Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorine-18 labelled Ruppert–Prakash reagent ([18F]Me3SiCF3) for the synthesis of 18F-trifluoromethylated compounds†
Anna
Pees
 a,
Maria J.W.D
Vosjan
b,
Neil
Vasdev
a,
Maria J.W.D
Vosjan
b,
Neil
Vasdev
 c,
Albert D.
Windhorst
c,
Albert D.
Windhorst
 a and
Danielle J.
Vugts
a and
Danielle J.
Vugts
 *a
*a
aAmsterdam UMC, VU University, Radiology and Nuclear medicine, Radionuclide Center, De Boelelaan 1085c, Amsterdam, The Netherlands. E-mail: d.vugts@amsterdamumc.nl
bBV Cyclotron VU, De Boelelaan 1081, 1081 HV Amsterdam, The Netherlands
cAzrieli Centre for Neuro-Radiochemistry, Brain Health Imaging Centre, Centre for Addiction and Mental Health & Department of Psychiatry, University of Toronto, 250 College St., Toronto M5T-1R8, ON, Canada
First published on 27th April 2021
Abstract
This article describes the first synthesis and application of fluorine-18 labelled Ruppert–Prakash reagent [18F]Me3SiCF3. [18F]Me3SiCF3 was synthesized from [18F]fluoroform with radiochemical yields of 85–95% and radiochemical purities of >95% within 20 minutes. 18F-trifluoromethylated compounds were successfully prepared by reaction of [18F]Me3SiCF3 with benzaldehydes, acetophenones and benzophenones.
Positron emission tomography (PET) is a molecular imaging technique that non-invasively visualizes biochemical processes in vivo.1–3 This highly sensitive technique is increasingly used for the diagnosis of diseases, evaluation of the efficacy of a drug treatment, and drug discovery.3 PET radiotracers incorporate positron-emitting radionuclides, the most frequently used nuclide being fluorine-18.4 Fluorine-18 has very favourable characteristics for PET imaging, such as a convenient half-life (109.7 minutes) and a low positron energy (Emax = 0.634 MeV), making it desirable for distribution to radiochemistry laboratories and imaging centres thereby enabling multi-centre clinical trials.5–7
There is a tremendous interest in the use of fluorine-19 in drug development.8,9 This is reflected by recent Food and Drug Administration (FDA) drug approvals: in 2018 almost 1/3 of the new drugs approved contained one or more fluorine atoms.10 The trifluoromethyl group is a prevalent motif as it can positively influence many characteristics of a given drug, e.g. metabolic stability, lipophilicity, and protein binding affinity.11–13
Since trifluoromethyl moieties are so often found in modern pharmaceuticals, much attention has been paid to the development of synthesis strategies for introducing fluorine-18 labelled trifluoromethyl groups into PET tracers. Aromatic [18F]trifluoromethylation is well established and multiple strategies have been reported.14–18 However, the reported methodologies mainly rely on [18F]fluoroform as [18F]trifluoromethylation agent. In organic chemistry, not many trifluoromethylation procedures report the use of [19F]fluoroform, as alternative trifluoromethylation agents are generally preferred. One of the most applied nucleophilic trifluoromethylation agents is the Ruppert–Prakash reagent (Me3SiCF3).9 It was first described in 1984 by Ruppert et al.19 and found its first application in 1989 by Prakash et al.20 We therefore envisioned that developing a synthesis strategy for 18F-labelled Ruppert–Prakash reagent would be very useful for the translation of CF3 functionalization reactions that have been developed in fluorine-19 chemistry to radiofluorination reactions and PET tracer synthesis.21
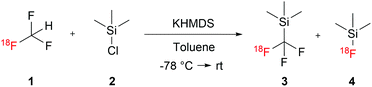
To synthesize [18F]Me3SiCF3 we followed a procedure reported by Prakash et al. that reacted fluoroform with trimethylsilyl chloride in presence of potassium hexamethyldisilazide (KHMDS) as the base in toluene.22 Initial experiments using [18F]fluoroform 1 synthesized according to our previously reported method18 showed successful formation of the desired product [18F]Me3SiCF33, in addition to unreacted [18F]fluoroform 1 and formation of a side product which was identified as [18F]trimethylsilyl fluoride 4 (see Scheme 1). To optimize the reaction towards full conversion to [18F]Me3SiCF33 several reaction parameters were varied.
