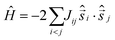Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
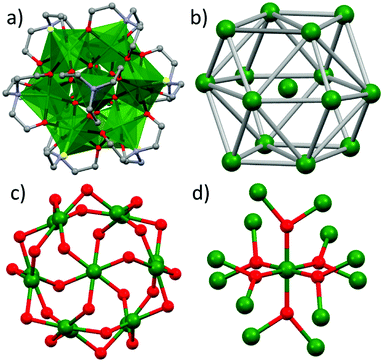
[Fe15]: a frustrated, centred tetrakis hexahedron†
Daniel J.
Cutler
 a,
Mukesh K.
Singh
a,
Mukesh K.
Singh
 a,
Gary S.
Nichol
a,
Gary S.
Nichol
 a,
Marco
Evangelisti
a,
Marco
Evangelisti
 b,
Jürgen
Schnack
b,
Jürgen
Schnack
 *c,
Leroy
Cronin
*c,
Leroy
Cronin
 *d and
Euan K.
Brechin
*d and
Euan K.
Brechin
 *a
*a
aEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, Scotland, UK. E-mail: ebrechin@ed.ac.uk
bInstituto de Nanociencia y Materiales de Aragón (INMA), CSIC – Universidad de Zaragoza, 50009 Zaragoza, Spain
cFakultät für Physik, Universität Bielefeld, Postfach 100131, D-33501 Bielefeld, Germany. E-mail: jschnack@uni-bielefeld.de
dSchool of Chemistry, The University of Glasgow, Joseph Black Building, Glasgow, G12 8QQ, Scotland, UK. E-mail: lee.cronin@glasgow.ac.uk
First published on 9th August 2021
Abstract
The combination of two different FeIII salts in a solvothermal reaction with triethanolamine results in the formation of a high symmetry [FeIII15] cluster whose structure conforms to a centred, tetrakis hexahedron.
Homometallic compounds of FeIII have played a central role in the history of molecular magnetism, proving key to the development and understanding of an array of physical properties. For example, the study of oxo-bridged [Fe2] dimers allowed the development of detailed magneto-structural correlations that can be translated to larger species,1 antiferromagnetically coupled [Fe6–12] ferric wheels revealed interesting quantum size effects manifested in stepped magnetisation,2 [Fe17/19] was an early example of a molecule possessing a very large spin ground state,3 [Fe8] was the second known example of a single-molecule magnet,4 [Fe14] was an early example of a compound displaying an enhanced magnetocaloric effect,5 and cages ranging in nuclearity from [Fe13] to [Fe34] have structures that conform to Archimedean and Platonic solids which aid understanding of the self-assembly of molecular oxides en route to mineral phases.6 High symmetry clusters are of particular interest as they may possess geometric spin frustration, a phenomenon whose definition has evolved from its strict initial derivation.7 Frustration can lead to some unusual and potentially useful low-temperature physics, a beautiful example being the [Mo72Fe30] icosidodecahedron which shows anomalous magnetisation behaviour in an applied magnetic field.8
One synthetic methodology proven to enable the construction of such species is hydro/solvothermal synthesis, which typically exploits superheating reaction solutions under autogenous pressure.9 In the chemistry of polynuclear cluster compounds of paramagnetic transition metal ions, the temperature regimes employed (which are typically below 250 °C) can lead to enhanced solubility, reduced solvent viscosity and increased reagent diffusion. The result is often the synthesis of metastable kinetic products of high symmetry, with slow cooling enabling pristine crystal growth directly from the reaction mixture.10
