen28 and Purgstaller et al.33 a quantitative description of the variations of KACMC with temperature and Mg content of ACMC can be obtained starting from the relationship between ACC solubility (KACC) and temperature (T in Kelvin) determined by Brečević and Nielsen:28
| log(KACC) = −0.0001096 T2 + 0.0545 T − 12.919 | (4) |
| log(KACMC) = 0.01629 × [Mg]ACMC − 0.0001096 T2 + 0.0545 T − 12.919 | (5) |
The dependence of ACMC solubility on the Mg content is similar to that of Mg-calcite (above 4 mol% Mg), where high Mg contents in calcite result in higher solubilities (for 25 °C: Busenberg and Plummer;19Fig. 6B). However, the impact of temperature on the solubility of HMC is not fully elucidated and to our knowledge only Bertram et al. (1991)57 studied the solubility of synthetic Mg-calcites with 1.9 to 15 mol% Mg between 25 to 64 °C and showed that the decrease in solubility of Mg-calcite with increasing temperature parallels that of calcite (see K data of Mg-calcite with 15 mol% Mg in Fig. 6A). No experimental data, however, exist for the solubility dependence of HMC with >15 mol% Mg at elevated temperatures. Fig. 6A and B shows the calculated K values of the final HMC (KHMC) from experiment T_20 °C and T_60 °C. The K-values were calculated using the chemical data and the HMC stoichiometry (based on [Mg]XRD, Table 1) at 1 week and 2 months of reaction time (Table S3†). At this time the system achieved near-equilibrium conditions (Table 2). Note that KHMC values were not calculated in experiments T_10 °C, T_40 °C and T_80 °C because the final solids of T_10 °C consist of two Mg-calcites with different Mg contents (Fig. 2A) and those of T_40 °C and T_80 °C include HMG (6–15 wt%, Table 1). As it can be seen in Fig. 6B, the HMC formed via ACMC at 20 °C (with 18 mol% Mg) is significantly more soluble (log(KHMC) = −7.83) than the biogenic and synthetic Mg-calcites of group II reported by Busenberg and Plummer,19 which exhibit similar Mg contents (e.g. log(KHMC) = −8.16 for biogenic Mg-calcite with 19 mol% Mg at 25 °C). Note that Busenberg and Plummer19 distinguished two groups of Mg-calcites with distinct solubilities, reflecting crystal structure defects (Fig. 6B). Group I consists of metamorphic and hydrothermal Mg-calcites and synthetic Mg-calcites prepared at high temperatures and pressures or low temperatures and low supersaturations (saturation index ≤0.2). Group II Mg-calcites are of either biogenic origin or synthetic Mg-calcites prepared at high supersaturations (saturation index ≥0.5). The Mg-calcites of group I are thought to be relatively free of structural defects, whereas group II Mg-calcites were described as more defective Mg-calcites which exhibit higher solubilities. The discrepancy between the reported solubility value of Busenberg and Plummer19 and of the present study might be explained by different physical defects originating from rapid crystal growth during HMC formation. Considering an ACMC transformation step, the solution in equilibrium with ACMC is highly supersaturated with respect to calcite (saturation index >2 (ref. 31) and 49)). Such high calcite supersaturation degrees probably promote rapid precipitation of HMC at the ACMC-solution interface. Indeed, in the present study, ACMC transformed into nanocrystalline HMC at relatively short time intervals (<60 min, Fig. 2 and 3). These solids are likely more defective and thus exhibit higher solubilities compared to well-crystallized HMC (Fig. 6B). The elevated solubilities of nanocrystalline HMC appears to be consist with recent results of Davis et al.58 who demonstrated, based on microscopic observations, enhanced solubilities of Mg-calcites at the nanometric scale.