The following reaction setup was used as a starting point: [18F]fluoroform 118 was trapped in a solution of 0.6M Me3SiCl 2 and 0.5M KHMDS in 650 μL toluene at −80 °C (prepared 15 minutes before trapping [18F]fluoroform 1, analogous to 19F-chemistry).22 After complete distillation (∼3 min) of [18F]fluoroform 1, the trapping vial was either actively warmed up to 20 °C or passively by removing it from the cooling bath.
[18F]Fluoroform was trapped well in the toluene solution and comparable RCYs to those previously reported (trapping in DMF) were obtained (up to 44 ± 0% vs 44 ± 1%, calculated from dry [18F]fluoride). The conversion of [18F]fluoroform to [18F]Me3SiCF3 was temperature sensitive and active warming to 20 °C resulted in complete conversion in less than 2.5 minutes, while passive warming required 15 minutes to reach completion at these reagent concentrations. Trapping at around −80 °C was crucial, since higher temperatures favoured the formation of the side product, [18F]Me3SiF.
Stirring of the precursor Me3SiCl together with the base KHMDS before the trapping was not necessary for [18F]Me3SiCF3 formation, nor was stirring during the reaction. Increase of the precursor concentration as well as the total amounts of precursor, base and solvent helped to push the reaction towards completion and shorten the reaction time with passive warming up to 5 min (see Table 1).
| Me3SiCl μmol | KHMDS μmol | Toluene μL | RCY CHF3a % | RCP Me3SiCF3b % | |
|---|---|---|---|---|---|
| Conditions: Trapping at −80 °C, passive warmup to rt in 5 min.a Isolated yield, average ± SD, decay corrected (dc), n = 3.b Determined by HPLC, average ± SD, dc, n = 3. | |||||
| 1 | 394 | 325 | 650 | n.d. | 0 ± 0 |
| 2 | 788 | 325 | 650 | 28 ± 1 | 74 ± 28 |
| 3 | 1576 | 650 | 1300 | 39 ± 0 | 95 ± 4 |
| 4 | 1182 | 488 | 975 | 44 ± 0 | 98 ± 2 |
| 5 | 1182 | 500 | 1000 | 43 ± 3 | 97 ± 3 |
After having established high-yielding reaction conditions for the formation of [18F]Me3SiCF3, we turned our attention to the isolation of the product for its use in subsequent [18F]trifluoromethylation reactions. As Me3SiCF3 has a low boiling point (54–55 °C)9 we investigated purification by distillation. Different distillation temperatures and flow rates were tested. An overview of the conditions is given in Table 2. The distillation efficiency increased with higher flow (entry 1–7). At flow rates of 30 mL min−1 and higher, >90% of [18F]Me3SiCF3 could be distilled into THF. Exploring different distillation temperatures showed that temperatures ≥70 °C led to the highest yields and at 50 °C the co-distilled precursor precipitated in the tubing and led to blockage during distillation (see entry 6, 8–10). The radiochemical purity of the distilled [18F]Me3SiCF3 was determined and only minor impurities (0–2%) of [18F]Me3SiF were found.
| Flow mL min−1 | Temp. °C | RCYa Me3SiCF3 % | |
|---|---|---|---|
| Conditions: 1182 μmol Me3SiCl, 500 μmol KHMDS, 1 mL toluene.a Average ± SD, dc, n = 3.b Distillation blocked in 2 out of 3 reactions. | |||
| 1 | 5 | 70 | 52 ± 6 |
| 2 | 10 | 70 | 69 ± 3 |
| 3 | 20 | 70 | 86 ± 3 |
| 4 | 30 | 70 | 93 ± 1 |
| 5 | 40 | 70 | 94 ± 1 |
| 6 | 50 | 70 | 95 ± 1 |
| 7 | 70 | 70 | 95 ± 1 |
| 8 | 50 | 50 | 75 ± 21b |
| 9 | 50 | 60 | 88 ± 6 |
| 10 | 50 | 80 | 95 ± 1 |
At higher flow rates and higher temperatures we noted a significant decrease of the volume in the first reaction vessel and the formation of a precipitate in the second vessel suggesting that precursor Me3SiCl (bp. 57 °C)23 and also solvent (toluene, bp. 111 °C) were co-distilled along with [18F]Me3SiCF3. Subsequently, we explored the possibility of further purification by distillation over solid phase extraction (SPE) cartridges. Our primary focus was on the removal of the precursor, Me3SiCl, to avoid potential reaction with the subsequent [18F]trifluoromethylation reaction. It was found that the silica plus long Sep-Pak® was able to retain the precursor while almost all [18F]Me3SiCF3 passed the SPE. The RCY of [18F]Me3SiCF3 was comparable to distillation without SPE (86 ± 1 vs. 88 ± 6%). However, the distillation time increased from 5 minutes to 10 minutes (see ESI† for more details). In summary, [18F]Me3SiCF3 was readily synthesized from [18F]fluoroform and obtained as a solution in THF with radiochemical yields of 85–95% and radiochemical purities of >95%.