The solvothermal reaction of FeCl3, Fe(ClO4)3·6H2O and teaH3 (triethanolamine) in a basic MeOH solution results in the formation of red/brown crystals upon cooling (see the Experimental section in the ESI† for full details). Crystals of [FeIII15O6(tea)8Cl6](OH)(ClO4)2 (1, Fig. 1 and Fig. S1, Table S1, ESI†) were found to be in a trigonal crystal system, and structure solution was performed in the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (see the Crystallographic section in the ESI† for full details). The metallic skeleton of 1 describes a centred tetrakis hexahedron (Fig. 2), a Catalan solid which is the dual of the truncated octahedron, an Archimedean solid. The central Fe ion (Fe4) is octahedral, being bonded to six oxide ions ([FeO6]; O5 and symmetry equivalent; Fe–O5 = 1.999 Å). O5 bridges to two further Fe ions in the peripheral shell (O5–Fe1 = 1.925 Å; O5–Fe2 = 2.025 Å) and is thus three coordinate and trigonal planar. There is a fourth, longer contact to Fe3 (O5–Fe3 = 2.492 Å), so O5 may be considered pseudo-tetrahedral if one considers this interaction significant (Fig. 2 and Fig. S2, S3, ESI†). The outer shell is decorated with a combination of halide and tea3− ions. The former are monodentate, coordinated to Fe2 (Fe2–Cl, 2.315 Å). The latter are tetradentate, chelating either Fe1 or Fe3 with each O-atom μ-bridging to a second Fe ion (Fe2). Thus Fe1 is five-coordinate ([FeO4N]) and trigonal pyramidal, Fe2 is six-coordinate ([FeO5Cl)] and octahedral, and Fe3 is four coordinate ([FeO3N]) and trigonal prismatic, or seven coordinate ([FeO6N)] and a capped octahedron if the Fe–O5 bonds are included (Fig. S3 and S4, ESI†). A review of the Cambridge Structural Database (CSD) for Fe–O bond lengths in any Fe–O–Fe moiety produces 3378 different compounds and 12
space group (see the Crystallographic section in the ESI† for full details). The metallic skeleton of 1 describes a centred tetrakis hexahedron (Fig. 2), a Catalan solid which is the dual of the truncated octahedron, an Archimedean solid. The central Fe ion (Fe4) is octahedral, being bonded to six oxide ions ([FeO6]; O5 and symmetry equivalent; Fe–O5 = 1.999 Å). O5 bridges to two further Fe ions in the peripheral shell (O5–Fe1 = 1.925 Å; O5–Fe2 = 2.025 Å) and is thus three coordinate and trigonal planar. There is a fourth, longer contact to Fe3 (O5–Fe3 = 2.492 Å), so O5 may be considered pseudo-tetrahedral if one considers this interaction significant (Fig. 2 and Fig. S2, S3, ESI†). The outer shell is decorated with a combination of halide and tea3− ions. The former are monodentate, coordinated to Fe2 (Fe2–Cl, 2.315 Å). The latter are tetradentate, chelating either Fe1 or Fe3 with each O-atom μ-bridging to a second Fe ion (Fe2). Thus Fe1 is five-coordinate ([FeO4N]) and trigonal pyramidal, Fe2 is six-coordinate ([FeO5Cl)] and octahedral, and Fe3 is four coordinate ([FeO3N]) and trigonal prismatic, or seven coordinate ([FeO6N)] and a capped octahedron if the Fe–O5 bonds are included (Fig. S3 and S4, ESI†). A review of the Cambridge Structural Database (CSD) for Fe–O bond lengths in any Fe–O–Fe moiety produces 3378 different compounds and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 bond lengths ranging from a minimum value of 1.651 Å to a maximum value of 2.629 Å, as depicted in the histogram in Fig. S5 (ESI†).
361 bond lengths ranging from a minimum value of 1.651 Å to a maximum value of 2.629 Å, as depicted in the histogram in Fig. S5 (ESI†).
