Open Access Article
Open Access ArticleAnion binding properties of a hollow PdL-cage†
Brian J. J.
Timmer
 and
Tiddo J.
Mooibroek
and
Tiddo J.
Mooibroek
 *
*
van ‘t Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, Amsterdam, 1098 XH, The Netherlands. E-mail: t.j.mooibroek@uva.nl
First published on 23rd June 2021
Abstract
The hollow [PdL][BArF]2 complex 1 of a tetra-pyridyl (py) ligand (L) has a [Pd(py)4]2+ coordination environment. Addition of coordinating anions resulted in the formation of a neutral species with Pd(py)2(anion)2 coordination environment (12A). These species bind further to the coordinating anions in the order Cl− > N3− > Br− > I− > AcO− with Ka1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ≤ 414 M−1. With relatively non-coordinating anions 1 remains intact and displays 1
1 ≤ 414 M−1. With relatively non-coordinating anions 1 remains intact and displays 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding behaviour dominated by the 1
2 binding behaviour dominated by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in the order NO3− (∼105 M−1) » ClO4− and BF4− (∼103 M−1). As evidenced by crystal structure data, DFT calculations and {1H–19F}-HOESY NMR with BF4−, the anions are bound by charge assisted [C–H]+···anion interactions.
1 stoichiometry in the order NO3− (∼105 M−1) » ClO4− and BF4− (∼103 M−1). As evidenced by crystal structure data, DFT calculations and {1H–19F}-HOESY NMR with BF4−, the anions are bound by charge assisted [C–H]+···anion interactions.
Anion recognition chemistry has potential applications in the biochemical domain, medicine, and catalysis, and anion binders can be used to detect/extract anionic pollutants from waste streams.1 Conceptual strategies to achieve selective anion recognition are plentiful1 and historically have revolved around the use of metal bonding,2 hydrogen bonding interactions,3 or a combination thereof.4 In recent years it has become clear that other types of interactions such as anion–π interactions,5 halogen bonds6 and other σ–hole interactions7 can be used to interact with anions. It has even been reported that the weakly polarized hydrogen atoms of C–H bonds can act as hydrogen bond donors for anions, especially when several such interactions work in concert.8 Unsurprisingly, employment of electron withdrawing groups such as a somewhat distant positive charge can reinforce these otherwise weak C–H⋯anion interactions.8b,9 Molecular frameworks that preorganize hydrogen bond donors are typically flexible or more rigid podand-like architectures,8i,10 or macrocyclic designs.8f,11 Discrete coordination compounds where the metal acts as organizing entity (e.g. large molecular knots and links,12 and pyridyl complexes13) have also been explored for their solution phase anion recognition potential. One interesting class of compounds in this regard are the so-called ‘M2L4 cages’ where ‘M’ is a transition metal ion and ‘L’ is a ditopic ligand.14 When M is a square-planar metal such as Pd2+ or Pt2+ and the ligand is a dipyridyl ligand of appropriate size, the resulting M2L4 coordination cage can bear a hollow interior suitable for hosting other molecules.14a–c Examples of guests include anti-cancer drugs,15 carbohydrates,16 and anions.17 Interestingly, anion binding with Pd2L4 complexes can be facilitated by (charge assisted) C–H⋯anion interactions,17a and M2L4 complexes have been reported as selective binders for nitrate17b or perchlorate.17c,17d
We recently reported on the new type of hollow molecule 1 shown in Fig. 1a for the purpose of binding carbohydrates.18 This hybrid-design of the type ‘PdL’ combines design principles from M2L4 coordination cages with those of potent covalent macrocyclic receptors.19 A particularly attractive feature of the design represented by 1 is that the formation of a hollow molecule is nearly stoichiometric as opposed to the often low-yielding macrocyclizations needed to make covalent macrocycles. Indeed, the addition of Pd(BArF)216b to the parent ligand gives 1 in a stoichiometric fashion based on NMR analysis.
 | ||
| Fig. 1 (a) Coordination compound 1 studied in this work for anion recognition. R = –(CH2)2-p-Ph-C(para-t-Bu-Ph)3 solubility handle and the counter anions are tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (BArF);18 (b) perspective views of the molecular electrostatic potential map of a fragment of 1 calculated at the DFT/ωB97X-D/6-31G* level of theory. The fragment was derived from an energy minimized structure of 1 and for clarity the ‘bottom’ biphenyl with four methylamides are omitted. The colour scale ranges from 41–167 kcal mol−1. | ||
It was observed that when 1 binds to monosaccharides, the polarized C–H bonds of the coordinated pyridyl ligands are typically involved in a (charge assisted) [C–H]+⋯O hydrogen bonding interaction. This made us wonder about the anion binding properties of 1 and the possible role of charge-assisted [C–H]+⋯anion interactions.
