Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoactivated release of membrane impermeant sulfonates inside cells†
Stuart T.
Caldwell
 a,
Sean N.
O'Byrne
a,
Sean N.
O'Byrne
 a,
Calum
Wilson
a,
Calum
Wilson
 b,
Filip
Cvetko
c,
Michael P.
Murphy
b,
Filip
Cvetko
c,
Michael P.
Murphy
 c,
John G.
McCarron
c,
John G.
McCarron
 b and
Richard C.
Hartley
b and
Richard C.
Hartley
 *a
*a
aSchool of Chemistry, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: richard.hartley@glasgow.ac.uk
bStrathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, 161 Cathedral Street, Glasgow G4 0RE, UK
cMRC Mitochondrial Biology Unit, Hills Road, University of Cambridge, CB2 0XY, UK
First published on 12th March 2021
Abstract
Photouncaging delivers compounds with high spatial and temporal control to induce or inhibit biological processes but the released compounds may diffuse out. We here demonstrate that sulfonate anions can be photocaged so that a membrane impermeable compound can enter cells, be uncaged by photoirradiation and trapped within the cell.
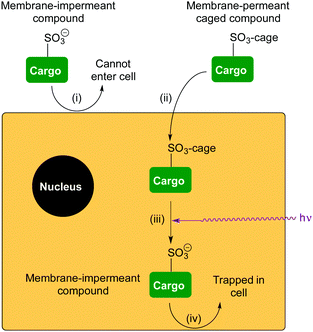
The delivery of drugs or probes to cells often relies on diffusion into the cell. Under these circumstances, the compound can subsequently freely diffuse out of the cell in the same way. Therefore, some chemical change is necessary to retain it. One approach is to protect the compound in a way that is susceptible to endogenous processes in the cell, such as enzyme-catalysed hydrolysis, and for the released compound to become membrane impermeant as a result of increased charge. This is successful for many sensors. For example, BAPTA-based calcium sensors can be caged as neutral acetoxymethyl (AM) esters,1 and when released the concentrated charge of four carboxylate anions prevents the sensor diffusing out of the cell again. In a similar way, esters have been combined with photocaged fluorophores2–4 and bioactive compounds5,6 (Fig. 1 for examples). Esterase release of the charged groups result in the photocaged compounds being trapped inside cells. Photouncaging using UV light generates the switched-on fluorophore or bioactive still trapped inside the cell. We believed a more general method would be to cage the ion of a strong acid incorporated into the structure of the compound that is to be retained in the cell. We now report that the delivery of membrane impermeant sulfonate compounds to cells followed by photochemical trapping is possible (Fig. 2).
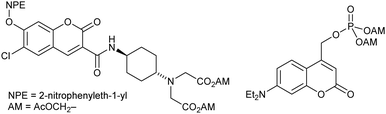 | ||
| Fig. 1 Examples of doubly caged compounds. Doubly caged coumarin (ref. 3) and doubly caged phosphate (ref. 6). | ||
Photouncaging of aryl sulfonates using short wavelength UV light of typically <330 nm7 has been used for the generation of acids or the deprotection of sulfonates.8 2-Nitrobenzyl sulfonate esters9 such as “caged sulfate”10 have also been used as photoacids, while irradiation of 1-(2-nitrophenyl)-ethylhexadecyl sulfonate11 has been used in vivo for intracellular acidification, but caging in this way has never been suggested for the delivery of membrane-impermeant compounds to cells. This may be because benzylic sulfonates are easily hydrolysed and are reactive alkylating agents. The Miller group12,13 has demonstrated that sulfonate anions can be caged as α-trifluoromethylbenzylic sulfonate esters, which are resistant to hydrolysis by SN1 or SN2. We reasoned that incorporation of an α-trifluoromethyl group would ensure stability of an ortho-nitrobenzylic photocage prior to activation and may increase the efficiency of uncaging.14 We chose to use a sulfonated dye as a proof of principle to demonstrate that the α-trifluoromethyl-ortho-nitrobenzyl (TFNB) group would allow membrane-impermeant sulfonates to enter cells and be retained upon uncaging. The use of a fluorophore allows visualization of uptake and also allows assessment of the effect of sulfonation on membrane permeation. To demonstrate the principle we chose sulforhodamine B 1 because it is one of the most widely utilized fluorescent dyes and is known to be cell impermeable.15,16 We proposed that bis-caged sulforhodamine (BCSR) 2 would be membrane permeant. Photouncaging would then lead to sulforhodamine B 1 being trapped within the cell, demonstrating the utility of TFNB for delivery of sulfonates (Scheme 1). (See ESI,† for synthesis).