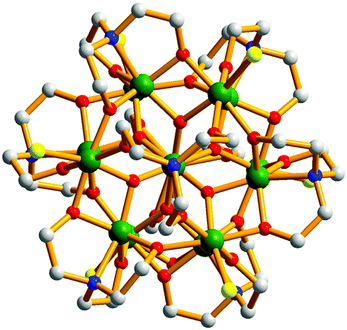 | ||
| Fig. 1 Molecular structure of the cation of 1 viewed down the c-axis of the unit cell. Colour code: Fe = green, O = red, N = blue, C = grey, Cl = yellow. H atoms omitted for clarity. | ||
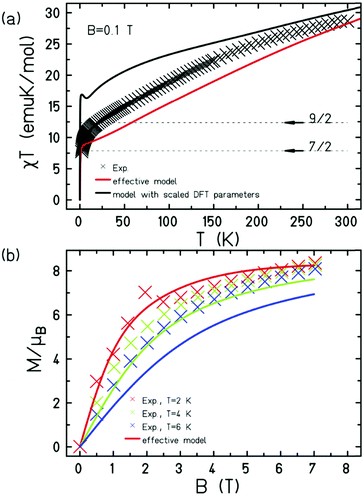 | ||
| Fig. 3 (a) Magnetic susceptibility (χT) versus temperature (T) data for 1 measured in an applied field, B = 0.1 T in the T = 300–2 K temperature range. (b) Magnetisation (M) versus field (B) data in the 2–6 K temperature and 0–7 T field ranges. The solid lines are a simulation of the experimental data (x) using the finite-temperature Lanczos method. The effective model denotes a Heisenberg model with high symmetry, as described in the main text. The black line in (a) denotes the model employing DFT parameters given in Table S3 (ESI†) in a Heisenberg model scaled by 25/9 taking into account the use of spins, S = 3/2. | ||
The [Fe15O30] core displays a breadth of different Fe–O–Fe angles, ranging from a minimum of 86.67° (Fe4–O5–Fe3) to a maximum of 140.82° (Fe4–O5–Fe2). Angles from the central Fe4 ion to the peripheral Fe1 and Fe2 ions via the O5 oxide are 140.82° and 117.41°, while those connecting the outer Fe1, Fe2 and Fe3 ions together via the oxides and alkoxides range between 86.7–129.39° (Table S2, ESI†). The closest intermolecular interactions are between the monodentate Cl ions on Fe2 and the C-atoms of the tea3− ligands on neighbouring molecules (Cl1⋯C7, ∼3.43 Å), and between the perchlorate O-atoms and the C-atoms of the tea3− ligands (O6⋯C4, ∼3.43 Å; Fig. S6 and S7, ESI†). This results in an aesthetically pleasing honeycomb-like network when viewed down the c-axis of the cell. A search of the CSD reveals that just three [Fe15] clusters have been reported previously,11 with 1 being the first example of a [centred] tetrakis hexahedron. Perhaps more surprisingly, given the widespread use of the H3tea ligand in 3d coordination chemistry, there are very few homometallic FeIII clusters of this ligand deposited. Indeed, they are limited to [Fe5],12 [Fe6] wheels (both unsupported13 and supported14), [Fe7],15 [Fe8]16 (including an [Fe8] cluster self-assembled into a [Fe64] cage17), and [Fe10].18 Heterometallic Fe–Ln species are far more prevalent.19
The direct-current (dc) molar magnetic susceptibility, χ, of a polycrystalline sample of 1 was measured in an applied magnetic field, B, of 0.1 T, over the 2–300 K temperature, T, range. The results are plotted in Fig. 3 in the form of χT product, where χ = M/B with M the magnetisation. At room temperature, the χT product is 28.67 emu K mol−1, much lower than the Curie constant expected for fifteen uncorrelated S = 5/2 centres (65.625 emu K mol−1) with g = 2. On lowering the temperature, the χT product decreases rapidly, reaching a value of 11.05 emu K mol−1) at T = 10 K, before decreasing even more abruptly to a value of 7.74 emu K mol−1 at T = 2 K. The data is therefore indicative of competing antiferromagnetic exchange interactions, and a ground state spin of S ≈ 9/2 (compare arrows in Fig. 3). Variable-temperature-variable-field (VTVB) dc magnetisation measurements in the temperature range 2–6 K and in applied magnetic fields up to 7 T reach a maximum value of just M = 8.35 μB (Fig. 3b), well below the upper limit expected for a ferromagnetically coupled system (M = 75 μB for g = 2). This behaviour is clearly indicative of relatively strong antiferromagnetic interactions between the FeIII ions, consistent with the Fe–O distances and Fe–O–Fe angles present.1
It is computationally impossible to quantitatively analyse the magnetic data of a molecule containing 15 × S = 5/2 spins via conventional matrix diagonalisation techniques since the dimension of the Hilbert space is 470,184,984,576 and thus we turn to the finite-temperature Lanczos method.20 Even here, several assumptions must be made. (A) Despite the presence of eight independent exchange interactions, we reduce this to four based on similar Fe–O bond lengths and Fe–O–Fe angles (Fig. S8, ESI†). These are: Jcube along edges of the cube; Jpyramid along the four edges from the top of each pyramid to the respective base square of the cube; Jc,cube from the central Fe inside the cube to vertices of the cube; and Jc,pyramid from the central Fe ion to the tops of pyramids. (B) We simulate the data using isotropic S = 3/2 spins rather than isotropic S = 5/2 spins and scale the resulting data accordingly. The corresponding isotropic spin-Hamiltonian is:
To further support the relative sign and magnitude of the coupling constants obtained, we have performed DFT calculations (see the ESI† for the Computational details) on a model complex, 1A, derived from complex 1 (Fig. S10 and S11, ESI†). These suggest that the eight independent exchange interactions are in the range |J| = 4.6–16.4 cm−1 (Table S3, ESI†), in good agreement with the experimental simulations. All are antiferromagnetic in nature, with the exception of the Fe4–(μ4-O2−)3–Fe3 interaction which affords J = +4.6 cm−1 on account of the large Fe–O bond lengths and small Fe–O–Fe bond angles present which lead to orbital orthogonality. Overlap integral calculations23 using metal-based singly occupied molecular orbitals (SOMOs) reveal that the strongest antiferromagnetic interactions occur where there are a higher number of strong or moderate overlap integrals, and vice versa (Fig. S12 and S13, ESI†). For the Fe4–(μ4-O2−)3–Fe3 interaction (J1 in Tables S3 and S4, ESI†) there is only one strong interaction (dz2||dxz) with the remaining 24 interactions being weak. The overall result is a weak/moderate ferromagnetic interaction. See the ESI† for more information. Spin density analysis suggests that strong spin delocalisation is present in 1 with FeIII spin densities ranging between 4.007–4.151 (Fig. S14, ESI†).
