Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cryogenic activity and stability of benzaldehyde lyase enzyme in lipidic mesophases-nanoconfined water†
Tao
Zhou
 a,
Yang
Yao
a,
Qin
Zhang
ab and
Raffaele
Mezzenga
a,
Yang
Yao
a,
Qin
Zhang
ab and
Raffaele
Mezzenga
 *ac
*ac
aDepartment of Health Sciences and Technology, ETH Zürich, 8092 Zurich, Switzerland. E-mail: raffaele.mezzenga@hest.ethz.ch
bInstitut des Sciences et Ingénierie Chimiques, EPFL, 1015 Lausanne, Switzerland
cDepartment of Materials, ETH Zurich, 8093 Zürich, Switzerland
First published on 11th May 2021
Abstract
Phytantriol-based lipidic mesophases (LMs) are introduced as a platform for cryoenzymology, which relies on the presence of liquid water in LMs at subzero temperatures. After incorporation into LMs, the model enzyme Benzaldehyde lyase (BAL) shows high cryogenic stability and activity. In contrast, BAL in bulk solution undergoes significant secondary structural transitions caused by low temperatures (cold denaturation), demonstrating the potential of this approach to enable in meso cryoenzymology.
Numerous organisms, especially microorganisms, live in cold ecosystems, such as polar and alpine regions.1 These microorganisms require enzymes to function at low and even subzero temperatures to maintain essential metabolic functions.2,3 Investigating enzyme performance at subzero temperatures, including assessing enzyme activity and stability, would deepen our understanding of life in these severe environmental conditions.4 In addition, cryo-enzymology is a powerful tool that allows us to comprehensively understand the catalytic mechanism of enzymes through the identification of various reaction intermediates.5,6 Yet, the main limitation of cryo-enzymology is the lack of suitable systems to host enzymatic reactions at subzero temperatures, since water freezes into ice at temperatures below 0 °C. Typically, an ideal host system needs to meet two main requirements: (i) water in the system should remain unfrozen at temperatures below 0 °C for enzymes to function;7 and (ii) enzymes in these systems are able to resist cold denaturation since enzymes can undergo structural transitions at low temperatures, leading to lower activity.8,9 To date, the most straightforward way to address this is by mixing water with organic solvents.10–12 However, introducing toxic organic solvents will unavoidably decrease enzyme stability. Other examples include using micelles or microemulsions, which have been used to host a limited number of cryo-enzymatic reactions.13–15 However, studies of enzyme stability at low temperatures, which are vital to understand the behaviours of enzymes at cryogenic temperatures, are lacking.
The temperature-induced structural transition is normally linked to the physical conditions of the medium, e.g., pH, ionic strength, and pressure, instead of the intrinsic molecular properties of proteins.16–20 Therefore, it is possible to design suitable media in which enzymes maintain their structural stability at low temperatures. Lipidic mesophases (LMs), which contain biomimetic lipidic bilayer structures separated by water channels, are promising hosts.21 Enzymes confined in LMs generally have higher stability without activity loss.22–25 Even within LMs, most of the lipid undergoes crystallization and the water confined inside freezes to ice at subzero temperatures. However, the application of LMs as a cryo-enzymology host has become feasible by the recent finding, in our group, of a novel unfrozen lipid which allows the water confined in LMs produced using this lipid, to resist crystallization at temperatures as low as 10 K.26 Yet, the complicate synthetic production of this lipid inhibits its broad application. Therefore, in the present work, we have used a commercially available lipid phytantriol, which can also remain unfrozen, to prepare lipidic mesophases (LMs) for cryo-enzymatic reactions.27 The high efficiency of enzyme benzaldehyde lyase (BAL) in catalysing the asymmetric condensation of 2-naphthaldehyde (NA) is successfully achieved in LMs at 25 °C, 4 °C, and remarkably at −5 °C. The access to cryo-enzymatic reactions in phytantriol-based LMs relies on the existence of water at subzero temperatures, as demonstrated by differential scanning calorimetry (DSC) measurements and already reported in our recent work.27 Water confined in Phy70–W30 (mixture of 70 wt% phytantriol and 30 wt% water) with BAL enzyme and NA substrates incorporated within, could remain completely unfrozen down to −15 °C. The crystallization temperature of water could be further decreased by reducing the water ratio of LMs. Moreover, Fourier-transform infrared spectroscopy (FTIR) quantitative analysis indicates that the cryogenic stability of the BAL enzyme is much higher in LMs than in bulk water at low temperatures (see for example Fig. 4 in what follows).
