 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A site-selective and stereospecific cascade Suzuki–Miyaura annulation of alkyl 1,2-bisboronic esters and 2,2′-dihalo 1,1′-biaryls†
Suzanne
Willems
a,
Georgios
Toupalas
 b,
Julia C.
Reisenbauer
b and
Bill
Morandi
b,
Julia C.
Reisenbauer
b and
Bill
Morandi
 *ab
*ab
aMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, Mülheim an der Ruhr 45470, Germany
bLaboratorium für Organische Chemie ETH Zürich, Vladimir-Prelog-Weg 3, HCI, Zürich 8093, Switzerland. E-mail: morandib@ethz.ch
First published on 15th March 2021
Abstract
A cascade Suzuki–Miyaura cross-coupling giving rise to 9,10-dihydrophenanthrenes has been developed. Using biaryls with unsymmetrical substitution-pattern full site-selectivity was observed. Furthermore, this cross-coupling of an alkyl 1,2-bisboronic pinacol ester proceeds through the challenging coupling of a secondary boronate with complete stereoretention.
The Suzuki–Miyaura cross-coupling has found broad use in the synthesis of drugs, agrochemicals, fine chemicals, and polymers.1–3 In combination with cascade annulations, in which multiple bonds are formed in a single reaction vessel, it offers the possibility to rapidly build up molecular complexity, limit chemical waste, and improve step-economy.4
Selected examples of cascade Suzuki–Miyaura cross-coupling reactions with alkenyl 1,2-bisboronic pinacol esters have been previously reported for the synthesis of polycyclic aromatic hydrocarbons (PAH) by Shimizu and Hiyama (Fig. 1).5,6 In addition, Wang has reported a cascade annulation with alkyl 1,1-bisboronic pinacol esters in the synthesis of 9H-fluorenes.7
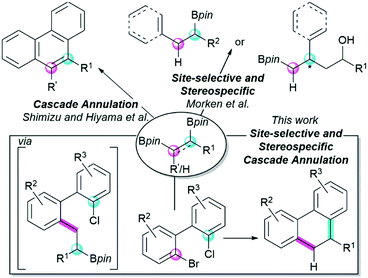 | ||
| Fig. 1 Reaction design of a cascade Suzuki–Miyaura cross-coupling based on alkyl 1,2-bisboronic pinacol esters. | ||
Alkyl 1,2-bisboronic pinacol esters can be readily accessed in racemic and stereoselective fashion using transition metal-catalyzed and organocatalytic approaches.8–17 The Morken group has reported a Suzuki–Miyaura cross-coupling of the alkyl 1,2-bisboronic pinacol esters in which either the primary or secondary position can be selectively functionalized (Fig. 1).18,19 However, despite their facile synthesis and intriguing site-selective reactivity, these reagents have not yet been evaluated in cascade annulation reactions.
Given the straightforward access and site-selective reactivity of the stable alkyl 1,2-bisboronic pinacol esters, we questioned whether these reagents could be successfully employed in site-selective annulation reactions. We reasoned that the direct, modular synthesis of tricyclic 9,10-dihydrophenanthrenes, could be achieved using a tailored annulation reaction that takes advantage of these reagents. Key to the development of this reaction would be the high site-selectivity of both cross-coupling events (Fig. 1) that would, crucially, take advantage of the matching reaction rates between the two different nucleophilic (1° vs. 2° C–Bpin) and electrophilic (C–Br vs. C–Cl) sites present on the two reagents.
Herein we report the development of a Pd-catalyzed site-selective and stereospecific cascade annulation of alkyl 1,2-bisboronic pinacol esters and 2-bromo-2′-chloro biaryls.