It is somewhat unusual for synthetic chemists to employ two different metal salts for the formation of homometallic cluster compounds containing paramagnetic 3d metals, since the anions are often considered solely as charge balancing moieties rather than structure-directing agents. This observation has certainly prompted us to re-examine a number of reactions to probe whether it may be of general applicability, or if it is of more limited scope. Here, the use of both FeCl3, Fe(ClO4)3·6H2O with teaH3 in a high temperature, high pressure reaction leads to the formation of an aesthetically pleasing [Fe15] cage conforming to a centred, tetrakis hexahedron. The high symmetry of the metallic skeleton leads to the presence of competing antiferromagnetic exchange interactions and spin frustration. Use of the finite temperature Lanczos method allows for “an order of magnitude” estimation of the exchange constants present, a computationally non-trivial task for a molecule containing fifteen S = 5/2 spins. Values of Jcube = −17.4 cm−1, Jpyramid = −17.4 cm−1, Jc,cube = −17.4 cm−1 and Jc,pyramid = −3.5 cm−1 are consistent with parameters obtained from DFT calculations which fall in the range +4.6 to −16.4 cm−1, and with low temperature heat capacity data which reflects the presence of a large density of low-lying spin states.
EKB and LC thank U21/EPSRC for funding a studentship (DJC). MKS would like to thank Edinburgh Compute and Data Facility (ECDF), and the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 832488. ME thanks the Spanish Ministry of Science and Innovation (Project RTI2018-098537-B-C22).
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Weihe and H. U. Güdel, J. Am. Chem. Soc., 1998, 120, 2870–2879 CrossRef CAS; C. Cañada-Vilalta, T. A. O'Brien, E. K. Brechin, M. Pink, E. R. Davidson and G. Christou, Inorg. Chem., 2004, 43, 5505–5521 CrossRef PubMed.
- R. W. Saalfrank, I. Bernt, E. Uller and F. Hampel, Angew. Chem., Int. Ed. Engl., 1997, 36, 2482–2485 CrossRef CAS; A. Caneschi, A. Cornia and S. J. Lippard, Angew. Chem., Int. Ed. Engl., 1995, 34, 467–469 CrossRef; K. L. Taft, C. D. Delfs, G. C. Papaefthymiou, S. Foner, D. Gatteschi and S. J. Lippard, J. Am. Chem. Soc., 1994, 116, 823–832 CrossRef; A. Caneschi, A. Cornia, A. C. Fabretti and D. Gatteschi, Angew. Chem., Int. Ed., 1999, 38, 1295–1297 CrossRef PubMed.
- A. K. Powell, S. L. Heath, D. Gatteschi, L. Pardi, R. Sessoli, G. Spina, F. Del Giallo and F. Pieralli, J. Am. Chem. Soc., 1995, 117, 2491–2502 CrossRef CAS.
- C. Delfs, D. Gatteschi, L. Pardi, R. Sessoli, K. Wieghardt and D. Hanke, Inorg. Chem., 1993, 32, 3099–3103 CrossRef CAS.
- R. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin and E. J. L. McInnes, Inorg. Chem., 2007, 46, 4968–4978 CrossRef CAS PubMed.