To explore the applicability of [18F]Me3SiCF3 as [18F]trifluoromethylation reagent, we demonstrated its use by reaction with aldehydes and ketones and adapted the procedure for 19F-reactions reported by Prakash et al.20 In this procedure, the aldehyde or ketone was reacted with Me3SiCF3 under addition of catalytic amounts of TBAF in THF. After hydrolysis with aqueous HCl, the desired trifluoromethylated product was obtained. 4-Nitrobenzaldehyde 6g was selected as a model substrate and the following reaction parameters were varied: reaction time and temperature, quantities of TBAF and precursor as well as the scale of the reaction. [18F]Me3SiCF3 was found to readily react with 4-nitrobenzaldehyde: Reaction of an aliquot of the [18F]Me3SiCF3 in THF (300 μL) with 100 μmol 4-nitrobenzaldehyde 6g and 200 μmol TBAF for 5 minutes at room temperature in a total of 0.5 mL THF resulted in 39 ± 4% RCY. [18F]CHF3 was formed as a side product, likely resulting from reaction of [18F]Me3SiCF3 with traces of water present in THF or the TBAF solution. The 1M TBAF solution in THF was therefore stored in small batches (10–20 mL) over molecular sieves (3Å) to minimize the water content as much as possible.23
Variation of reaction time (2.5–20 min) and temperature (0–80 °C) did not have an effect on the radiochemical yield under our conditions. The control of the TBAF concentration however was important since low concentrations (≤200 μM) resulted in incomplete release of [18F]CF3− from [18F]Me3SiCF3 and therefore lower yields, whereas high concentrations (≥600 μM) were not beneficial for the reaction, and is likely attributed to increasing amounts of water in the TBAF solution which is responsible for promoting [18F]CHF3 formation.
Next, the effect of precursor concentration was investigated and showed that the higher the concentration of precursor, the higher the radiochemical yield (64 ± 5% RCY with 400 μmol precursor; see ESI† for details). To adapt the reaction for relevance to PET tracer syntheses the scale of the reaction was reduced compared to the initial conditions. It was observed that scaling down the reaction to 250 μL and 125 μL total volume resulted in comparable to even slightly higher radiochemical yields than the initial conditions (see Table S6 in ESI†). Due to easier handling and abundance of the model precursors we moved on to explore the substrate scope with the following conditions: 250 μL THF, 200 μmol precursor, 100 μmol TBAF (ESI,† Table S6, entry 7).
Three different groups of substrates were investigated, benzaldehydes, acetophenones and benzophenones, each with no substituents, electron withdrawing (4-MeO-) and electron donating (3-NO2-, 4-NO2-) substituents. An overview of all products and the corresponding RCYs is shown in Scheme 2. Two general trends were observed: 1. Electron withdrawing groups had a positive influence on the RCY. 2. Benzaldehydes reacted very well whereas benzophenones only resulted in low RCYs (<10%). The first observation can be explained by the electron density. The trifluoromethyl group attacks the positively polarised C atom of the carbonyl group. Electron withdrawing groups reduce the electron density of the carbonyl C atom and facilitate the nucleophilic attack whereas electron donating groups exert the opposite effect. The second observation can be explained by the reaction kinetics. It has been described that the rate constants of trifluoromethylation reactions observe the following rank order: k(benzaldehyde) > k(acetophenone) > k(benzophenone); and a competing reaction is the formation of fluoroform by reaction of Me3SiCF3 with trace amounts of water.24 The reaction with benzaldehydes is likely fast enough to outcompete the [18F]fluoroform formation whereas with benzophenones [18F]fluoroform formation predominates.