The successful incorporation of BAL into LMs was first demonstrated by SAXS measurements. A lipidic cubic mesophase with double-diamond symmetry (Pn3m group) (diffraction peaks follow the sequence of √2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √3
√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √4
√4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √6
√6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √8
√8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √9), is formed by mixing 70% phytantriol and 30% buffer solution (Fig. 1a). The lattice parameter is 6.8 nm and the water channel diameter is 2.49 nm. After loading the hydrophilic BAL enzyme into the LM, the lattice parameter remains identical, indicating that incorporation of BAL into the LM does not affect the long-rage order of the mesophase. The structure of the host LM system is also maintained during the reaction process.
√9), is formed by mixing 70% phytantriol and 30% buffer solution (Fig. 1a). The lattice parameter is 6.8 nm and the water channel diameter is 2.49 nm. After loading the hydrophilic BAL enzyme into the LM, the lattice parameter remains identical, indicating that incorporation of BAL into the LM does not affect the long-rage order of the mesophase. The structure of the host LM system is also maintained during the reaction process.
 | ||
| Fig. 1 (a) Small-angle X-ray scattering (SAXS) profile of lipidic mesophase (LM) Phy70–W30. Black: pure LM prepared by mixing 70% phytantriol and 30% phosphate buffer solution; blue: 0.42 mg mL−1 benzaldehyde lyase (BAL) (PDB code: 2AG0) enzyme loaded in the LM; wine: 100 mM reactant 2-naphthaldehyde (NA) loaded in the LM; orange: 0.42 mg mL−1 enzyme and 100 mM reactant loaded in the LM. SAXS was measured after enzymatic reaction completion. (b) Schematic representation of the condensation of NA confined in LMs catalysed by BAL; the enzyme position is hypothetically sketched for visual purposes since its distribution within the LMs is unknown. (c) The yield of the enzymatic reaction in LMs with different water ratios and in a bulk solution mixture of 80% DMSO and 20% buffer. In all LMs, the concentrations of BAL enzyme and NA substrate are maintained at 0.42 mg mL−1 and 100 mM, respectively. In the bulk solution, the concentrations of BAL and NA are 0.01 mg mL−1 and 2.5 mM, respectively. (d) Kinetic curves of the enzymatic reaction in LMs with different water ratios. | ||
The high efficiency of BAL enzyme in catalysing the asymmetrical condensation of 2-Naphthaldehyde is characterized in the bulk solution and LMs (Fig. 1b and c). In DMSO20–W80 (mixture of 20 wt% DMSO and 80 wt% water buffer solution), the reaction yield is 93%. However, because of the super hydrophobicity of the NA substrate, the maximum NA concentration is only 2.5 mM. Higher DMSO levels would completely denature BAL. When the medium is changed to LMs, the NA concentration can be increased to 100 mM, which is 40 times higher than that in DMSO20–W80. This is due to the existence of hydrophobic (lipidic bilayer) and hydrophilic (water channel) regions, which can host hydrophobic and hydrophilic chemicals. Importantly, BAL still shows high catalytic efficiency in the presence of 100 mM NA, with a reaction yield of 86% (Fig. 1c). Moreover, BAL enzyme performance in LMs is highly dependent on the water content. A clear decrease in both yield and reaction rate is observed with decreasing water content (Fig. 1c and d). This is because the lower water ratio will reduce the size of the LM water channel, leading to a lower diffusion coefficient24 (Fig. S2 and S3, ESI†).