Particularly as the inwards facing C–H fragments are very polarized due to the dicationic nature of the [Pd(py)4]2+ complex that gives structure to the cavity. As is shown in Fig. 1b, an electrostatic potential map of a fragment of 1 indicates that the positive potential on these C–H fragments (+150 kcal mol−1) is similar to that on the adjacent amidic N–H protons (+167 kcal mol−1).
Binding of 1 to anions was studied by monitoring the 1H-NMR resonances of 1 as a function of increasing concentration of the +N(n-Bu)4 salts listed in Table 1.
| Entry | An. | K a (M−1) for 12A | Goodness of fit (r2) |
K
a
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (M−1) 1 (M−1) |
K
a
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (M−1) 2 (M−1) |
Goodness of fit (r2) |
|---|---|---|---|---|---|---|
| for 1 | ||||||
| 1 | Cl− | 414 | 0.9979 | — | ||
| 2 | Br− | 169 | 0.9982 | — | ||
| 3 | I− | 74 | 0.9942 | — | ||
| 4 | N3− | 193 | 0.9887 | — | ||
| 5 | AcO− | 15 | 0.9777 | — | ||
| 6 | NO3− | — | 91.960 | 2.484 | 0.9976 | |
| 7 | ClO4− | — | 6.102 | 33 | 0.9941 | |
| 8 | BF4− | — | 4.141 | 24 | 0.9965 | |
| 9 | PF6− | — | — | |||
As is shown in Fig. 2a, addition of one equivalent of Cl− led to the disappearance of resonances that belong to the 1 with the proportional appearance of an unsymmetrical species. This is particularly evident for the resonances belonging to the inwards pointing s3-NH (10.5 ppm) and C–H p2 (9.5 ppm). Both resonances are replaced by two sets of four resonances in the region 10.7–10.5 and 9.2–9.8 ppm respectively (highlighted with red lines). Addition of more Cl− caused the gradual disappearance of these eight resonances with the concomitant emergence of two sets of new resonances around 10.5 ppm in a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio. These resonances shifted about 0.4 ppm downfield upon addition of more Cl− salt, and the shifts could be fitted to a 1
1.5 molar ratio. These resonances shifted about 0.4 ppm downfield upon addition of more Cl− salt, and the shifts could be fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model with Ka = 414 M−1 as listed in entry 1 of Table 1 (see also Fig. S1, ESI†). These observations are consistent with pyridyl ligand (py) displacement by one anion (forming [Pd(py)3(anion)]+, 1A) followed by a second (forming [Pd(py)2(anion)2], 12A). Given that for the species 12A, two sets of signals were observed, it is likely that these originate from cis- and trans-isomers. A similar phenomenon has been observed before with Pd2L4 cages, but leading exclusively to trans-coordinated neutral rings.20 Species like 12A were also obtained in the titrations with Br−, I−, N3−, and AcO− with accompanying affinities given in entries 2–5 of Table 1. These affinities are ordered Cl− > N3− > Br− > I− > AcO−, which is likely a reflection of the relative ‘hardness’21 of these anions.