5. Conclusions
In the present study, the transformation of ACMC with near-dolomite stoichiometry (47.9 ± 1.5 mol% Mg) into HMC was investigated in a MgCl2–NaHCO3 buffered solution at pH 7.6 and in the temperature range from 10 to 80 ± 1 °C. The results provide an advanced understanding of the complex interplay between the chemical composition of ACMC, the corresponding solution and the finally formed Mg-calcite. At first, the experimental data indicate that the apparent solubility of ACMC is lower at higher temperature and the relative increase of solubility as a function of the Mg content of ACMC is similar from 10 to 80 °C which can be assessed by eqn 5. At ambient temperatures, the Mg content of HMC formed via the transformation of ACMC is as low as 18 mol%, whereas at 60 and 80 °C, the final HMCs reach Mg levels comparable with those of VHMC (40 mol%). Hence, the Mg content of the HMC is not pre-determined by the Mg content of the amorphous precursor, but strongly influenced by the reaction temperature during ACMC transformation. The transformation of ACMC to HMC proceeds via a dissolution and re-precipitation mechanism where at higher temperatures the amount of Mg incorporated into calcite is enhanced due to (i) the reduced solvation energy barrier of aqueous Mg2+ and (ii) the high Mg2+/Ca2+ ratio of the reactive solution after its reaction with the ACMC. The high Mg2+/Ca2+ ratio of the latter solution can be explained by low solubility of ACMC at elevated temperatures. The obtained solubilities of the final HMC are higher compared to those of well-crystalline Mg-bearing calcites, which is most likely caused by nanometric-sized HMC crystals with structural defects originated from fast crystal growth.Abbreviations
| ACC | Amorphous calcium carbonate |
| ACMC | Amorphous calcium magnesium carbonate |
| ARG | Aragonite |
| FWHM | Full-width at half-maximum |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| LMC | Low Mg-calcite |
| HMC | High Mg-calcite |
| HMG | Hydromagnesite |
| SEM | Scanning electron microscope |
| TGA | Thermogravimetric analyses |
| XRD | X-ray diffraction |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Stefanie Eichinger for her support with the analytical work and to Isaac Kell-Duivestein for providing the Coorong dolomite sample. Chemical analyses were conducted at NAWI Graz Central Lab for Water, Minerals and Rocks. This work has been financially supported by Austrian Science Fund (FWF) project number T920-N29, and in part by DFG-FWF collaborative research initiative CHARON II (DFG Forschergruppe 1644; FWF I3028-N29) and by NAWI Graz.References
- J. W. Morse and F. T. Mackenzie, in Developments in Sedimentology 48, Elsevier, 1990, p. 725 Search PubMed.
- R. J. Reeder, in Reviews in Mineralogy, Mineralogical Society of America, 11th edn, 1990, p. 399 Search PubMed.
- F. T. Mackenzie, W. D. Bischoff, F. C. Bishop, M. Loijens, J. Schoonmaker and R. Wollast, Carbonates Mineral. Chem., 1983, pp. 97–144 Search PubMed.
- W. D. Bischoff, S. K. Sharma and F. T. MacKenzie, Am. Mineral., 1985, 70, 581–589 CAS.
- J. M. Gregg, D. L. Bish, S. E. Kaczmarek and H. G. Machel, Sedimentology, 2015, 1749–1769 CrossRef CAS.
- M. E. Tucker and V. P. Wright, Carbonate Sedimentology, Blackwell Scientific Publications, Boston, 1990 Search PubMed.
- V. Mavromatis, R. Botz, M. Schmidt, V. Liebetrau and C. Hensen, Int. J. Earth Sci., 2014, 103, 1831–1844 CrossRef CAS.
- A. Paul and S. W. Lokier, Sediment. Geol., 2017, 352, 1–13 CrossRef CAS.
- S. Bentov and J. Erez, Geochem., Geophys., Geosyst., 2006, 7, Q01P08 CrossRef.
- S. Raz, S. Weiner and L. Addadi, Adv. Mater., 2000, 12, 38–42 CrossRef CAS.
- M. C. Nash, U. Troitzsch, B. N. Opdyke, J. M. Trafford, B. D. Russell and D. I. Kline, Biogeosciences, 2011, 8, 3331–3340 CrossRef CAS.
- S. Gayathri, R. Lakshminarayanan, J. C. Weaver, D. E. Morse, R. Manjunatha Kini and S. Valiyaveettil, Chem. – Eur. J., 2007, 13, 3262–3268 CrossRef CAS.
- J. H. Schroeder, E. J. Dwornik and J. J. Papike, Geol. Soc. Am. Bull., 1969, 80, 1613–1616 CrossRef CAS.