In line with the previous work from our group,27 after coupling enzyme BAL and substrate NA, the host (Phy70–W30) could still remain in the cubic phase while the temperature decreased from 25 °C to below 0 °C, although the symmetry changed from Pn3m to Ia3d (Fig. 2a). Detailed DSC experiments were performed to elucidate the state of water in this system. A water crystallization peak on the cooling curve and an ice melting peak on the heating curve are observed, indicating water confined in Phy70–W30 coupled with BAL and the substrates crystallizes at −22 °C (Fig. 2b). To exclude the supercooling effect, the DSC annealing experiments were performed and the results indicate that water confined in Phy70–W30 coupled with BAL and the substrates could remain unfrozen at temperatures as low as −15 °C; only below these temperatures water starts to crystallize (Fig. 2c). The freezing temperature of water could be further reduced by decreasing the water ratio. In Phy90–W10 with BAL and the substrates inside, water could still remain unfrozen down to −60 °C (Fig. S6 and S7, ESI†). The enzymatic reaction was then performed at 4 °C and −5 °C (Fig. 2d). The result shows that, the reaction rate when performed at 4 °C and −5 °C is slower than when performed at 25 °C. This is what is anticipated based on the classical Arrhenius theory. Importantly, the high efficiency of the BAL enzyme is maintained at such low temperatures. The yield at 4 °C reaches 87%, which is comparable to the 86% yield observed at 25 °C. At −5 °C, a 67% yield is achieved after 120 h, but since the kinetics curve has a positive slope still after such a long time (Fig. 2d), this shows that while the kinetics is slowed down, the enzymatic reaction still progresses continuously and the yield could be further increased by increasing reaction time.
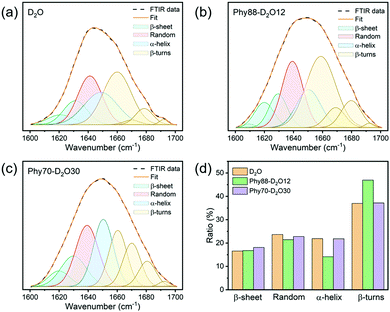
To gain a full understanding of the cryogenic performance of BAL in different host matrices, it is vital to investigate the structural stability of BAL enzyme. This is achieved using Fourier-transform infrared spectroscopy (FTIR). Specifically, the amide I band region from 1600 cm−1 to 1700 cm−1, which originates from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the amide group and is directly related to the backbone conformation, is used to quantify the secondary structural composition of proteins.28–31 Since phytantriol does not contain any double bonds, the phytantriol in LMs will not interfere the FTIR signal of BAL in this region (Fig. S9, ESI†). The disturbance from H2O is also eliminated by using D2O. Using this approach, we managed to obtain the BAL FTIR signal and obtain quantifiable information about the secondary structures of BAL in bulk D2O and LMs (Fig. S10 and Fig. 3a–c, ESI†). The results show that the secondary structure distribution of BAL in Phy88–D2O12 and Phy70–D2O30 is almost identical to that in D2O, though a slight reduction in the α-helical ratio and increase in the β-turn ratio were observed for BAL in Phy88–D2O12 (Fig. 3d). This proves that the biomimetic LMs are superior host matrices for enzymes and the structure of the incorporated enzyme is well preserved, especially in more hydrated cubic LMs.
O stretching vibration of the amide group and is directly related to the backbone conformation, is used to quantify the secondary structural composition of proteins.28–31 Since phytantriol does not contain any double bonds, the phytantriol in LMs will not interfere the FTIR signal of BAL in this region (Fig. S9, ESI†). The disturbance from H2O is also eliminated by using D2O. Using this approach, we managed to obtain the BAL FTIR signal and obtain quantifiable information about the secondary structures of BAL in bulk D2O and LMs (Fig. S10 and Fig. 3a–c, ESI†). The results show that the secondary structure distribution of BAL in Phy88–D2O12 and Phy70–D2O30 is almost identical to that in D2O, though a slight reduction in the α-helical ratio and increase in the β-turn ratio were observed for BAL in Phy88–D2O12 (Fig. 3d). This proves that the biomimetic LMs are superior host matrices for enzymes and the structure of the incorporated enzyme is well preserved, especially in more hydrated cubic LMs.