1 binding model with Ka = 414 M−1 as listed in entry 1 of Table 1 (see also Fig. S1, ESI†). These observations are consistent with pyridyl ligand (py) displacement by one anion (forming [Pd(py)3(anion)]+, 1A) followed by a second (forming [Pd(py)2(anion)2], 12A). Given that for the species 12A, two sets of signals were observed, it is likely that these originate from cis- and trans-isomers. A similar phenomenon has been observed before with Pd2L4 cages, but leading exclusively to trans-coordinated neutral rings.20 Species like 12A were also obtained in the titrations with Br−, I−, N3−, and AcO− with accompanying affinities given in entries 2–5 of Table 1. These affinities are ordered Cl− > N3− > Br− > I− > AcO−, which is likely a reflection of the relative ‘hardness’21 of these anions.
 | ||
Fig. 2 (a) Partial 1H-NMR spectra of 1 titrated with Cl− salt. The top spectrum of 1 is assigned (see inset figure for labels) and the red lines are added as a guide to the eye. For larger scale graphic see Fig. S1 (ESI†); (b) partial 1H-NMR spectra of 1 titrated with NO3− salt. The top spectrum of 1 is assigned (see inset figure for labels and Fig. S6, ESI† for larger scale graphic); (c) HypNMR fit of peak shifting involving the indicated signals of 1 during the titration with nitrate salt. Speciation is also giving as coloured lines. Fitting to all 168 data point gave r2 = 0.9976 and Ka2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 36 M−1; Ka1 1 = 36 M−1; Ka1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 91.960 M−1 and Ka1 2 = 91.960 M−1 and Ka1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 2.484 M−1. Solvent = in CD2Cl2 with 5% DMSO-d6 (v/v). 2 = 2.484 M−1. Solvent = in CD2Cl2 with 5% DMSO-d6 (v/v). | ||
The titrations of 1 with salts of the relatively non-coordinating NO3−,22 ClO4−, BF4− and PF6− anions did not result in the formation of new species. Instead, as is illustrated for nitrate in Fig. 2b, only peak shifting occurred. Notably, the resonance of the inwards pointing pyridinic C–H p2 around 9.5 ppm shifted upfield to about 9.1 ppm after addition of about one equivalent of nitrate. Addition of more nitrate caused the resonance to shift an additional ∼0.1 ppm. Contrariwise, the major shift of the outwards pointing pyridinic C–H p3 was observed after adding one equivalent of nitrate, and occurred in a downfield direction. The upfield shift of p2 can be seen as atypical13,22 and likely originates from displacement of interior bound DMSO, and/or a conformational change of the pyridyl rings upon binding of the nitrate (p5 also shifted significantly).
These shifts are highly indicative of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host (1) to guest (nitrate) binding stoichiometry with very strong 1
2 host (1) to guest (nitrate) binding stoichiometry with very strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding to the interior of 1 (p2 shifts first) and weaker exterior 1
1 binding to the interior of 1 (p2 shifts first) and weaker exterior 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding (p3 shifts later). The shifts of s3-NH, p2, p3, p5, b2 and s4 were used simultaneously for curve-fitting analysis with HypNMR,23 as is detailed in Fig. S6 (ESI†). As anticipated, straightforward fitting to a 1
2 binding (p3 shifts later). The shifts of s3-NH, p2, p3, p5, b2 and s4 were used simultaneously for curve-fitting analysis with HypNMR,23 as is detailed in Fig. S6 (ESI†). As anticipated, straightforward fitting to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model of 1vs nitrate was not possible. Unexpectedly, assuming a 1
1 binding model of 1vs nitrate was not possible. Unexpectedly, assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 model did not give an accurate fit (r2 = 0.9328). As is shown in Fig. 2c, also incorporating 2
2 model did not give an accurate fit (r2 = 0.9328). As is shown in Fig. 2c, also incorporating 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding resulted in an excellent fit to give Ka2
1 binding resulted in an excellent fit to give Ka2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 36 M−1, Ka1
1 = 36 M−1, Ka1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 91.960 M−1 and Ka1
1 = 91.960 M−1 and Ka1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 2.484 M−1 (r2 = 0.9976 over 168 data points). Presumably, nitrate anions can act as a bridge between two molecules of 1 by binding to the exterior of the cage. Such a 2
2 = 2.484 M−1 (r2 = 0.9976 over 168 data points). Presumably, nitrate anions can act as a bridge between two molecules of 1 by binding to the exterior of the cage. Such a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 species would be present only in the very beginning of the titration, when 1 is in excess.
1 species would be present only in the very beginning of the titration, when 1 is in excess.