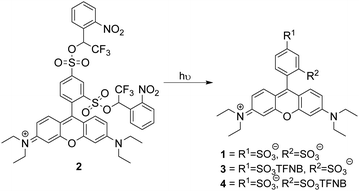 | ||
| Scheme 1 Sulforhodamine bis-TFNB ester 2 and potential products from photochemical uncaging. TFNB = α-trifluoro-ortho-nitrobenzyl. | ||
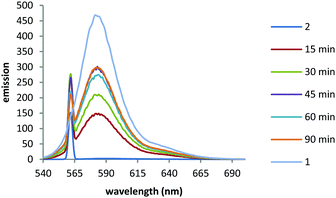
We first monitored the uncaging using fluorescence. Fluorescence emission of BCSR 2 in pH 7.4 buffer was almost completely quenched in buffer due to photoinduced electron transfer (PeT) quenching by the nitroarene groups. This increased 118-fold upon irradiation at 365 nm (330 W m−2) for 90 min (Fig. 3).
LCMS analysis of the uncaging revealed a mixture of products due to incomplete uncaging and some partial deethylation of the amines.17–19 Within 15 minutes only 1% of the starting material remained with 24% 1, 50% 3 and 25% 4 (including products from loss of the ethyl group) observed (Scheme 1), showing that removal of the first caging group was rapid and this was followed by a slower second uncaging. Integration of the LC chromatogram showed that 70% full uncaging had occurred within 60 min irradiation, the remainder being a mixture of monouncaged products 3 and 4 (Fig. S1B and A, ESI,† for assignment of LCMS peaks). BSCR 2 was relatively stable in pH 7.4 buffer remaining 85% unchanged after 4 days at 37 °C in the dark (Fig. S1D, ESI†). On the other hand, ortho-nitrobenzyl derivatives lacking the α-trifluoro group were unstable.
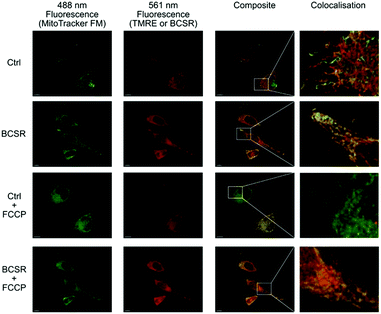
Next, we used BSCR 2 to show that the TFNB group allowed uptake, release and retention of membrane impermeant sulfonates. BCSR 2 was taken up by HeLa cells. Excitation at 488 nm revealed a pattern of fluorescence for BCSR 2 that was colocalised with the mitochondrial dye, MitoTracker green, (Fig. 4). This confirmed that caging the sulfonates with TFNB allowed an otherwise membrane-impermeant compound to enter cells. Many rhodamine-based dyes accumulate several-hundred-fold in the mitochondrial matrix20 because they are lipophilic cations and there is a 120–160 mV membrane potential across the mitochondrial inner membrane, which is negative inside. Therefore, we compared the behaviour of BCSR 2 against another rhodamine dye, TMRE, as a control. The control compound colocalised with MitoTracker green, and as expected this colocalisation was lost when the membrane potential was removed by FCCP. On the other hand, colocalisation of BCSR 2 and MitoTracker green was unchanged by the addition of FCCP. This demonstrates that the colocalisation of BSCR 2 with mitochondria is not the result of membrane-potential driven uptake into the matrix.
