Open Access Article
Open Access ArticleA photochemical ring expansion of 6- to 8-membered nitrogen heterocycles by [1,3]-sigmatropic rearrangement†‡
Morgan A.
Manning
,
Wei
Sun
,
Mark E.
Light
and
David C.
Harrowven
 *
*
Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK. E-mail: dch2@soton.ac.uk
First published on 30th March 2021
Abstract
A new route to azocines and benzoazocines from furopyridinones is described through a photochemically induced [1,3]-sigmatropic rearrangement. The method gives access to these 8-membered nitrogen heterocycles from dimethyl squarate in four stages and with excellent atom economy by sequencing thermal and photochemical ring expansion steps under continuous flow.
Azocanes and their unsaturated analogues form a class of 8-membered nitrogen heterocycles that includes the manzamine alkaloids and other natural products.1–5 Though they have the attributes of a privileged structure in medicinal chemistry,6 they remain underexploited in that context due to challenges associated with their synthesis.7 In particular, syntheses based on ‘end-to-end’ cyclisation strategies have to overcome transannular strain and the loss of entropy on ring closure,3,7,8 making it necessary to employ high dilution or pseudo-high dilution conditions to reduce competing intermolecular reactions.9 Herein we describe a new route to azocines 3 and benzoazocines 6 by photo-induced ring expansion of vinyl- and aryl-furopyridinones 2 and 5 respectively (Scheme 1).10,11 In addition we show how the same products can be formed directly from alkynylcyclobutenones 1 and 4 by sequencing thermal and photochemical rearrangements under flow.11,12
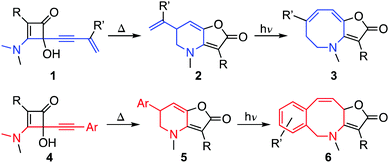 | ||
| Scheme 1 Sequential thermal and photochemical ring expansion reactions for the synthesis of azocines and benzoazocines from cyclobutenones. | ||
The discovered was made during a follow-up study on the thermal rearrangement of aminocyclobutenones 1/4 to furopyridinones 2/5.10,12 The presence of an extended chromophore in the products prompted us to examine their photochemistry.13 Pleasingly, when an acetonitrile solution of 2a was irradiated with UVA light (λ = 370 nm, 36 W) under continuous flow, using a set-up akin to that described by Booker-Milburn and Berry et al.,13–15 it gave furoazocine 3a in 48% yield (Scheme 2). Similarly, furopyridinones 2b and 2c gave furoazocines 3b and 3c in 47% and 54% yield respectively on irradiation.
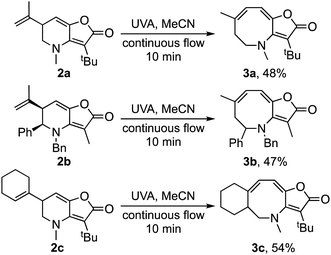 | ||
| Scheme 2 Photochemical ring expansions of furopyridinones to azocines.16,17 | ||
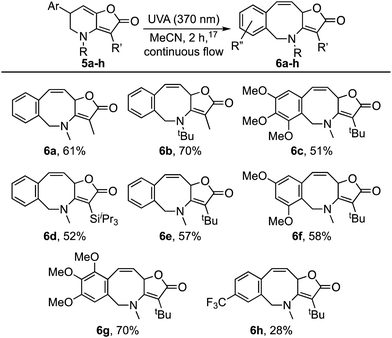
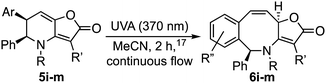
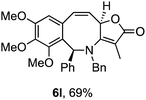
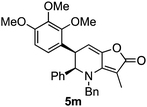
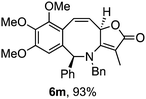
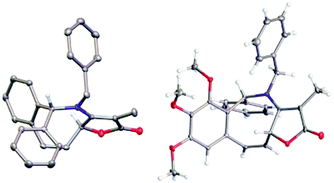
Attention next turned to aryl-substituted furopyridinones 5a–h, which were readily prepared by thermal rearrangement of the corresponding alkynylcyclobutenones 4 (Scheme 4 and ESI‡).10 Each underwent ring expansion on irradiation with UVA to give the corresponding benzoazocines 6a–h (Tables 1 and 2) with a skipped diene unit. Yields were typically in the range of 51–74%, except for substrate 5h with the electron deficient arene which was significantly lower (28%). Cases where the migrating bond was between two benzylic centres, e.g.5i–n (Table 2), were also high yielding and notably gave benzoazocines 6i–n as single diastereoisomers. Their relative stereochemistry was confirmed by x-ray crystallographic analysis of 6i and 6l (Fig. 1).