 | ||
| Scheme 2 Reaction of [18F]Me3SiCF3 with aldehydes and ketones; Reaction conditions: 200 μmol precursor, 250 μL THF, 5 min, rt, 100 μmol TBAF or 80 μmol TBAT; RCY = average ± SD, n = 3. | ||
Attempts to increase the RCY of the benzophenone reactions by further optimization of the previously varied reaction conditions (temperature, time, TBAF amount) were not fruitful. We therefore turned our attention to alternative initiators. Based on work of Johnston et al.24 we chose two initiators, KOPh and TBAT. Reactions initiated by K+-containing initiators were expected to have a faster turnover rate than with NH4+ and could improve the RCY of the slow-reacting benzophenones. Anhydrous TBAT was reported to result in more reproducible yields compared to TBAF, due to lower water content.
Under our radiochemistry conditions KOPh resulted in very low yields for all substrate groups, and is likely due to poor solubility of KOPh in THF. However, TBAT proved to be an excellent initiator (see Scheme 2). Reactions with nitro-substituted benzaldehydes and acetophenones resulted in ≥90% RCY of the corresponding products. It is noteworthy that three of these compounds previously failed to label using [18F]fluoroform.16 Furthermore, the unsubstituted and methoxy-substituted benzaldehydes were [18F]trifluoromethylated with decent to good yields (73 ± 4% and 43 ± 6%, respectively). Unsubstituted and methoxy-substituted acetophenones and benzophenones still showed low yields (≤10%). On the contrary to the nitro-substituted derivatives, these substrates are reported to react very well with [18F]fluoroform.
All optimization reactions were carried out using aliquots of a [18F]Me3SiCF3 stock solution. To confirm that the [18F]trifluoromethylation reactions work using the full batch of [18F]Me3SiCF3 and to determine the overall RCY and molar activity (Am), [18F]Me3SiCF3 was synthesized from 5 GBq [18F]fluoride. It was trapped after distillation in a reaction vessel containing THF, TBAT and 4-nitrobenzaldehyde and reacted for 5 min at rt after complete trapping. It was found that higher TBAT amounts (556 μmol) were required to fully release CF3− from [18F]Me3SiCF3, but the precursor amount could be kept at 200 μmol. The product was purified by preparative HPLC and the collected product fraction was measured for radioactivity and analysed by HPLC. The overall radiochemical yield (with regard to aqueous [18F]fluoride) was 11 ± 3% (dc) and the molar activity was 13 ± 2 GBq/μmol (n = 3). The molar activity compares well to the Am reported for [18F]fluoroform18 at the same starting amount of [18F]fluoride. The low overall yield is mainly due to losses during [18F]fluoroform formation. Details on the reaction procedure and molar activity calculations can be found in the ESI.†
As a final note it should be mentioned that for the chosen model reaction it was not necessary to distil [18F]Me3SiCF3 over a silica SPE cartridge to remove co-distilling Me3SiCl since RCYs of the subsequent [18F]trifluoromethylation reaction were comparable with and without Me3SiCl present. Surprisingly, without any intermediate purification of [18F]Me3SiCF3 the [18F]trifluoromethylation of 4-nitrobenzaldehyde still proceeds very well. We were able to [18F]trifluoromethylate 4-nitrobenzaldehyde in a one-pot synthesis from [18F]fluoroform that was synthesized on a commercial automated radiofluorination platform (Neptis module)18 with RCYs of 58 ± 10% (n = 3, determined by HPLC, with regard to [18F]fluoroform) (see ESI†). The purification might be a crucial point of consideration when developing trifluoromethylation reactions for other substrates where Me3SiCl, KHMDS and/or toluene could contaminate the desired reaction.
In conclusion, we report the first synthesis and application of fluorine-18 labelled Ruppert–Prakash reagent. [18F]Me3SiCF3 was synthesized with radiochemical yields of over 90% and radiochemical purities of >95% (starting from [18F]fluoroform) within 20 minutes. Reaction with benzaldehydes, acetophenones and benzophenones provided a complementary substrate scope to the previously reported method using [18F]fluoroform,25 enabling the synthesis of compounds that were not previously accessible. It should be noted that the relatively high amounts of precursor (200 μmol) require further optimization for routine application in PET tracer synthesis, and will be the focus of our future work. Since Ruppert–Prakash reagent is a widely used trifluoromethylation agent in organic synthesis, the development of [18F]Me3SiCF3 will open doors to the development of many new [18F]trifluoromethylation strategies.