In the titrations with ClO4− and BF4− significant shifting of resonances was also observed (Fig. S7 and S8, ESI†). The resulting peak shifts could be fitted accurately to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding model without incorporation of the small 2
2 binding model without incorporation of the small 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding constant that was necessary in the case of nitrate. The resulting binding constants are listed in Table 1 and are about two orders of magnitude less than observed for nitrate. The weaker binding can rationalize why the 2
1 binding constant that was necessary in the case of nitrate. The resulting binding constants are listed in Table 1 and are about two orders of magnitude less than observed for nitrate. The weaker binding can rationalize why the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry did not have to be incorporated in the fit for ClO4− and BF4−. For NO3−, ClO4− and BF4− the 1
1 stoichiometry did not have to be incorporated in the fit for ClO4− and BF4−. For NO3−, ClO4− and BF4− the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was significantly larger than 1
1 stoichiometry was significantly larger than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding (entries 6–8 in Table 1). The 1
2 binding (entries 6–8 in Table 1). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry likely signifies binding of nitrate with the interior of 1 (i.e.: p2 is shifting while p3 is stationary), followed by 1
1 stoichiometry likely signifies binding of nitrate with the interior of 1 (i.e.: p2 is shifting while p3 is stationary), followed by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding to the exterior of 1 (i.e.: p2 is stationary while p3 is shifting). Additional evidence for this dual binding mode was obtained in the form of a {1H–19F}-HOESY NMR spectrum of a sample of 1 with BF4− (Fig. S10, ESI†). Clear intermolecular nuclear Overhauser effect (nOe) cross peaks were observed between BF4− and the inwards pointing s3-NH, p2, and s4, as well as with the outwards pointing p3. In the titration with PF6− (Table 1, entry 9) only relatively small shifts were observed, which could not be fitted accurately to obtain a binding constant (see Fig. S9, ESI†). Apparent, like the BArF anion, PF6− does not have any specific interactions with 1. This was confirmed by {1H–19F}-HOESY NMR spectroscopy of a sample of 1 containing PF6−, where no intermolecular nOe was observed (Fig. S10, ESI†).
2 binding to the exterior of 1 (i.e.: p2 is stationary while p3 is shifting). Additional evidence for this dual binding mode was obtained in the form of a {1H–19F}-HOESY NMR spectrum of a sample of 1 with BF4− (Fig. S10, ESI†). Clear intermolecular nuclear Overhauser effect (nOe) cross peaks were observed between BF4− and the inwards pointing s3-NH, p2, and s4, as well as with the outwards pointing p3. In the titration with PF6− (Table 1, entry 9) only relatively small shifts were observed, which could not be fitted accurately to obtain a binding constant (see Fig. S9, ESI†). Apparent, like the BArF anion, PF6− does not have any specific interactions with 1. This was confirmed by {1H–19F}-HOESY NMR spectroscopy of a sample of 1 containing PF6−, where no intermolecular nOe was observed (Fig. S10, ESI†).
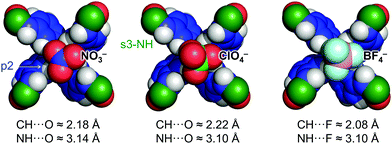
The binding mode of NO3−, ClO4− and BF4− to the interior of 1 was modelled with density functional theory (DFT) calculations and (parts of) the resulting molecular models are shown in Fig. 3. In all three cases, the average C–H⋯O/F distance involving pyridinic C–H p2 are about 1 Å shorter than the average N–H⋯O/F distance with the amide s3-NH. Actually, the average C–H⋯O/F distances are about 0.4 Å shorter than the sum of the van der Waals radii for H (1.09 Å) and O (1.52 Å) or F (1.47 Å). The average N–H⋯O/F distances on the other hand, are about 0.5 Å longer than this benchmark.
It is thus likely that interior binding of 1 for anions is established predominantly by charge-assisted [C–H]+⋯anion interactions (as was also evidenced for BF4− with HOESY NMR). Moreover, a model of [1·PF6−]+ shown in Fig. S11 (ESI†) reveals that PF6− barely fits inside 1 and likely experiences F⋯π repulsion with the biphenyl part of 1. This may offer a rational for the lack of binding observed with PF6−.
