Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Tethered CAAC–CAAC dimers: oxidation to persistent radical cations and bridging-unit dependent reactivity/stability of the dications
Mithilesh Kumar
Nayak
a,
Simon
Suhr
bc,
Nicolas
Chrysochos
d,
Hemant
Rawat
a,
Carola
Schulzke
*d,
Vadapalli
Chandrasekhar
*ae,
Biprajit
Sarkar
*bc and
Anukul
Jana
*a
aTata Institute of Fundamental Research Hyderabad, Gopanpally, Hyderabad-500046, Telangana, India. E-mail: ajana@tifrh.res.in
bInstitut für Chemie und Biochemie, Freie Universität Berlin, Fabeckstraße 34-36, 14195 Berlin, Germany
cUniversität Stuttgart, Fakultät Chemie, Lehrstuhl für Anorganische Koordinationschemie, Institut für Anorganische Chemie, Pfaffenwaldring 55, Stuttgart, D-70569, Germany. E-mail: biprajit.sarkar@iac.uni-stuttgart.de
dInstitut für Biochemie, Universität Greifswald, Felix-Hausdorff-Straße 4, Greifswald, D-17489, Germany. E-mail: carola.schulzke@uni-greifswald.de
eDepartment of Chemistry, Indian Institute of Technology Kanpur, Kanpur-208016, India. E-mail: vc@iitk.ac.in
First published on 7th May 2021
Abstract
Correction for ‘Tethered CAAC–CAAC dimers: oxidation to persistent radical cations and bridging-unit dependent reactivity/stability of the dications’ by Mithilesh Kumar Nayak et al., Chem. Commun., 2021, 57, 1210–1213, DOI: 10.1039/D0CC07385G.
The authors regret that some data in the original article was mis-interpreted.
In case of 2-electron oxidation, the ethylene bridged CAAC–CAAC dimer leads to the dication 6Et with the concurrent elimination of two hydrogens. For the trans-1,2-cyclohexylene bridged CAAC–CAAC dimer, however, it leads to the dication 5Cy instead of 6Cy as reported in the original article. Consequently, sections of the text in the manuscript should be adjusted according to this change, and these are detailed below.
The CCDC number, 1971758, refers to compound 5Cy rather than compound 6Cy as originally reported.
Scheme 3 has been revised to reflect this change
The caption of Fig. 3 should read “Fig. 3 Solid-state structures of 4Cy (left) and 5Cy (right). Hydrogen atoms are omitted for clarity.”
The paragraph on page 1212 beginning “The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reactions of 3Et and 3Cy with AgOTf did not lead to the isolation of the expected dications 5Et and 5Cy…” should be correctly given as “The 1
2 reactions of 3Et and 3Cy with AgOTf did not lead to the isolation of the expected dications 5Et and 5Cy…” should be correctly given as “The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reactions of 3Et and 3Cy with AgOTf led to the isolation of 6Et (72%) and 5Cy (80%), respectively (Scheme 3). The elimination of two hydrogen atoms for 3Et is most likely driven forward by the gain of aromaticity. The theoretical calculation at the B3LYP/def2-TZVP level of theory for the ethylene bridged analogue as a representative example suggests that the reaction is exergonic by ΔG = −0.92 kcal mol−1.18 Crystallographic analysis showed that the central C4N2-six-membered ring is planar for 6Et and non-planar for 5Cy with a N1–C4–C3–N2 dihedral angle of 33.72° (Fig. S67, ESI,‡ and Fig. 3).”
2 reactions of 3Et and 3Cy with AgOTf led to the isolation of 6Et (72%) and 5Cy (80%), respectively (Scheme 3). The elimination of two hydrogen atoms for 3Et is most likely driven forward by the gain of aromaticity. The theoretical calculation at the B3LYP/def2-TZVP level of theory for the ethylene bridged analogue as a representative example suggests that the reaction is exergonic by ΔG = −0.92 kcal mol−1.18 Crystallographic analysis showed that the central C4N2-six-membered ring is planar for 6Et and non-planar for 5Cy with a N1–C4–C3–N2 dihedral angle of 33.72° (Fig. S67, ESI,‡ and Fig. 3).”
The paragraph on page 1212 beginning “Surprisingly, the results obtained from electrochemistry and spectroelectrochemistry are in contrast to the chemical reduction of 6Et/6Cy…” should be correctly given as “The results obtained from electrochemistry and spectroelectrochemistry are in line with the chemical reduction of 6Et (ESI‡).”
In the sentence on page 1212 beginning “In contrast to 3Et and 3Cy, the oxidation of 3Pr…”, 3Cy should be removed.
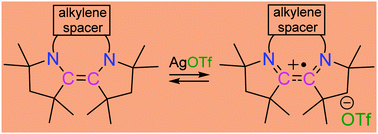
The corrected table of contents entry is shown below:
The electronic supplementary information and crystal structure data have been updated to reflect this correction.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2021 |