We entered our studies by identifying a suitable model alkyl 1,2-bisboronic pinacol ester (Scheme 1). Initial exploration focused on the use of symmetrical 2,2′-dibromobiphenyl 2b, as this would simplify reaction monitoring and allow rapid identification of promising conditions for this highly challenging annulation reaction. Using previously reported reaction conditions,19 the octyl-1,2-bisboronic pinacol ester 1a only gave trace amounts of the cyclized product, further highlighting the challenges in developing such a process. A sequence of experiments allowed us to find a promising Pd2(dba)3/IPr HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) catalytic system in THF which gave 35% of 9-hexyl-9,10-dihydrophenanthrene 3a in the presence of KOH (Scheme 1 and Table 1, entry 1). We next explored the reactivity of other electrophilic coupling partners in the cyclization reaction. 2,2′-Dichlorobiphenyl 2c gave a cleaner reaction and a similar amount of annulated product compared to the dibromide 2b (entry 1 and 2). In contrast, the diiodide 2d gave a diminished product yield (entry 3). 2,2′-Biphenol derived ditriflate 2e and dimesylate 2f were unreactive and starting materials were recovered (entry 4 and 5). Combining these preliminary observations, we envisioned that optimizing conditions for 2-bromo-2′-chloro biphenyl 2a may set the stage for the subsequent development of a fully site-selective annulation process.20,21
4) catalytic system in THF which gave 35% of 9-hexyl-9,10-dihydrophenanthrene 3a in the presence of KOH (Scheme 1 and Table 1, entry 1). We next explored the reactivity of other electrophilic coupling partners in the cyclization reaction. 2,2′-Dichlorobiphenyl 2c gave a cleaner reaction and a similar amount of annulated product compared to the dibromide 2b (entry 1 and 2). In contrast, the diiodide 2d gave a diminished product yield (entry 3). 2,2′-Biphenol derived ditriflate 2e and dimesylate 2f were unreactive and starting materials were recovered (entry 4 and 5). Combining these preliminary observations, we envisioned that optimizing conditions for 2-bromo-2′-chloro biphenyl 2a may set the stage for the subsequent development of a fully site-selective annulation process.20,21
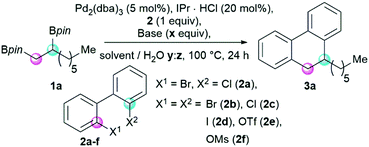 | ||
| Scheme 1 Optimization of the annulation reaction using alkyl 1,2-bisboronic pinacol ester 1a and biaryls 2 to yield 9,10-dihydrophananthrene 3a. | ||
| Entry | Base (x equiv.) | 2a–f X1, X2 | Solvent/H2O (y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z) z) |
3a [%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2 (0.1 mmol), Pd2(dba)3 (0.005 mmol), IPr HCl (0.02 mmol), base (0.2–0.3 mmol), THF/H2O (0.1 mol L−1), 100 °C, 24 h. b GC yield using n-tetradecane as internal standard. c THF/H2O (0.04 mol L−1). d Without Pd2(dba)3 and IPr HCl. | ||||
| 1 | KOH (3) | Br, Br | THF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
35 |
| 2 | KOH (3) | Cl, Cl | THF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
33 |
| 3 | KOH (3) | I, I | THF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 |
| 4 | KOH (3) | OTf, OTf | THF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4 |
| 5 | KOH (3) | OMs, OMs | THF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 6 | KOH (3) | Br, Cl | THF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
48 |
| 7 | Ba(OH)2 (2) | Br, Cl | THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 |
| 8 | Ba(OH)2 (2) | Br, Cl | Dioxane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
31 |
| 9 | Ba(OH)2 (2) | Br, Cl | H2O | 53 |
| 10c | Ba(OH) 2 (2) | Br, Cl |
THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1) |
76 |
| 11c | — | Br, Cl | THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
| 12cd | Ba(OH)2 (2) | Br, Cl | THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 |
Excitingly, using 2-bromo-2′-chloro biphenyl 2a, the yield increased to 48% (entry 6). Further optimization showed that the use of barium hydroxide as a base under more dilute reaction conditions was crucial to increase the yield of the double Suzuki–Miyaura to 76% (entry 12). The availability of hydroxides plays a pivotal role in the efficiency of the Suzuki–Miyaura cross-coupling as discussed by Amatore and Jutand,22 as well as Hartwig.23 Based on these studies, we postulate that changing the counterion of the hydroxide-source may increase the activity of the Pd-catalyst during transmetalation due to a weaker binding affinity of the barium-compared to the potassium-counterion.24,25 Furthermore, barium hydroxide might release hydroxide ions more gradually as compared to potassium hydroxide due to its lower solubility.26
Having optimized the reaction conditions, we next investigated whether we could verify the central hypothesis of this work, namely that the two unsymmetrically substituted reagents can undergo a site-selective cross-coupling reaction.