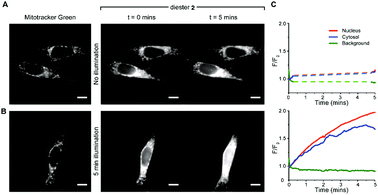
Uptake and colocalisation with MitoTracker green was also observed when HEK 293T cells (Fig. S2, ESI†) were incubated with BCSR 2. Localised irradiation of mitochondria at 355 nm using a laser gave no increase in fluorescence but instead showed a loss of fluorescence. However, surprisingly, wide field excitation at 555 nm (rhodamine excitation wavelength used for imaging) for 5 min led to an approximate 2-fold increase in fluorescence emission and this increase in emission remained within the cell (Fig. 5A and B and Videos 1, 2, ESI†). Cells that were not irradiated with 555 nm light showed negligible increase in fluorescence over the same time period (Fig. 5C and Videos 3, 4, ESI†). Therefore, the observed increase in fluorescence was spatially controlled by visible light and is consistent with photouncaging and is not via an alternative hydrolysis mechanism. Notably, the fluorescence increased throughout the cell, indicating redistribution of the dye, consistent with the decrease in fluorescence upon localised irradiation of mitochondria.
The observed redistribution within the cell upon uncaging (Fig. 5 and Videos 1–4, ESI†) is consistent with BCSR 2 associating with the outer surface of the mitochondrial membrane and the highly water-soluble sulforhodamine 1 dissociating upon uncaging. To confirm that this redistribution was due to full uncaging to sulforhodamine B 1, we confirmed that the monocaged compound 3 showed a similar localisation pattern to BCSR 2 (Fig. S3, ESI†).
Photouncaging of 2-nitrobenzyl groups is typically achieved using UV light21 but substituted nitrobenzyl groups have been uncaged at 420 nm22 so the increase in fluorescence observed when irradiating TFNB sulfonate diester 2 in cells with 555 nm light was unexpected. We therefore wished to confirm the photouncaging in vitro. The UV/Vis absorption spectrum of TFNB sulfonate diester 2 in buffer showed a maximum absorption at ∼586 nm (Fig. S4, ESI†). We therefore irradiated a 10 μM degassed solutions of sulfonate diester 2 at 595 nm (∼57 W m−2) with a ±5 nm bandpass filter in pH 7.4 buffer and monitored the reaction by LCMS and fluorescence. BCSR 2 was almost completely converted to a mixture of sulfonate monoesters 3 and 4 and sulforhodamine B 1 within 30 min (plus compounds arising from partial deethylation of the amines) giving a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 1
1 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (3 + 4)
(3 + 4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. S1C, ESI†). After 90 min irradiation at 595 nm 62% of the dye was fully uncaged and showed a 170 fold increase in fluorescence emission (Fig. S5, ESI†). The greater increase in emission intensity upon uncaging with 595 nm light compared to 365 nm light is presumably due to less photobleaching when using low energy long wavelength light. Thus, the increase in fluorescence when cells treated with diester 2 are irradiated with 555 nm light is congruent with photouncaging and formation of sulforhodamine B 1. The observed fluorescence increase when BCSR 2 is irradiated with visible light is due to photouncaging. However, this cannot proceed via the usual mechanism of uncaging of 2-nitrobenzyl groups since there is insufficient energy to form the intermediate aci–nitro23 complex. We therefore propose that the unexpected uncaging using visible light (555–595 nm) proceeds via a SRN1 mechanism.24–27 PeT28 from the excited state rhodamine to the nitrobenzyl group results in an aryl radical anion which ultimately cleaves to give the uncaged sulfonate. The uncaging of a picolinium salts has been reported to proceed via a similar mechanism.29–31
