Tertiary cyclopropyl carbagermatranes: synthesis and cross-coupling†
Abstract
The construction of the cyclopropyl quaternary carbon center can afford a series of 1,1-olefin bioisosteres. Here, we report tertiary cyclopropyl carbagermatranes, which can be easily obtained by the zinc-mediated decarboxylation of NHP esters. In addition, they exhibit efficient reactivity in the palladium-catalyzed cross-coupling reaction and orthogonal reactivity with boron reagents, therefore acting as robust nucleophiles for the synthesis of tertiary cyclopropane and efficient intermediates for the formation of quaternary centers.
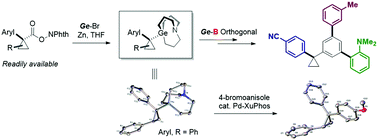
- This article is part of the themed collections: 10th Anniversary of the Youth Innovation Promotion Association of the Chinese Academy of Science and Chemical Communications HOT articles


 Please wait while we load your content...
Please wait while we load your content...