Finally, as is detailed in Section S5 (ESI†), a survey of the Cambridge Structure Database revealed that [C–H]+⋯anion interactions involving complexes of the type [Pd(py)4]2+ are rather common. The survey also indicated a clear preference of such interactions in the order NO3− > ClO4− ≈ BF4− » PF6−, which is consistent with the observed order in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding affinities (Table 1). Three concrete examples of crystal structures with NO3− (FEDYOF),24 ClO4− (YUPCUK),25 and BF4− (TIFXEM)26 are shown in Fig. 4. In each case, the anion is situated very similarly as observed in the models obtained by DFT (Fig. 3) and short C–H⋯O/F distances are present. Interestingly, in the di-acetone solvate complex [Pd(pyridine)4][NO3][PF6] FEDYOF, the PF6− anions are not located near Pd, which implies that [Pd(py)4]2+ complexes are selective for nitrate over PF6− in the solid state. This is consistent with the strong binding of 1 observed for NO3− and the absence of binding for PF6−. Moreover, the nitrate anions act as a bridge in between [Pd(pyridine)4]2+ complexes to form an infinite one dimensional chain in the crystal structure. This can be seen as evidence for the feasibility of a 1
1 binding affinities (Table 1). Three concrete examples of crystal structures with NO3− (FEDYOF),24 ClO4− (YUPCUK),25 and BF4− (TIFXEM)26 are shown in Fig. 4. In each case, the anion is situated very similarly as observed in the models obtained by DFT (Fig. 3) and short C–H⋯O/F distances are present. Interestingly, in the di-acetone solvate complex [Pd(pyridine)4][NO3][PF6] FEDYOF, the PF6− anions are not located near Pd, which implies that [Pd(py)4]2+ complexes are selective for nitrate over PF6− in the solid state. This is consistent with the strong binding of 1 observed for NO3− and the absence of binding for PF6−. Moreover, the nitrate anions act as a bridge in between [Pd(pyridine)4]2+ complexes to form an infinite one dimensional chain in the crystal structure. This can be seen as evidence for the feasibility of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry in solution. The observed bridging function of nitrate also lends further credence to the 2
2 stoichiometry in solution. The observed bridging function of nitrate also lends further credence to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry that was needed to accurately fit the titration data with NO3− (Fig. 2c). The dual binding mode to a [Pd(pyridine)4]2+ complex was also observed in TIFXEM with BF4−, but the anion does not bridge two [Pd(pyridine)4]2+ complexes. This is in line with the model used to fit the titration data with BF4− to a 1
1 stoichiometry that was needed to accurately fit the titration data with NO3− (Fig. 2c). The dual binding mode to a [Pd(pyridine)4]2+ complex was also observed in TIFXEM with BF4−, but the anion does not bridge two [Pd(pyridine)4]2+ complexes. This is in line with the model used to fit the titration data with BF4− to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 model without the use of a 2
2 model without the use of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
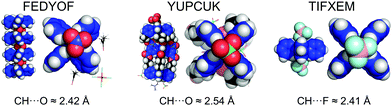 | ||
| Fig. 4 Crystal structures of [Pd(py)4]2+ complexes with a nitrate (FEDYOF),24 perchlorate (YUPCUK)25 and tetrafluoroborate (TIFXEM)26 anion bound to the Pd-complex with [C–H]+⋯anion interactions very similar to those modelled with DFT for 1 (Fig. 3). | ||
In summary, Pd-complex 1 reacts with coordinating anions to eventually form charge neutral species with a Pd(py)2(anion)2 coordination environment (12A). These species bind further to the coordinating anions in the order Cl− > N3− > Br− > I− > AcO− with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry and affinities below 103 M−1.
1 binding stoichiometry and affinities below 103 M−1.