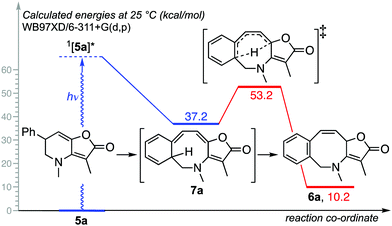
The mechanistic course of the reaction was next examined by TD-DFT,18 using 5a → 6a as the exemplar. Calculations showed that the singlet excited state 1[5a]* could relax directly to azocine 7avia a 1,3-sigmatropic rearrangement (Fig. 2),19 before giving benzoazocine 6avia a thermal [1,5]-sigmatropic H-shift (estimated Ea = 16.0 kcal mol−1).20
Interestingly, for the related thiophene derivative 5n the conjugated tetraene 7n was evidenced as an intermediate by 1H NMR, albeit as a mixture with 6n. On standing that sample underwent isomerization to give tris-heterocycle 6n as the sole product (Scheme 3). TD-DFT analysis indicated that the barrier for the [1,5]-H-shift, 7n → 6n (26.7 kcal mol−1, see ESI‡), was significantly higher than for 7a → 6a, suggesting that isomerization may be by protonation and deprotonation in this case. The method was then extended to the 2-thiophenyl and 3-pyridyl analogues, 5o and 5p with both giving a tris-heterocyclic product, 6o and 6p, albeit in low yield with the electron deficient heteroaromatic (Scheme 4).
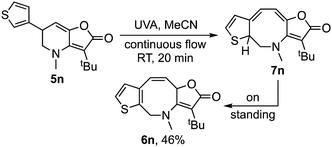 | ||
| Scheme 3 Evidence for the intermediacy of polyene 7 was provided by extension to the heteroaromatic analogue 5n.17 | ||
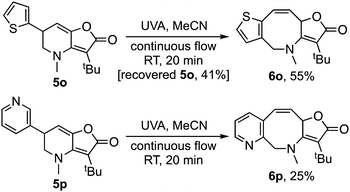 | ||
| Scheme 4 Further examples involving heteroaromatic ring systems.17 | ||
Finally, we have been able to produce benzoazocines 6 from alkynylcyclobutenones 4, directly and in high yield, by sequencing the respective thermal and photochemical rearrangements under flow (Scheme 5). Thus, dioxane solutions of cyclobutenones 4a, e, g, i, l were first subjected to thermolysis at 210 °C for a residence time of 100 min, then irradiated with UVA light from 6 × 1.7 W LEDs for 10 min to give the corresponding benzoazocines 6a, e, g, i, l in 74–84% yield. Notably, the efficiency with which each starting material was prepared ensured that these four-stage sequences from dimethyl squarate 8 each proceeded in ∼50% overall yield.21
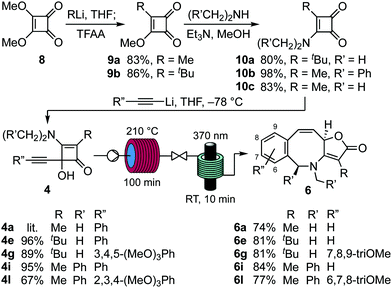 | ||
| Scheme 5 Preparation of cyclobutenones 4 and conversion to benzoazocines 6 by sequenced thermal and photochemical rearrangement under continuous flow. | ||
Sequential thermal and photochemical rearrangements were also effective with alkynylcyclobutenone 1c (Scheme 6). In this case it was found advantageous to conduct the thermolysis in two stages due to its poor conversion to the intermediate furopyridinone 2c following a single pass at 160 °C. As with the aforementioned examples, the overall yield of azocine 3c given after sequencing these steps under continuous flow was substantially higher than that achieve using stepwise procedures.21
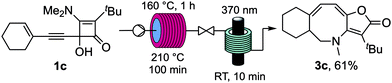 | ||
| Scheme 6 Sequenced thermal and photochemical rearrangement of cyclobutenone 1c to azocine 3c under continuous flow. | ||
In conclusion, we have developed a new route to azocines and benzoazocines involving the photochemical ring expansion of furopyridinones. The ease with which these products can be prepared from dimethyl squarate 8 in high yield and diastereoselectivity, and with excellent atom economy, makes this an attractive entry to a class of nitrogen heterocycles that is difficult to access using classical procedures.