- A. Bino, M. Ardon, D. Lee, B. Spingler and S. J. Lippard, J. Am. Chem. Soc., 2002, 124, 4578–4579 CrossRef CAS PubMed; O. Sadeghi, L. N. Zakharov and M. Nyman, Science, 2015, 347, 1359–1362 CrossRef PubMed; G. W. Powell, H. N. Lancashire, E. K. Brechin, D. Collison, S. L. Heath, T. Mallah and W. Wernsdorfer, Angew. Chem., Int. Ed., 2004, 43, 5772–5775 CrossRef PubMed; C. Vecchini, D. H. Ryan, L. M. D. Cranswick, M. Evangelisti, W. Kockelmann, P. G. Radaelli, A. Candini, M. Affronte, I. A. Gass, E. K. Brechin and O. Moze, Phys. Rev. B, 2008, 77, 224403 CrossRef; O. Nachtigall, M. Kusserow, R. Clérac, W. Wernsdorfer, M. Menzel, F. Renz, J. Mrozinski and J. Spandl, Angew. Chem., Int. Ed., 2015, 54, 10361–10364 CrossRef PubMed; A. E. Dearle, D. J. Cutler, H. W. L. Fraser, S. Sanz, E. Lee, S. Dey, I. F. Diaz-Ortega, G. S. Nichol, H. Nojiri, M. Evangelisti, G. Rajaraman, J. Schnack, L. Cronin and E. K. Brechin, Angew. Chem., Int. Ed., 2019, 58, 16903–16906 CrossRef PubMed; N. A. G. Bandeira, O. Sadeghi, T. J. Woods, Y.-Z. Zhang, J. Schnack, K. Dunbar, M. Nyman and X. Bo, J. Phys. Chem. A, 2017, 121, 1310–1318 CrossRef PubMed.
- G. Toulouse, Comm. Phys., 1977, 2, 115–119 Search PubMed; S. Kirkpatrick, Phys. Rev. B, 1977, 16, 4630–4641 CrossRef CAS; A. P. Ramirez, Annu. Rev. Mater. Sci., 1994, 24, 453 CrossRef; O. Kahn, Chem. Phys. Lett., 1997, 265, 109–114 CrossRef; C. Schröder, H. Nojiri, J. Schnack, P. Hage, M. Luban and P. Kögerler, Phys. Rev. Lett., 2005, 94, 017205 CrossRef PubMed; J. Schnack, Dalton Trans., 2010, 39, 4677–4686 RSC; M. L. Baker, G. A. Timco, S. Piligkos, J. S. Mathieson, H. Mutka, F. Tuna, P. Kozłowski, M. Antkowiak, T. Guidi, T. Gupta, H. Rath, R. J. Woolfson, G. Kamieniarz, R. G. Pritchard, H. Weihe, L. Cronin, G. Rajaraman, D. Collison, E. J. L. McInnes and R. E. P. Winpenny, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 19113–19118 CrossRef PubMed; J. W. Sharples, D. Collison, E. J. L. McInnes, J. Schnack, E. Palacios and M. Evangelisti, Nat. Commun., 2014, 5, 5321 CrossRef PubMed; M. Murugesu, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2005, 44, 6678–6682 CrossRef PubMed.
- V. O. Garlea, S. E. Nagler, J. L. Zarestky, C. Stassis, D. Vaknin, P. Kögerler, D. F. McMorrow, C. Niedermayer, D. A. Tennant, B. Lake, Y. Qiu, M. Exler, J. Schnack and M. Luban, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 024414 CrossRef.
- M. I. Khan and J. Zubieta, Prog. Inorg. Chem., 1995, 43, 1–149 CAS.
- R. H. Laye and E. J. L. McInnes, Eur. J. Inorg. Chem., 2004, 2811–2818 CrossRef CAS.
- Y. Hou, X. Fang, K. D. Kwon, L. J. Criscenti, D. Davis, T. Lambert and M. Nyman, Eur. J. Inorg. Chem., 2013, 1780–1787 CrossRef CAS; E. Freire, M. Quintero, D. Vega and R. Baggio, Inorg. Chim. Acta, 2013, 394, 229–236 CrossRef; A.-Q. Zhang and L.-L. Liu, Transit. Met. Chem., 2017, 42, 753–761 CrossRef.
- W. Schmitt, L. Zhang, C. E. Anson and A. K. Powell, Dalton Trans., 2010, 39, 10279–10285 RSC.