In an attempt to observe a single regioisomer, we used unsymmetrically substituted biaryls and performed an annulation with alkyl 1,2-bisboronic pinacol esters (Scheme 2). Excitingly, introduction of a Me- or F-substituent on the biaryl substrate indeed led to a single regioisomer (3b–3i). Additionally, an electron-poor mono CF3-substituted biaryl and an electron-rich methoxy-substituted biaryl also gave the corresponding products with high regioselectivity (3j, 3k). We rationalize this selectivity with the different reaction rates of the reactive centers in both bifunctional reagents (1° vs. 2° C–Bpin, C–Br vs. C–Cl). Initially, a C–C bond between the primary C–Bpin and Ar–Br is formed, followed by the intramolecular cyclization between the secondary C–Bpin and Ar–Cl.
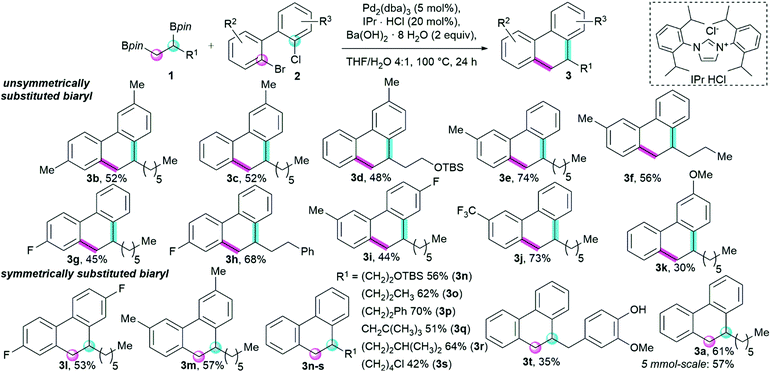 | ||
| Scheme 2 Scope of the double Suzuki–Miyaura cross coupling of alkyl 1,2-bisboronic pinacol esters 1 with 2-bromo-2′-chloro biaryls 2 to afford 9,10-dihydrophenanthrenes 3. Yields are for isolated products. See ESI,† for further information. | ||
Biaryls with a symmetric substitution pattern (3l, 3m) were also well tolerated. An attempt to cyclize 2,2′-dibromo-1,1′-binaphtalene did not afford any annulated product. The ability to rotate freely around the biaryl bond might therefore be crucial to the efficiency of the second coupling step. Furthermore, an extension to heterocyclic biaryls proved unsuccessful.
Turning our focus to the alkyl 1,2-bisboronic pinacol ester scope, sterically more encumbered substituents showed good compatibility with the annulation reaction (3n–s). A dinucleophile containing a primary chloride (3s) and a eugenol-derivative (3t) were efficiently converted to the annulated product. Unfortunately, nitrile, ester or ketone functionalities were not tolerated. Similarly, double secondary, ethane- or methane-derived dinucleophiles did not yield cyclized products. Finally, the model reaction could be scaled up to a 5 mmol scale yielding 57% (0.76 g) of the annulated product 3a.