Dr Wei Sun and Morgan Manning contributed equally in respect of the experimental work, with Dr Mark Light performing the X-ray analyses and Prof. David Harrowven supervising the work.
We gratefully acknowledge financial support from the European Regional Development Fund [ERDF Interreg Va programme (Project 121)] and EPSRC [EP/P013341/1, EP/L003325/1 and EP/K039466/1].
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- R. Sakai, T. Higa, C. W. Jefford and G. Bernardinelli, J. Am. Chem. Soc., 1986, 108, 6404 CrossRef CAS; P. Jakubec, A. Hawkins, W. Felzmann and D. J. Dixon, J. Am. Chem. Soc., 2012, 134, 17482 CrossRef PubMed; J. E. Baldwin, T. D. W. Claridge, F. A. Heupel and R. C. Whitehead, Tetrahedron Lett., 1992, 33, 2059 CrossRef; P. Jakubec, A. F. Kyle, J. Calleja and D. J. Dixon, Tetrahedron Lett., 2011, 52, 6094 CrossRef; J. D. Winkler and J. M. Axten, J. Am. Chem. Soc., 1998, 120, 6425 CrossRef PubMed; M. Nakagawa, J. Heterocycl. Chem., 2000, 37, 567 CrossRef; S. F. Martin, J. M. Humphrey, A. Ali and M. C. Hillier, J. Am. Chem. Soc., 1999, 121, 866 CrossRef; J. M. Humphrey, Y. Liao, A. Ali, T. Rein, Y. L. Wong, H. J. Chen, A. K. Courtney and S. F. Martin, J. Am. Chem. Soc., 2002, 124, 8584 CrossRef PubMed; T. Toma, Y. Kita and T. Fukuyama, J. Am. Chem. Soc., 2010, 132, 10233 CrossRef PubMed; J. E. Baldwin, T. D. W. Claridge, A. J. Culshaw, F. A. Heupel, V. Lee, D. R. Spring and R. C. Whitehead, Chem. – Eur. J., 1999, 5, 3154 CrossRef; B. Zhang, R. Higuchi, T. Miyamoto and R. W. M. Van Soest, Chem. Pharm. Bull., 2008, 56, 866 CrossRef PubMed; Y. Kita, T. Toma, T. Kan and T. Fukuyama, Org. Lett., 2008, 10, 3251 CrossRef PubMed; R. A. Stockman, P. J. McDermott, A. F. Newton and P. Magnus, Synlett, 2010, 559 CrossRef.
- Lundurine A and B: A. Nash, X. Qi, P. Maity, K. Owens and U. K. Tambar, Angew. Chem., Int. Ed., 2018, 57, 6888 CrossRef CAS PubMed; S. Jin, J. Gong and Y. Qin, Angew. Chem., Int. Ed., 2015, 54, 2228 CrossRef PubMed; M. Hoshi, O. Kaneko, M. Nakajima, S. Arai and A. Nishida, Org. Lett., 2014, 16, 768 CrossRef PubMed Nakadomarin A: P. Jakubec, D. M. Cockfield and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 16632 CrossRef PubMed; P. Jakubec, A. F. Kyle, J. Calleja and D. J. Dixon, Tetrahedron Lett., 2011, 52, 6094 CrossRef; J. N. I. Kobayashi, D. Watanabe, N. Kawasaki and M. Tsuda, J. Org. Chem., 1997, 62, 9236 CrossRef; J. Kobayashi, Pure Appl. Chem., 1999, 71, 1123 Search PubMed; I. S. Young and M. A. Kerr, J. Am. Chem. Soc., 2007, 129, 1465 CrossRef PubMed.