The cryogenic stability of BAL in bulk D2O and LMs was further investigated (Fig. S11–S13, ESI†). In bulk D2O, a significant secondary structural transition is observed. By decreasing the temperature from 25 to 4 °C, the α-helical ratio jumps from 22 to 63%, and further increases to 71% when the temperature is decreased to −5 °C (Fig. 4a). Correspondingly, the ratios of the other three structures (β-sheet, random, and β-turns) are significantly decreased. Interestingly, the secondary structures of BAL remain relatively stable within the confinement of LMs. In both the cubic phase (Phy70–D2O30) and the lamellar phase (Phy88–D2O12) a slight reduction in the α-helical content and increase in the β-turn ratio were observed while decreasing the temperature (Fig. 4b and c). Given that BAL still shows high activity in Phy70–W30 at 4 and −5 °C, such small structural changes do not appear to have a great effect on BAL performance. Overall, the BAL enzyme shows higher cryogenic stability in LMs than it does in bulk D2O; because this strategy does not require any co-solvent, this route offers a robust alternative to co-mixing water with organic solvent to expand the range of enzymatic reach.
 | ||
| Fig. 4 Temperature dependence of the secondary structural distribution of benzaldehyde lyase (BAL) (a) in D2O; (b) in Phy88–D2O12; (c) and in Phy70–D2O30. | ||
In summary, we have shown that BAL cryogenic activity and stability can be greatly preserved by incorporation of this enzyme into unfrozen phytantriol-based LMs. The concentration of the NA superhydrophobic substrate in LMs could reach 40 times higher values than that in the DMSO/H2O mixture without affecting the activity of BAL. More remarkably, this platform could host BAL enzyme and preserve its function at cryogenic temperatures, because of the presence of unfrozen water combined to a high BAL cryogenic stability in LMs. Water confined in Phy70–W30 with BAL enzyme and NA substrate inside remains unfrozen at −15 °C, expanding the width of application of enzymes at low temperatures. In addition, as shown by FTIR measurements, the BAL secondary structure changes in bulk D2O induced by low temperatures are much more dramatic than that in LMs, demonstrating the enhanced cryogenic stability of BAL in LMs. This work confirms and expands our earlier findings27 on the efficiency of phytantriol-based lipidic mesophases to host and run cryo-enzymatic reactions involving cascading and hydrolysis reactions at sub-zero temperatures, and demonstrates that efficiency of cryo-enzymatic reactions is largely due to the preserved secondary structure of enzymes at low temperatures. Together, these results highlight the broad applications of phytantriol-based lipidic mesophase in hosting diverse enzymes and other biomolecules at cryogenic temperatures, preserving them against cold denaturation and significantly expanding the scope of cryogenic enzymatic research.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Feller and C. Gerday, Nat. Rev. Microbiol., 2003, 1, 200 CrossRef CAS PubMed.
- L. J. Rothschild and R. L. Mancinelli, Nature, 2001, 409, 1092 CrossRef CAS PubMed.
- A. Clarke, Int. J. Astrobiol., 2014, 13, 141 CrossRef CAS.
- M. J. Liszka, M. E. Clark, E. Schneider and D. S. Clark, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 77 CrossRef CAS PubMed.
- A. L. Fink, S. J. Cartwright and P. Douzou, Crit. Rev. Biochem., 1981, 11, 145 CrossRef CAS PubMed.
- F. Travers and T. Barman, Biochimie, 1995, 77, 937–948 CrossRef CAS.