Intrigued by the high efficiency and selectivity of the transformation, we monitored the model reaction more closely. The chlorinated reaction intermediate 4 could be isolated when stopping the reaction after three hours (Scheme 3A). In contrast, the corresponding brominated intermediate was not observable. NMR studies showed that compound 4 exists as a mixture of two atropoisomers that are interconvertible at elevated temperature (see ESI,† for details). Using the intermediate 4 as a starting material in our reaction, the annulated product was observed in high yield (Scheme 3A). Interestingly, using potassium hydroxide as a base gave a comparable conversion (77%, see ESI†) to the cyclized product in this single-step, intramolecular coupling reaction. The intramolecular nature of the coupling between the secondary boronic pinacol ester and the aryl chloride may be key to facilitate the challenging secondary cross-coupling. To substantiate this hypothesis, an intermolecular control reaction was performed. As expected, both reactions using the corresponding homobenzylic boronic pinacol ester and either bromobenzene or chlorobenzene did not yield any of the cross-coupled product (see ESI,† for details). Usually, the Pd-catalyzed Suzuki–Miyaura cross-coupling of aliphatic, chiral coupling reagents is stereospecific.27 Thus, using the Pt(dba)3/TADDOL-protocol developed by Morken et al. for the enantioselective diborylation of terminal alkenes, we synthesized an enantioenriched dinucleophile (S)-1b and performed the annulation reaction (Scheme 3B).9 The product was obtained with complete enantiospecificity.28 Derivatization and crystallization of the major enantiomer after preparative HPLC revealed the absolute configuration of enantiomer (S)-3n, showing that the configuration is retained during the annulation reaction. This result is in accordance with literature precedent showing that stereoretentive transmetalation is the predominant pathway in this type of cross-coupling reactions.27
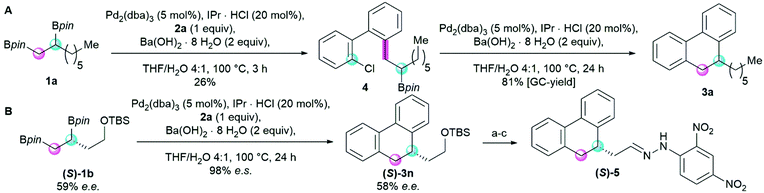 | ||
| Scheme 3 Mechanistic studies of the Suzuki–Miyaura annulation reaction. (A) Isolation of the key reaction intermediate 4 and a mono-coupling to the annulated 9,10-dihydrophenanthrene 3a. (B) Cascade cross-coupling of the enantioenriched alkyl 1,2-bisboronic pinacol ester (S)-1b and the derivatization to crystallize (S)-5 (CCDC #2054485†): (a) TBAF (2 equiv.), THF, r.t., 3 h, (b) DMP (1.25 equiv.), DCM, 0 °C to r.t., 1.5 h, (c) DNP (1.2 equiv.), HCl (35% in H2O, 2 equiv.), MeOH, r.t., 24. | ||
In summary, we have reported a site-selective double Suzuki–Miyaura cross-coupling using non-symmetric bifunctional reagents. This reaction allows for easy access to twenty different 9,10-dihydrophenanthrenes, a biologically-relevant scaffold.29 The high stereospecificity of our annulation reaction shows that it offers a direct access to enantiomerically enriched 9,10-dihydrophenanthrenes. We have shown that a notoriously challenging cross-coupling of a secondary pinacol boronic ester and the aryl chloride is enabled by the proximity of the reactive sites which plays a key role to the success of this annulation. Thus, this cascade reaction also suggests a new avenue to facilitate the otherwise challenging cross-coupling of secondary aliphatic boron nucleophiles.30
Financial support from the Max-Planck-Gesellschaft and Max-Planck-Institut für Kohlenforschung and ETH Zürich. Prof. List is acknowledged for sharing analytical equipment and the technical departments of MPI KOFO are acknowledged for assistance and the ETH D-CHAB small molecule crystallography center for single-crystal analysis. G. T. acknowledges a fellowship of the Studienstiftung des deutschen Volkes. J. C. R. acknowledges a fellowship of the Stipendienfonds Schweizerische Chemische Industrie (SSCI). We thank the Morandi group members for proof reading of the manuscript. Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS PubMed.
- G. Marzano, C. V. Ciasca, F. Babudri, G. Bianchi, A. Pellegrino, R. Po and G. M. Farinola, Eur. J. Org. Chem., 2014, 6583–6614 CrossRef CAS.
- S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed.
- P. A. Wender, Tetrahedron, 2013, 69, 7529–7550 CrossRef CAS PubMed.
- M. Shimizu and T. Hiyama, Eur. J. Org. Chem., 2013, 8069–8081 CrossRef CAS.