- Buflavine: C. Hoarau, A. Couture, E. Deniau and P. Grandclaudon, J. Org. Chem., 2002, 67, 5846 CrossRef CAS PubMed; P. Sahakitpichan and S. Ruchirawat, Tetrahedron Lett., 2003, 44, 5239 CrossRef; F. Viladomat, J. Bastida, C. Codina, W. E. Campbell and S. Mathee, Phytochemistry, 1995, 40, 307 CrossRef; P. A. Patil and V. Snieckus, Tetrahedron Lett., 1998, 39, 1325 CrossRef; Y. Ishida, Y. Sasaki, Y. Kimura and K. Watanabe, J. Pharmacobio-Dyn, 1985, 8, 917 CrossRef PubMed; S. Kobayashi, M. Kihara, S. Shizu, S. Katayama, H. Ikeda, K. Kitahiro and H. Matsumoto, Chem. Pharm. Bull., 1977, 25, 3312 CrossRef PubMed.
- Magallanesine: E. Valencia, V. Fajardo, A. J. Freyer and M. Shamma, Tetrahedron Lett., 1985, 26, 993 CrossRef CAS; R. Yoneda, Y. Sakamoto, Y. Oketo, K. Minami, S. Harusawa and T. Kurihara, Tetrahedron Lett., 1994, 35, 3749 CrossRef; R. Yoneda, Y. Sakamoto, Y. Oketo, S. Harusawa and T. Kurihara, Tetrahedron, 1996, 52, 14563 CrossRef; F. G. Fang, G. B. Feigelson and S. J. Danishefsky, Tetrahedron Lett., 1989, 30, 2743 CrossRef.
- Apparicine: M. L. Bennasar, E. Zulaica, D. Solé and S. Alonso, Chem. Commun., 2009, 3372 RSC; M. L. Bennasar, E. Zulaica, D. Solé, T. Roca, D. García-Díaz and S. Alonso, J. Org. Chem., 2009, 74, 8359 CrossRef CAS PubMed.
- C. Zhao, Z. Ye, Z. Ma, S. A. Wildman, S. A. Blaszczyk, L. Hu, I. A. Guizei and W. Tang, Nat. Commun., 2019, 10, 4015 CrossRef CAS PubMed; A. Hussain, S. K. Yousuf and D. Mukherjee, RSC Adv., 2014, 4, 43241 RSC; R. A. Bauer, T. A. Wenderski and D. S. Tan, Nat. Chem. Biol., 2013, 9, 21 CrossRef PubMed; A. Sharma, P. Appukkuttan and E. Van Der Eycken, Chem. Commun., 2012, 48, 1623 RSC; K. R. Romines, K. D. Watenpaugh, P. K. Tomich, W. J. Howe, J. K. Morris, K. D. Lovasz, A. M. Mulichak, B. C. Finzel, J. C. Lynn, M. M. Homg, F. J. Schwende, M. J. Ruwart, G. L. Zipp, K. T. Chong, L. A. Dolak, L. N. Toth, G. M. Howard, B. D. Rush, K. F. Wilkinson, P. L. Possert, R. J. Dalga and R. R. Hinshaw, J. Med. Chem., 1995, 38, 1884 CrossRef PubMed; E. D. Thorsett, E. E. Harris, S. D. Aster, E. R. Peterson, J. P. Snyder, J. P. Springer, J. Hirshfield, E. W. Tristram, A. A. Patchett, E. H. Ulm and T. C. Vassil, J. Med. Chem., 1986, 29, 251 CrossRef PubMed; M. E. Rogers, H. H. Ong, E. L. May and W. A. Klee, J. Med. Chem., 1975, 18, 1036 CrossRef PubMed; H. H. Ong and E. L. May, J. Org. Chem., 1973, 38, 924 CrossRef; E. J. Lien, L. L. Lien and G. L. Tong, J. Med. Chem., 1971, 14, 846 CrossRef PubMed.