- Y. Ma, B. Aichmayer, O. Paris, P. Fratzl, A. Meibom, R. A. Metzler, Y. Politi, L. Addadi, P. U. P. A. Gilbert and S. Weiner, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6048–6053 CrossRef CAS.
- F. C. Furman, R. E. Woody, M. A. Rasberry, D. J. Keller and J. M. Gregg, Proc. Sixth Symp. Geol. Bahamas, 1993, 47–54 Search PubMed.
- C. Vasconcelos and J. A. McKenzie, J. Sediment. Res., 1997, 67, 378–390 CAS.
- J. B. Sumrall, E. B. Larson and J. E. Mylroie, Carbonates Evaporites, 2017, 32, 123–133 CrossRef CAS.
- D. Fussmann, A. J. E. Von Hoyningen-Huene, A. Reimer, D. Schneider, H. Babková, R. Peticzka, A. Maier, G. Arp, R. Daniel and P. Meister, Biogeosciences, 2020, 17, 2085–2106 CrossRef CAS.
- E. Busenberg and L. Niel Plummer, Geochim. Cosmochim. Acta, 1989, 53, 1189–1208 CrossRef CAS.
- F. Lippmann, Sedimentary carbonate minerals, Springer-Verlag Berlin, 1973, vol. 12 Search PubMed.
- R. A. Berner, Geochim. Cosmochim. Acta, 1975, 39, 489–504 CrossRef CAS.
- L. Fernandez-Diaz, A. Putnis, M. Prieto and C. V. Putnis, J. Sediment. Res., 1996, 66, 482–491 CAS.
- K. E. Goetschl, B. Purgstaller, M. Dietzel and V. Mavromatis, Geochim. Cosmochim. Acta, 2019, 265, 505–519 CrossRef CAS.
- G. D. Markham, J. P. Glusker, C. L. Bock, M. Trachtman and C. W. Bock, J. Phys. Chem., 1996, 100, 3488–3497 CrossRef CAS.
- A. Bleuzen, P. A. Pittet, L. Helm and A. E. Merbach, Magn. Reson. Chem., 1997, 35, 765–773 CrossRef CAS.
- J. Schott, O. Pokrovsky and E. Oelkers, Rev. Mineral. Geochem., 2009, 70, 207–258 CrossRef.
- T. Ogino, T. Suzuki and K. Sawada, J. Cryst. Growth, 1990, 100, 159–167 CrossRef CAS.
- L. Brečević and A. E. Nielsen, J. Cryst. Growth, 1989, 98, 504–510 CrossRef.
- L. Addadi, S. Raz and S. Weiner, Adv. Mater., 2003, 15, 959–970 CrossRef CAS.
- Y. Politi, D. R. Batchelor, P. Zaslansky, B. F. Chmelka, J. C. Weaver, I. Sagi, S. Weiner and L. Addadi, Chem. Mater., 2010, 22, 161–166 CrossRef CAS.
- J. D. Rodriguez-Blanco, S. Shaw and L. G. Benning, Am. Mineral., 2015, 100, 1172–1181 CrossRef.
- A. V. Radha, A. Fernandez-Martinez, Y. Hu, Y. S. Jun, G. A. Waychunas and A. Navrotsky, Geochim. Cosmochim. Acta, 2012, 90, 83–95 CrossRef CAS.
- B. Purgstaller, K. E. Goetschl, V. Mavromatis and M. Dietzel, CrystEngComm, 2019, 21, 155–164 RSC.
- J. D. Rodriguez-Blanco, S. Shaw, P. Bots, T. Roncal-Herrero and L. G. Benning, J. Alloys Compd., 2012, 536, S477–S479 CrossRef CAS.
- C. J. Lin, S. Y. Yang, S. J. Huang and J. C. C. Chan, J. Phys. Chem. C, 2015, 119, 7225–7233 CrossRef CAS.
- J. M. Xto, H. Du, C. N. Borca, E. Amstad, J. A. Van Bokhoven and T. Huthwelker, Cryst. Growth Des., 2019, 19, 4385–4394 CrossRef CAS.
- X. Long, Y. Ma and L. Qi, Cryst. Growth Des., 2011, 11, 2866–2873 CrossRef CAS.
- H. Yang, S. Chai, Y. Zhang and Y. Ma, CrystEngComm, 2015, 18, 157–163 RSC.