2 (Fig. S1C, ESI†). After 90 min irradiation at 595 nm 62% of the dye was fully uncaged and showed a 170 fold increase in fluorescence emission (Fig. S5, ESI†). The greater increase in emission intensity upon uncaging with 595 nm light compared to 365 nm light is presumably due to less photobleaching when using low energy long wavelength light. Thus, the increase in fluorescence when cells treated with diester 2 are irradiated with 555 nm light is congruent with photouncaging and formation of sulforhodamine B 1. The observed fluorescence increase when BCSR 2 is irradiated with visible light is due to photouncaging. However, this cannot proceed via the usual mechanism of uncaging of 2-nitrobenzyl groups since there is insufficient energy to form the intermediate aci–nitro23 complex. We therefore propose that the unexpected uncaging using visible light (555–595 nm) proceeds via a SRN1 mechanism.24–27 PeT28 from the excited state rhodamine to the nitrobenzyl group results in an aryl radical anion which ultimately cleaves to give the uncaged sulfonate. The uncaging of a picolinium salts has been reported to proceed via a similar mechanism.29–31
Taken together, these results confirmed that TFNB can be used to cage a membrane impermeant sulfonate allowing it to enter the cells and be photouncaged within cells to release the membrane impermeant sulfonate, which is retained (Fig. 4, 5 and Videos, ESI†). TFNB can be removed in the usual way with UV light (Fig. 3 and Fig. S1B, ESI†), opening the way for the usual spatiotemporal control,21 and in the specific case of the dye used to illustrate delivery, it could also be removed with visible light (Fig. S1C and S5, ESI†) to give release only in the irradiated cells (Fig. 5 and Videos, ESI†).
In summary, we have demonstrated for the first time that sulfonic acids can be successfully photocaged as their TFNB sulfonates to deliver membrane-impermeant compounds to cells. The TFNB sulfonate diester of the membrane impermeant dye, sulforhodamine B 1, can enter cells and can there be uncaged not only by UV light, but also by yellow light. This unusual uncaging at a long wavelength is believed to proceed via photoinduced electron transfer from the rhodamine to the TFNB cage. The ability to trap compounds within cells by generating a salt of a strong acid in situ using light without the need for endogenous enzymes or nucleophiles allows for the delivery of drug and probes that can be retained near their area of action without diffusing out of the cell. Coupled with the spatial and temporal control typically endowed by photoactivation,21 this new method of delivery will be of great use.
This work was supported by Wellcome Trust awards to RCH (092292/B/10/Z and 110158/Z/15/Z), JGM (202924/Z/16/Z), CW (204682/Z/16/Z), MPM (110159/A/15/Z) and FC (PhD programme in Metabolic and Cardiovascular Diseases, RG88195), and by the Medical Research Council UK (MC_U105663142) for MPM.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Grynkiewicz, M. Poenie and R. Y. Tsien, J. Biol. Chem., 1985, 260, 3440–3450 CrossRef CAS.
- T. Kobayashi, Y. Urano, M. Kamiya, T. Ueno, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 6696–6697 CrossRef CAS PubMed.
- Y. Zhao, Q. Zheng, K. Dakin, K. Xu, M. L. Martinez and W.-H. Li, J. Am. Chem. Soc., 2004, 126, 4653–4663 CrossRef CAS PubMed.
- L. M. Heckman, J. B. Grimm, E. R. Schreiter, C. Kim, M. A. Verdecia, B. C. Shields and L. D. Lavis, Angew. Chem., Int. Ed., 2016, 55, 8363–8366 CrossRef CAS PubMed.
- A. P. Gorka, T. Yamamoto, J. Zhu and M. J. Schnermann, ChemBioChem, 2018, 19, 1239–1243 CrossRef CAS PubMed.
- C. Herbivo, Z. Omran, J. Revol, H. Javot and A. Specht, ChemBioChem, 2013, 14, 2277–2283 CrossRef CAS PubMed.