- A. K. Clarke and W. P. Unsworth, Chem. Sci., 2020, 11, 2876 RSC; J. R. Donald and W. P. Unsworth, Chem. – Eur. J., 2017, 23, 8780 CrossRef CAS PubMed; A. V. Listratova and L. G. Voskressensky, Synthesis, 2017, 3801 Search PubMed; A. Deiters and S. F. Martin, Chem. Rev., 2004, 104, 2199 CrossRef; M. E. Maier, Angew. Chem., Int. Ed., 2000, 39, 2073 CrossRef PubMed; L. Yet, Chem. Rev., 2000, 100, 2963 CrossRef PubMed; P. A. Evans and B. Holmes, Tetrahedron, 1991, 47, 9131 CrossRef; C. J. Roxburgh, Tetrahedron, 1993, 49, 10749 CrossRef; G. Illuminati and L. Mandolini, Acc. Chem. Res., 1981, 14, 95 CrossRef; M. L. Bennasar, E. Zulaica, D. Solé and S. Alonso, Tetrahedron, 2007, 63, 861 CrossRef.
- A. Hussain, S. K. Yousuf and D. Mukherjee, RSC Adv., 2014, 4, 43241 RSC; G. A. Molander, Acc. Chem. Res., 1998, 31, 603 CrossRef CAS; M. A. Casadei, C. Galli and L. Mandolini, J. Am. Chem. Soc., 1984, 106, 1051 CrossRef; H. Jacobson and W. H. Stockmayer, J. Chem. Phys., 1950, 18, 1600 CrossRef.
- C. J. White and A. K. Yudin, Nat. Chem., 2011, 3, 509 CrossRef CAS PubMed; K. C. Majumdar, RSC Adv., 2011, 1, 1152 RSC; H. Bieräugel, T. P. Jansen, H. E. Schoemaker, H. Hiemstra and J. H. van Maarseveen, Org. Lett., 2002, 4, 2673 CrossRef PubMed; G. Rousseau, Tetrahedron, 1995, 51, 2777 CrossRef; J. Fastrez, J. Phys. Chem., 1989, 93, 2635 CrossRef; M. Antonietta Casadei, C. Galli and L. Mandolini, J. Org. Chem., 1981, 46, 3127 CrossRef.
- W. Sun, D. C. Wilson, M. E. Light and D. C. Harrowven, Org. Lett., 2018, 20, 4346 CrossRef CAS PubMed; W. Sun, D. C. Wilson and D. C. Harrowven, Synthesis, 2017, 3091 Search PubMed See also: T. C. Stephens and W. P. Unsworth, Synlett, 2020, 133 Search PubMed; L. G. Baud, M. A. Manning, H. L. Arkless, T. C. Stephens and W. P. Unsworth, Chem. – Eur. J., 2017, 23, 2225 CrossRef PubMed; D. C. Harrowven, N. L’Helias, J. D. Moseley, N. J. Blumire and S. R. Flanagan, Chem. Commun., 2003, 2658 RSC.
- J. P. Knowles, L. D. Elliott and K. I. Booker-Milburn, Beilstein J. Org. Chem., 2012, 8, 2025 CrossRef CAS PubMed; K. Gilmore and P. H. Seeberger, Chem. Rec., 2014, 14, 410 CrossRef PubMed; C. Sambiagio and T. Noël, Trends Chem., 2020, 2, 92 CrossRef; M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796 CrossRef PubMed; D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276 CrossRef PubMed; F. Politano and G. Oksdath-Mansilla, Org. Process Res. Dev., 2018, 22, 1045 CrossRef; T. H. Rehm, ChemPhotoChem, 2019, 3, 1 CrossRef; N. Hoffmann, Chem. Rev., 2008, 108, 1052 CrossRef PubMed; M. Oelgemoeller, Chem. Eng. Technol., 2012, 35, 1144 CrossRef.
- E. Packard, D. D. Pascoe, J. Maddaluno, T. P. Gonçalves and D. C. Harrowven, Angew. Chem., Int. Ed., 2013, 52, 13076 CrossRef CAS PubMed; D. C. Harrowven, M. Mohamed, T. P. Gonçalves, R. J. Whitby, D. Bolien and H. F. Sneddon, Angew. Chem., Int. Ed., 2012, 51, 4405 CrossRef PubMed; M. Mohamed, T. P. Gonçalves, R. J. Whitby, H. F. Sneddon and D. C. Harrowven, Chem. – Eur. J., 2011, 17, 13698 CrossRef PubMed; D. C. Harrowven, D. D. Pascoe, D. Demurtas and H. O. Bourne, Angew. Chem., Int. Ed., 2005, 44, 1221 CrossRef PubMed.