The project is financially supported by the Dutch Research Council (NWO) grant no. 731.015.413 and BV Cyclotron VU. We also thank the Azrieli Foundation, Canada Foundation for Innovation, Ontario Research Fund and the Canada Research Chairs Program as well as members of the CAMH Brain Health Imaging Centre for support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- W. Wadsak and M. Mitterhauser, Eur. J. Radiol., 2010, 73, 461–469 CrossRef CAS PubMed.
- Z. Li and P. S. Conti, Adv. Drug Delivery Rev., 2010, 62, 1031–1051 CrossRef CAS PubMed.
- X. Deng, J. Rong, L. Wang, N. Vasdev, L. Zhang, L. Josephson and S. H. Liang, Angew. Chem., Int. Ed., 2019, 58, 2580–2605 CrossRef CAS PubMed.
- P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 8998–9033 CrossRef CAS PubMed.
- P. J. Riss and F. I. Aigbirhio, Chem. Commun., 2011, 47, 11873–11875 RSC.
- M. Tredwell and V. Gouverneur, Angew. Chem., Int. Ed., 2012, 51, 11426–11437 CrossRef CAS PubMed.
- M. Conti and L. Eriksson, EJNMMI Phys., 2016, 3, 8 CrossRef PubMed.
- C. Alonso, E. Martínez De Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847–1935 CrossRef CAS PubMed.
- X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683–730 CrossRef CAS.
- B. G. de la Torre and F. Albericio, Molecules, 2019, 24, 809 CrossRef PubMed.
- L. Chu and F. L. Qing, Acc. Chem. Res., 2014, 47, 1513–1522 CrossRef CAS PubMed.
- G. B. Li, C. Zhang, C. Song and Y. D. Ma, Beilstein J. Org. Chem., 2017, 14, 155–181 CrossRef PubMed.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. Del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- M. Huiban, M. Tredwell, S. Mizuta, Z. Wan, X. Zhang, T. L. Collier, V. Gouverneur and J. Passchier, Nat. Chem., 2013, 5, 941–944 CrossRef CAS PubMed.
- P. Ivashkin, G. Lemonnier, J. Cousin, V. Grégoire, D. Labar, P. Jubault and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 9514–9518 CrossRef CAS PubMed.
- D. van der Born, C. Sewing, J. D. M. Herscheid, A. D. Windhorst, R. V. A. Orru and D. J. Vugts, Angew. Chem., Int. Ed., 2014, 53, 11046–11050 CrossRef CAS PubMed.
- T. Rühl, W. Rafique, V. T. Lien and P. J. Riss, Chem. Commun., 2014, 50, 6056–6059 RSC.
- A. Pees, A. D. Windhorst, M. J. W. D. Vosjan, V. Tadino and D. J. Vugts, Eur. J. Org. Chem., 2020, 1177–1185 CrossRef CAS.
- I. Ruppert, K. Schlich and W. Volbach, Tetrahedron Lett., 1984, 25, 2195–2198 CrossRef CAS.
- G. K. S. Prakash, R. Krishnamurti and G. A. Olah, J. Am. Chem. Soc., 1989, 111, 393–395 CrossRef CAS.
- A. D. Dilman and V. V. Levin, Mendeleev Commun., 2015, 25, 239–244 CrossRef CAS.
- G. K. S. Prakash, P. V. Jog, P. T. D. Batamack and G. A. Olah, Science, 2012, 338, 1324–1327 CrossRef CAS PubMed.
- G. K. S. Prakash and M. Mandal, J. Fluorine Chem., 2001, 112, 123–131 CrossRef CAS.
- C. P. Johnston, T. H. West, R. E. Dooley, M. Reid, A. B. Jones, E. J. King, A. G. Leach and G. C. Lloyd-Jones, J. Am. Chem. Soc., 2018, 140, 11112–11124 CrossRef CAS PubMed.
- D. van der Born, J. K. D. M. Herscheid, R. V. a Orru and D. J. Vugts, Chem. Commun., 2013, 49, 4018–4020 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc01789f |
| This journal is © The Royal Society of Chemistry 2021 |