- M. Schmidt, S. Xeflide, R. Botz and S. Mann, Geochim. Cosmochim. Acta, 2005, 69, 4665–4674 CrossRef CAS.
- I. J. Kelleher and S. A. T. Redfern, Mol. Simul., 2002, 28, 557–572 CrossRef CAS.
- F. Konrad, F. Gallien, D. E. Gerard and M. Dietzel, Cryst. Growth Des., 2016, 16, 6310–6317 CrossRef CAS.
- B. Purgstaller, M. Dietzel, A. Baldermann and V. Mavromatis, Geochim. Cosmochim. Acta, 2017, 217, 128–143 CrossRef CAS.
- F. Konrad, B. Purgstaller, F. Gallien, V. Mavromatis, P. Gane and M. Dietzel, J. Cryst. Growth, 2018, 498, 381–390 CrossRef CAS.
- J. R. Goldsmith, D. L. Graf and H. C. Heard, Am. Mineral., 1961, 46, 453–459 CAS.
- C. R. Blue and P. M. Dove, Geochim. Cosmochim. Acta, 2015, 148, 23–33 CrossRef CAS.
- G. Montes-Hernandez, F. Renard, R. Chiriac, N. Findling and F. Toche, Cryst. Growth Des., 2012, 12, 5233–5240 CrossRef CAS.
- W. Bischoff, F. Bishop and F. Mackenzie, Am. Mineral., 1983, 68, 1183–1188 CAS.
- F. Zhang, H. Xu, H. Konishi and E. E. Roden, Am. Mineral., 2010, 95, 1650–1656 CrossRef CAS.
- B. Purgstaller, V. Mavromatis, A. Immenhauser and M. Dietzel, Geochim. Cosmochim. Acta, 2016, 174, 180–195 CrossRef CAS.
- B. Purgstaller, F. Konrad, M. Dietzel, A. Immenhauser and V. Mavromatis, Cryst. Growth Des., 2017, 17, 1069–1078 CrossRef CAS.
- C. R. Blue, A. Giuffre, S. Mergelsberg, N. Han, J. J. De Yoreo and P. M. Dove, Geochim. Cosmochim. Acta, 2017, 196, 179–196 CrossRef CAS.
- V. Mavromatis, Q. Gautier, O. Bosc and J. Schott, Geochim. Cosmochim. Acta, 2013, 114, 188–203 CrossRef.
- F. Di Lorenzo, R. M. Rodríguez-Galán and M. Prieto, Mineral. Mag., 2014, 78, 1363–1372 CrossRef.
- N. Han, C. R. Blue, J. J. De Yoreo and P. M. Dove, Procedia Earth Planet. Sci., 2013, 7, 223–227 CrossRef CAS.
- N. Plummer and E. Busenberg, Geochim. Cosmochim. Acta, 1982, 46, 1011–1040 CrossRef.
- P. Bénézeth, G. D. Saldi, J. L. Dandurand and J. Schott, Chem. Geol., 2011, 286, 21–31 CrossRef.
- M. A. Bertram, F. T. Mackenzie, F. C. Bishop and W. D. Bischoff, Am. Mineral., 1991, 76, 1889–1896 CAS.
- K. J. Davis, P. M. Dove and J. J. De Yoreo, Science, 2000, 290, 1134–1137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: ICSD database code and reference used for mineral phase quantification; Table S2: concentrations of free Ca2+ and Mg2+ ions and calculated Mg2+/Ca2+ ratios of the experimental solutions; Table S3: activities of Ca2+, Mg2+ and CO32− ions in the experimental solutions and calculated solubility product values (K) for ACMC and HMC; Table S4: summary of solubility data of amorphous Ca-(Mg-)carbonates; Fig. S1: XRD pattern, SEM image and thermal analysis of synthesized ACMC; Fig. S2: temporal evolution of pH and HCl solution addition to the experimental solution; Fig. S3: XRD patterns of solids collected during ACMC transformation experiments at a 2θ range from 4° to 65°; Fig. S4: XRD pattern and SEM image of the reference Coorong dolomite. See DOI: 10.1039/d0ce01679a |
| This journal is © The Royal Society of Chemistry 2021 |