With relatively non-coordinating anions, complex 1 remains intact and displays clear binding in the order NO3− (∼105 M−1) » ClO4− and BF4− (∼103 M−1), while no binding was observed for PF6−. The dominant binding stoichiometry is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is likely binding to the interior of 1. For NO3−, ClO4− and BF4−, a weaker 1
1, which is likely binding to the interior of 1. For NO3−, ClO4− and BF4−, a weaker 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry was also observed, while for NO3− an additional and very weak 2
2 stoichiometry was also observed, while for NO3− an additional and very weak 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry had to be included in the fit. {1H–19F}-HOESY NMR of a sample of 1 and BF4− confirmed the 1
1 stoichiometry had to be included in the fit. {1H–19F}-HOESY NMR of a sample of 1 and BF4− confirmed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding mode of 1. Several crystal structures also support such 1
2 binding mode of 1. Several crystal structures also support such 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 geometries as well as the 2
2 geometries as well as the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for a nitrate anion bridging two [Pd(py)4]2+ complexes. The crystal structure data, as well as DFT calculation of 1 further evidence that NO3−, ClO4− and BF4− anions are bound to [Pd(py)4]2+ complexes by charge assisted [C–H]+⋯anion interactions. We conclude that 1 is highly selective for nitrate, but likely too labile for actual application purposes. Adjustments of the parent ligand of 1 (e.g. to a di-picolinic acid derivative) in conjunction with the employment of octahedral metals might result in such more stable neutral species.
1 stoichiometry for a nitrate anion bridging two [Pd(py)4]2+ complexes. The crystal structure data, as well as DFT calculation of 1 further evidence that NO3−, ClO4− and BF4− anions are bound to [Pd(py)4]2+ complexes by charge assisted [C–H]+⋯anion interactions. We conclude that 1 is highly selective for nitrate, but likely too labile for actual application purposes. Adjustments of the parent ligand of 1 (e.g. to a di-picolinic acid derivative) in conjunction with the employment of octahedral metals might result in such more stable neutral species.
BJJT conducted the experimental work and helped write the paper. TJM wrote the paper and directed the study.
This research was financially supported by the Netherlands Organization for Scientific Research (NWO) with VIDI grant number 723.015.006.
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS PubMed.
- (a) B. J. Holliday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022–2043 CrossRef CAS; (b) E. J. O'Neil and B. D. Smith, Coord. Chem. Rev., 2006, 250, 3068–3080 CrossRef.
- (a) V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 RSC; (b) K. Kavallieratos, C. M. Bertao and R. H. Crabtree, J. Org. Chem., 1999, 64, 1675–1683 CrossRef CAS PubMed.
- D. J. Mercer and S. J. Loeb, Chem. Soc. Rev., 2010, 39, 3612–3620 RSC.
- (a) M. Staffilani, K. S. B. Hancock, J. W. Steed, K. T. Holman, J. L. Atwood, R. K. Juneja and R. S. Burkhalter, J. Am. Chem. Soc., 1997, 119, 6324–6335 CrossRef CAS; (b) D. X. Wang and M. X. Wang, J. Am. Chem. Soc., 2013, 135, 892–897 CrossRef CAS PubMed; (c) B. L. Schottel, H. T. Chifotides and K. R. Dunbar, Chem. Soc. Rev., 2008, 37, 68–83 RSC; (d) A. Frontera, P. Gamez, M. Mascal, T. J. Mooibroek and J. Reedijk, Angew. Chem., Int. Ed., 2011, 50, 9564–9583 CrossRef CAS PubMed; (e) H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2013, 46, 894–906 CrossRef CAS PubMed; (f) A. Bauza, T. J. Mooibroek and A. Frontera, CrystEngComm, 2016, 18, 10–23 RSC.
- (a) G. Cavallo, P. Metrangolo, T. Pilati, G. Resnati, M. Sansotera and G. Terraneo, Chem. Soc. Rev., 2010, 39, 3772–3783 RSC; (b) M. J. Langton, S. W. Robinson, I. Marques, V. Felix and P. D. Beer, Nat. Chem., 2014, 6, 1039–1043 CrossRef CAS PubMed.
- (a) A. Bauza, T. J. Mooibroek and A. Frontera, ChemPhysChem, 2015, 16, 2496–2517 CrossRef CAS PubMed; (b) P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2013, 15, 11178–11189 RSC; (c) S. Benz, M. Macchione, Q. Verolet, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2016, 138, 9093–9096 CrossRef CAS PubMed.