- T. P. Gonçalves, M. Mohamed, R. J. Whitby, H. F. Sneddon and D. C. Harrowven, Angew. Chem., Int. Ed., 2015, 54, 4531 CrossRef CAS PubMed; D. E. Collin, E. H. Jackman, N. Jouandon, W. Sun, M. E. Light, D. C. Harrowven and B. Linclau, Synthesis, 2021, 1307 Search PubMed.
- B. D. A. Hook, W. Dohle, P. R. Hirst, M. Pickworth, M. B. Berry and K. I. Booker-Milburn, J. Org. Chem., 2005, 70, 7558 CrossRef CAS PubMed.
- L. D. Elliott, M. Berry, B. Harji, D. Klauber, J. Leonard and K. I. Booker-Milburn, Org. Process Res. Dev., 2016, 20, 1806 CrossRef CAS; C. A. Clark, D. S. Lee, S. J. Pickering, M. Poliakoff and M. W. George, Org. Process Res. Dev., 2016, 20, 1792 CrossRef; L. D. Elliott, J. P. Knowles, P. J. Koovits, K. G. Maskill, M. J. Ralph, G. Lejeune, L. J. Edwards, R. I. Robinson, I. R. Clemens, B. Cox, D. D. Pascoe, G. Koch, M. Eberle, M. B. Berry and K. I. Booker-Milburn, Chem. – Eur. J., 2014, 20, 15226 CrossRef PubMed.
- Examples of the rearrangements 2 → 3 were limited by the availability of enynes from commercial suppliers, and on-going COVID restrictions. Compound 6k was contaminated with an unknown impurity accounting for ca. 5% of its mass.
- The photochemical experiments described in Tables 1 and 2 were conducted using a 36 W Philips UVA lamp [PL36/10/4P] that delivered ∼10 W UVA irradiation for a reactor volume of 120 mL [UVA irradiation density ∼0.08 W mL−1]. In Schemes 2–6, a bespoke UVA LED reactor was used that delivered ∼12 W UVA irradiation for a reactor volume of 10 mL. The irradiation density in this case was substantially higher at ∼1.2 W mL−1. See ESI‡ for details.
- K. G. Maskill, J. P. Knowles, L. D. Elliott, R. W. Alder and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2013, 52, 1499 CrossRef CAS PubMed.
- A. C. Cope, M. M. Martin and M. A. McKervey, Q. Rev. Chem. Soc., 1966, 20, 119 RSC; R. B. Woodward and R. Hoffmann, J. Am. Chem. Soc., 1965, 87, 2511 CrossRef CAS; R. Hoffmann and R. B. Woodward, Acc. Chem. Res., 1968, 1, 17 CrossRef; R. B. Woodward and R. Hoffmann, Angew. Chem., Int. Ed. Engl., 1969, 8, 781 CrossRef; N. G. Rondon and K. N. Houk, Tetrahedron Lett., 1984, 25, 2519 CrossRef; J. E. Baldwin, P. A. Leber and T. W. Lee, J. Org. Chem., 2001, 66, 5269 CrossRef PubMed; B. A. Hess and J. E. Baldwin, J. Org. Chem., 2002, 67, 6025 CrossRef PubMed; P. Rademacher and P. Choudhary Mohr, Org. Biomol. Chem., 2007, 5, 2698 RSC; L. Stéhelin, J. Lhomme and G. Ourisson, J. Am. Chem. Soc., 1971, 93, 1650 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A significant loss of mass balance to mixed fractions occurs on purification of furopyridinones 2 and 5 by column chromatography. We believe this to be the primary reason for the yield elevation observed when the thermal and photochemical steps are sequenced.
Footnotes |
| † Dedicated to Prof. Kevin Booker-Milburn on the occasion of his retirement. |
| ‡ Electronic supplementary information (ESI) available: Experimental accounts, spectral and analytical details together with copies of 1H and 13C NMR spectra. CCDC 1969077 and 2025763. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc00393c |
| This journal is © The Royal Society of Chemistry 2021 |