- M. Shimizu, I. Nagao, Y. Tomioka, T. Kadowaki and T. Hiyama, Tetrahedron, 2011, 67, 8014–8026 CrossRef CAS.
- S. Xu, X. Shangguan, H. Li, Y. Zhang and J. Wang, J. Org. Chem., 2015, 80, 7779–7784 CrossRef CAS PubMed.
- T. B. Marder and N. C. Norman, Top. Catal., 1998, 5, 63–73 CrossRef CAS.
- L. T. Kliman, S. N. Mlynarski and J. P. Morken, J. Am. Chem. Soc., 2009, 131, 13210–13211 CrossRef CAS PubMed.
- Y. Lee, H. Jang and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 18234–18235 CrossRef CAS PubMed.
- A. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás and E. Fernández, Angew. Chem., Int. Ed., 2011, 50, 7158–7161 CrossRef CAS PubMed.
- A. Bonet, C. Sole, H. Gulyás and E. Fernández, Org. Biomol. Chem., 2012, 10, 6621–6623 RSC.
- A. Farre, K. Soares, R. A. Briggs, A. Balanta, D. M. Benoit and A. Bonet, Chem. – Eur. J., 2016, 22, 17552–17556 CrossRef CAS PubMed.
- S. K. Bose, S. Brand, H. O. Omoregie, M. Haehnel, J. Maier, G. Bringmann and T. B. Marder, ACS Catal., 2016, 6, 8332–8335 CrossRef CAS.
- D. J. Blair, D. Tanini, J. M. Bateman, H. K. Scott, E. L. Myers and V. K. Aggarwal, Chem. Sci., 2017, 8, 2898–2903 RSC.
- X.-X. Wang, L. Li, T.-J. Gong, B. Xiao, X. Lu and Y. Fu, Org. Lett., 2019, 21, 4298–4302 CrossRef CAS PubMed.
- X. Wang, Y. Wang, W. Huang, C. Xia and L. Wu, ACS Catal., 2021, 11, 1–18 CrossRef CAS.
- T. P. Blaisdell and J. P. Morken, J. Am. Chem. Soc., 2015, 137, 8712–8715 CrossRef CAS PubMed.
- S. N. Mlynarski, C. H. Schuster and J. P. Morken, Nature, 2014, 505, 386–390 CrossRef CAS PubMed.
- F. Barrios-Landeros, B. P. Carrow and J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 8141–8154 CrossRef CAS PubMed.
- J. Almond-Thynne, D. C. Blakemore, D. C. Pryde and A. C. Spivey, Chem. Sci., 2017, 8, 40–62 RSC.
- C. Amatore, G. L. Duc and A. Jutand, Chem. – Eur. J., 2013, 19, 10082–10093 CrossRef CAS PubMed.
- B. P. Carrow and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 2116–2119 CrossRef CAS PubMed.
- C. Amatore, A. Jutand and G. Le Duc, Chem. – Eur. J., 2011, 17, 2492–2503 CrossRef CAS PubMed.
- C. Amatore, A. Jutand and G. Le Duc, Chem. – Eur. J., 2012, 18, 6616–6625 CrossRef CAS PubMed.
- N. Wiberg and A. F. Holleman, Kapitel XVII. Die Gruppe der Erdalkalimetalle, De Gruyter, 2020, pp. 1215–1258 DOI:10.1515/9783110206845-019.
- X. Ma, B. Murray and M. R. Biscoe, Nat. Rev. Chem., 2020, 4, 584–599 CrossRef CAS PubMed.
- A. H. Cherney, N. T. Kadunce and S. E. Reisman, Chem. Rev., 2015, 115, 9587–9652 CrossRef CAS PubMed.
- A. Kovacs, A. Vasas and J. Hohmann, Phytochemistry, 2008, 69, 1084–1110 CrossRef CAS PubMed.
- D. Leonori and V. K. Aggarwal, Angew. Chem., Int. Ed., 2015, 54, 1082–1096 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2054485. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc00648g |
| This journal is © The Royal Society of Chemistry 2021 |