- (a) M. Nishio, Phys. Chem. Chem. Phys., 2011, 13, 13873–13900 RSC; (b) J. J. Cai and J. L. Sessler, Chem. Soc. Rev., 2014, 43, 6198–6213 RSC; (c) Y. Choi, T. Kim, S. Jang and J. Kang, New J. Chem., 2016, 40, 794–802 RSC; (d) H. W. Wu, Y. Y. Chen, C. H. Rao and C. X. Liu, Prog. Chem., 2016, 28, 1501–1514 CAS; (e) Y. X. Yuan, N. N. Wu, Y. F. Han, X. Z. Song and H. B. Wang, J. Cent. South Univ., 2016, 23, 1023–1031 CrossRef CAS; (f) Y. J. Li and A. H. Flood, Angew. Chem., Int. Ed., 2008, 47, 2649–2652 CrossRef CAS PubMed; (g) P. Molina, F. Zapata and A. Caballero, Chem. Rev., 2017, 117, 9907–9972 CrossRef CAS PubMed; (h) T. S. Pandian and J. Kang, Tetrahedron Lett., 2015, 56, 4191–4194 CrossRef CAS; (i) M. Lisbjerg, H. Valkenier, B. M. Jessen, H. Al-Kerdi, A. P. Davis and M. Pittelkow, J. Am. Chem. Soc., 2015, 137, 4948–4951 CrossRef CAS PubMed.
- B. W. Tresca, R. J. Hansen, C. V. Chau, B. P. Hay, L. N. Zakharov, M. M. Haley and D. W. Johnson, J. Am. Chem. Soc., 2015, 137, 14959–14967 CrossRef CAS PubMed.
- (a) P. R. Brotherhood and A. P. Davis, Chem. Soc. Rev., 2010, 39, 3633–3647 RSC; (b) J. A. Cooper, S. T. G. Street and A. P. Davis, Angew. Chem., Int. Ed., 2014, 53, 5609–5613 CrossRef CAS PubMed; (c) H. Y. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurcek, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- (a) M. J. Chmielewski and J. Jurczak, Chem. – Eur. J., 2005, 11, 6080–6094 CrossRef CAS PubMed; (b) K. H. Choi and A. D. Hamilton, Coord. Chem. Rev., 2003, 240, 101–110 CrossRef CAS.
- J. F. Ayme, J. E. Beves, C. J. Campbell, G. Gil-Ramirez, D. A. Leigh and A. J. Stephens, J. Am. Chem. Soc., 2015, 137, 9812–9815 CrossRef CAS PubMed.
- (a) C. R. Bondy, P. A. Gale and S. J. Loeb, Chem. Commun., 2001, 729–730, 10.1039/b101440o; (b) C. R. Bondy, P. A. Gale and S. J. Loeb, J. Supramol. Chem., 2002, 2, 93–96 CrossRef CAS; (c) C. R. Bondy, P. A. Gale and S. J. Loeb, J. Am. Chem. Soc., 2004, 126, 5030–5031 CrossRef CAS PubMed.
- (a) M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848–1860 RSC; (b) A. Schmidt, A. Casini and F. E. Kuhn, Coord. Chem. Rev., 2014, 275, 19–36 CrossRef CAS; (c) F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Nat. Rev. Chem., 2019, 3, 204–222 CrossRef; (d) V. Marti-Centelles, R. L. Spicer and P. J. Lusby, Chem. Sci., 2020, 11, 3236–3240 RSC.
- (a) C. Y. Zhu, M. Pan and C. Y. Su, Isr. J. Chem., 2019, 59, 209–219 CrossRef CAS; (b) F. Kaiser, A. Schmidt, W. Heydenreuter, P. J. Altmann, A. Casini, S. A. Sieber and F. E. Kuhn, Eur. J. Inorg. Chem., 2016, 5189–5196, DOI:10.1002/ejic.201600811; (c) A. Schmidt, V. Molano, M. Hollering, A. Pothig, A. Casini and F. E. Kuhn, Chem. – Eur. J., 2016, 22, 2253–2256 CrossRef CAS PubMed.
- (a) M. Yamashina, M. Akita, T. Hasegawa, S. Hayashi and M. Yoshizawa, Sci. Adv., 2017, 3, 6 Search PubMed; (b) X. Schaapkens, E. O. Bobylev, J. N. H. Reek and T. J. Mooibroek, Org. Biomol. Chem., 2020, 18, 4734–4738 RSC; (c) X. Schaapkens, E. O. Bobylev, J. H. Holdener, J. Tolboom, J. N. H. Reek and T. J. Mooibroek, ChemPhysChem, 2021, 22, 1187–1192 CrossRef CAS PubMed.