- A. Baniodeh, Y. Liang, C. E. Anson, N. Magnani, A. K. Powell, A.-N. Unterreiner, S. Seyfferle, M. Slota, M. Dressel, L. Bogani and K. Goß, Adv. Funct. Mater., 2014, 24, 6280–6290 CrossRef CAS.
- R. W. Saalfrank, I. Bernt, E. Uller and F. Hampel, Angew. Chem., Int. Ed. Engl., 1997, 36, 2482–2485 CrossRef CAS.
- L. F. Jones, P. Jensen, B. Moubaraki, K. J. Berry, J. F. Boas, J. R. Pilbrow and K. S. Murray, J. Mater. Chem., 2006, 16, 2690–2697 RSC.
- O. Waldmann, R. Koch, S. Schromm, J. Schülein, P. Müller, I. Bernt, R. W. Saalfrank, F. Hampel and E. Balthes, Inorg. Chem., 2001, 40, 2986–2995 CrossRef CAS PubMed; G. Xiong, Y.-L. Hou, J.-Z. Cui and B. Zhao, Inorg. Chem. Commun., 2013, 35, 89–91 CrossRef; A. M. Ako, O. Waldmann, V. Mereacre, F. Klower, I. J. Hewitt, C. E. Anson, H. U. Güdel and A. K. Powell, Inorg. Chem., 2007, 46, 756–766 CrossRef PubMed; O. Botezat, J. van Leusen, V. Ch. Kravtsov, A. Ellern, P. Kögerler and S. G. Baca, Dalton Trans., 2015, 44, 20753–20762 RSC; I. A. Gass, C. J. Milios, A. Collins, F. J. White, L. Budd, S. Parsons, M. Murrie, S. P. Perlepes and E. K. Brechin, Dalton Trans., 2008, 2043–2053 RSC; M. Murugesu, K. A. Abboud and G. Christou, Dalton Trans., 2003, 4552–4556 RSC.
- T. Liu, Y.-J. Zhang, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2008, 130, 10500–10501 CrossRef CAS PubMed.
- S.-J. Liu, S.-D. Han, J.-M. Jia, L. Xue, Y. Cui, S.-M. Zhang and Z. Chang, CrystEngComm, 2014, 16, 5212–5215 RSC.
- M. Murugesu, A. Mishra, W. Wernsdorfer, K. A. Abboud and G. Christou, Polyhedron, 2006, 25, 613–625 CrossRef CAS; O. Botezat, J. van Leusen, V. Ch. Kravtsov, P. Kögerler and S. G. Baca, Inorg. Chem., 2017, 56, 1814–1822 CrossRef PubMed.
- J. Jaklič and P. Prelovšek, Phys. Rev. B, 1994, 49, 5065–5068 CrossRef CAS PubMed; J. Schnack and O. Wendland, Eur. Phys. J. B, 2010, 78, 535–541 CrossRef; J. Schnack, J. Richter and R. Steinigeweg, Phys. Rev. Res., 2020, 2, 013186 CrossRef.
- M. K. Singh and G. Rajaraman, Inorg. Chem., 2019, 58, 3175–3188 CrossRef CAS PubMed; K. J. Mitchell, K. A. Abboud and G. Christou, Inorg. Chem., 2016, 55, 6597 CrossRef PubMed.
- A. Baniodeh, N. Magnani, Y. Lan, G. Buth, C. E. Anson, J. Richter, M. Affronte, J. Schnack and A. K. Powell, npj Quant. Mater., 2018, 3, 10 CrossRef.
- M. Coletta, S. Sanz, D. J. Cutler, S. J. Teat, K. J. Gagnon, M. K. Singh, E. K. Brechin and S. J. Dalgarno, Dalton Trans., 2020, 49, 14790–14797 RSC; M. Coletta, T. G. Tziotzi, M. Gray, G. S. Nichol, M. K. Singh, C. J. Milios and E. K. Brechin, Chem. Commun., 2021, 57, 4122–4125 RSC; S. Hazra, S. Bhattacharya, M. K. Singh, L. Carrella, E. Rentschler, T. Weyhermueller, G. Rajaraman and S. Mohanta, Inorg. Chem., 2013, 52, 12881–12892 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, X-ray diffraction data, computational details. CCDC 2090901. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc03919a |
| This journal is © The Royal Society of Chemistry 2021 |