- F. Franks, Biophysics and biochemistry at low temperatures. Cambridge University Press, Cambridge, 1985 Search PubMed.
- P. L. Privalov, Crit. Rev. Biochem. Mol. Biol., 1990, 25, 281 CrossRef CAS PubMed.
- G. Graziano, Phys. Chem. Chem. Phys., 2014, 16, 21755–21767 RSC.
- A. L. Fink, J. Theor. Biol., 1976, 61, 419 CrossRef CAS PubMed.
- A. L. Fink and K. J. Angelides, Biochemistry, 1976, 15, 5287 CrossRef CAS PubMed.
- A. L. Fink and A. Ahmed, Nature, 1976, 263, 294 CrossRef CAS PubMed.
- P. Douzou, E. Keh and C. Balny, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 681 CrossRef CAS PubMed.
- P. Debey, C. Balny and P. Douzou, FEBS Lett., 1976, 69, 231 CrossRef CAS.
- P. Douzou, in Advances in Enzymology, ed. A. Meister, John Wiley and Sons, New York, 1980, vol. 51, p. 1 Search PubMed.
- W. Kauzmann, Adv. Protein Chem., 1959, 14, 1–63 CrossRef CAS PubMed.
- G. Graziano, F. Catanzano, A. Riccio and G. Barone, J. Biochem., 1997, 122, 395 CrossRef CAS PubMed.
- P. De Los Rios and G. Caldarelli, Phys. Rev. E, 2001, 63, 031802 CrossRef CAS PubMed.
- V. Bianco and G. Franzese, Phys. Rev. Lett., 2015, 115, 108101 CrossRef PubMed.
- R. Yan, P. D. Rios, A. Pastore and P. A. Temussi, Commun. Chem., 2018, 1, 13 CrossRef.
- N. Garti, P. Somasundaran and R. Mezzenga, Self-assembled Supermolecular Architectures: Lyotropic Liquid Crystals, A John Wiley & Sons Inc., New York, America, 2012, vol. 1 Search PubMed.
- T. Zhou, J. J. Vallooran, S. Assenza, A. Szekrenyi, P. Clapés and R. Mezzenga, ACS Catal., 2018, 7, 5810 CrossRef.
- W. J. Sun, J. J. Vallooran, A. Zabara and R. Mezzenga, Nanoscale, 2014, 6, 6853 RSC.
- W. J. Sun, J. J. Vallooran and R. Mezzenga, Langmuir, 2015, 31, 4558 CrossRef CAS PubMed.
- W. J. Sun, J. J. Vallooran, W. K. Fong and R. Mezzenga, J. Phys. Chem. Lett., 2016, 7, 1507 CrossRef CAS PubMed.
- L. S. Manni, S. Assenza, M. Duss, J. J. Vallooran, F. Juranyi, S. Jurt, O. Zerbe, E. M. Landau and R. Mezzenga, Nat. Nanotechnol., 2019, 14, 609 CrossRef PubMed.
- Y. Yao, T. Zhou, R. Färber, U. Grossner, G. Floudas and R. Mezzenga, Nat. Nanotechnol., 2021 DOI:10.1038/s41565-021-00893-5.
- J. L. Arrondo, A. Muga, J. Castresana and F. M. Goni, Prog. Biophys. Mol. Biol., 1993, 59, 23 CrossRef CAS PubMed.
- J. Kong and S. Yu, Acta Biochim. Biophys. Sin., 2007, 39, 549 CrossRef CAS PubMed.
- Y. J. Jiang, C. Li, X. Nguyen, S. Muzammil, E. Towers, J. Gabrielson and L. Narhi, J. Pharm. Sci., 2011, 100, 4631 CrossRef CAS PubMed.
- H. Y. Yang, S. N. Yang, J. L. Kong, A. C. Dong and S. N. Yu, Nat. Protoc., 2015, 10, 382 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc01315g |
| This journal is © The Royal Society of Chemistry 2021 |