- J. Andraos, G. G. Barclay, D. R. Medeiros, M. V. Baldovi, J. C. Scaiano and R. Sinta, Chem. Mater., 1998, 10, 1694–1699 CrossRef CAS.
- Y. Kageyama, R. Ohshima, K. Sakurama, Y. Fujiwara, Y. Tanimoto, Y. Yamada and S. Aoki, Chem. Pharm. Bull., 2009, 57, 1257–1266 CrossRef CAS PubMed.
- M. S. Kim and S. L. Diamond, Bioorg. Med. Chem. Lett., 2006, 16, 4007–4010 CrossRef CAS PubMed.
- A. Barth and J. E. T. Corrie, Biophys. J., 2002, 83, 2864–2871 CrossRef CAS PubMed.
- M. Carbone, T. Zlateva and L. Quaroni, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830, 2989–2993 CrossRef CAS PubMed.
- A. Choi and S. C. Miller, Org. Biomol. Chem., 2017, 15, 1346–1349 RSC.
- L. Rusha and S. C. Miller, Chem. Commun., 2011, 47, 2038–2040 RSC.
- A. Specht and M. Goeldner, Angew. Chem., Int. Ed., 2004, 43, 2008–2012 CrossRef CAS PubMed.
- R. J. Lee, S. Wang, M. J. Turk and P. S. Low, Biosci. Rep., 1998, 18, 69–78 CrossRef CAS PubMed.
- W. Viricel, A. Mbarek and J. Leblond, Angew. Chem., Int. Ed., 2015, 54, 12743–12747 CrossRef CAS PubMed.
- S. G. Cohen, A. Parola and G. H. Parsons, Chem. Rev., 1973, 73, 141–161 CrossRef CAS.
- J. Hu, J. Wang, T. H. Nguyen and N. Zheng, Beilstein J. Org. Chem., 2013, 9, 1977–2001 CrossRef PubMed.
- M. Wang, Y. Na, M. Gorlov and L. Sun, Dalton Trans., 2009, 6458–6467 RSC.
- A. T. Hoye, J. E. Davoren, P. Wipf, M. P. Fink and V. E. Kagan, Acc. Chem. Res., 2008, 41, 87–97 CrossRef CAS PubMed.
- C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed.
- M. J. Hansen, W. A. Velema, M. M. Lerch, W. Szymanski and B. L. Feringa, Chem. Soc. Rev., 2015, 44, 3358–3377 RSC.
- Y. V. Il'ichev, M. A. Schwörer and J. Wirz, J. Am. Chem. Soc., 2004, 126, 4581–4595 CrossRef PubMed.
- R. A. Rossi, A. B. Pierini and A. B. Peñéñory, Chem. Rev., 2003, 103, 71–168 CrossRef CAS PubMed.
- E. Arceo, I. D. Jurberg, A. Álvarez-Fernández and P. Melchiorre, Nat. Chem., 2013, 5, 750–756 CrossRef CAS PubMed.
- M. Julliard and M. Chanon, Chem. Rev., 1983, 83, 425–506 CrossRef CAS.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77 CrossRef CAS PubMed.
- J. E. T. Corrie, B. C. Gilbert, V. R. N. Munasinghe and A. C. Whitwood, J. Chem. Soc., Perkin Trans. 2, 2000, 2483–2491, 10.1039/B005073N.
- J. B. Edson, L. P. Spencer and J. M. Boncella, Org. Lett., 2011, 13, 6156–6159 CrossRef CAS PubMed.
- K. A. Korzycka, P. M. Bennett, E. J. Cueto-Diaz, G. Wicks, M. Drobizhev, M. Blanchard-Desce, A. Rebane and H. L. Anderson, Chem. Sci., 2015, 6, 2419–2426 RSC.
- C. Sundararajan and D. E. Falvey, J. Org. Chem., 2004, 69, 5547–5554 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc07713e |
| This journal is © The Royal Society of Chemistry 2021 |