- (a) T. Y. Kim, N. T. Lucas and J. D. Crowley, Supramol. Chem., 2015, 27, 734–745 CrossRef; (b) L. P. Zhou and Q. F. Sun, Chem. Commun., 2015, 51, 16767–16770 RSC; (c) E. Sone, M. Sato, M. Mochizuki, C. Kamio, K. Yamanishi and M. Kondo, CrystEngComm, 2016, 18, 5004–5011 RSC; (d) P. J. Steel and D. A. McMorran, Chem. – Asian J., 2019, 14, 1098–1101 CrossRef CAS PubMed; (e) C. Y. Fu, Y. Q. Li, L. Chen, Y. G. Wang and L. R. Lin, Inorg. Chim. Acta, 2019, 495, 10 CrossRef.
- B. J. J. Timmer, A. Kooijman, A. Schaapkens and T. J. Mooibroek, Angew. Chem., Int. Ed., 2021, 60, 11168–11172 CrossRef PubMed.
- (a) T. J. Mooibroek, M. P. Crump and A. P. Davis, Org. Biomol. Chem., 2016, 14, 1930–1933 RSC; (b) P. Rios, T. J. Mooibroek, T. S. Carter, C. Williams, M. R. Wilson, M. P. Crump and A. P. Davis, Chem. Sci., 2017, 8, 4056–4061 RSC; (c) O. Francesconi, M. Martinucci, L. Badii, C. Nativi and S. Roelens, Chem. – Eur. J., 2018, 24, 6828–6836 CrossRef CAS PubMed; (d) P. Stewart, C. M. Renney, T. J. Mooibroek, S. Ferheen and A. P. Davis, Chem. Commun., 2018, 54, 8649–8652 RSC; (e) O. Francesconi and S. Roelens, ChemBioChem, 2019, 20, 1329–1346 CrossRef CAS PubMed; (f) R. A. Tromans, T. S. Carter, L. Chabanne, M. P. Crump, H. Y. Li, J. V. Matlock, M. G. Orchard and A. P. Davis, Nat. Chem., 2019, 11, 52–56 CrossRef CAS PubMed; (g) A. P. Davis, Chem. Soc. Rev., 2020, 49, 2531–2545 RSC; (h) O. Francesconi, F. Milanesi, C. Nativi and S. Roelens, Angew. Chem., Int. Ed., 2021, 60, 11168–11172 CrossRef CAS PubMed.
- (a) R. M. Zhu, J. Lubben, B. Dittrich and G. H. Clever, Angew. Chem., Int. Ed., 2015, 54, 2796–2800 CrossRef CAS PubMed; (b) D. Preston, A. Fox-Charles, W. K. C. Lo and J. D. Crowley, Chem. Commun., 2015, 51, 9042–9045 RSC.
- (a) T. L. Ho, Chem. Rev., 1975, 75, 1–20 CrossRef CAS; (b) P. K. Chattaraj, H. Lee and R. G. Parr, J. Am. Chem. Soc., 1991, 113, 1855–1856 CrossRef CAS.
- (a) W. M. Bloch, J. J. Holstein, B. Dittrich, W. Hiller and G. H. Clever, Angew. Chem., Int. Ed., 2018, 57, 5534–5538 CrossRef CAS PubMed; (b) R. Sekiya, M. Fukuda and R. Kuroda, J. Am. Chem. Soc., 2012, 134, 10987–10997 CrossRef CAS PubMed.
- C. Frassineti, S. Ghelli, P. Gans, A. Sabatini, M. S. Moruzzi and A. Vacca, Anal. Biochem., 1995, 231, 374–382 CrossRef CAS PubMed.
- U. Siriwardane and F. Fronczek, CSD Communication, 2017, CCDC deposition Nr 1562063 Search PubMed.
- G. Sarada, A. Kim, D. Kim and O. S. Jung, Dalton Trans., 2020, 49, 6183–6190 RSC.
- A. De Leon, J. Pons, X. Solans and M. Font-Bardia, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, M2164–U1264 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc02663a |
| This journal is © The Royal Society of Chemistry 2021 |