Pentanuclear clusters resembling the cubane-dangler connectivity in the native oxygen-evolving center of photosystem II†
Chao
Yang
,
Shenyu
Wang
,
Fusheng
Sai
,
Dingqi
Liu
,
Fuxing
Sun
,
Yu
Gu
and
Gang
Wu
 *
*
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China. E-mail: wug@jlu.edu.cn
First published on 23rd November 2020
Abstract
A series of pentametallic “cubane-plus-dangler” complexes have been target synthesized. Among them, the [Fe3Ni2] aggregate strongly resembled the native oxygen-evolving center by mimicking the “cubane-plus-dangler” skeleton, the aqua binding site, and the connectivity between the pendent ion and the parent cubane. Our synthetic strategy that uses tri-substituted methanol as the “cubane-generator” and carboxylate as the pendant ligand provides a feasible approach for accessing model compounds of biological catalyst systems.
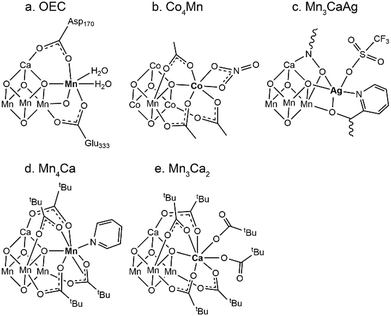
The oxidation of water into dioxygen by plants, algae, and cyanobacteria is performed at the oxygen-evolving center (OEC) of photosystem II (PSII).1 X-Ray diffraction (XRD) studies revealed that the core of the OEC consists of a low-symmetry Mn3CaO4 cubane motif and a “dangler” Mn ion (Scheme 1a).2 To better understand the mechanism behind the formation of dioxygen and the proposed intermediates during the water oxidation process, synthetic coordination clusters as OEC mimics are highly desired. Early efforts mainly focused on reproducing the cubane structure of the native OEC by using tetra-manganese aggregations.3 Cubane analogs containing various metal ions and/or bridging atoms have also exhibited certain catalytic activity under homogeneous oxidation conditions, demonstrating the vital role of the cubane structural motif in multielectron redox processes.4 Following the biochemical studies on the essential role of calcium ions in the water oxidation activity of the OEC,5 more accurate OEC models with Mn/Ca heterometallic compounds have also been targeted.6 In the native OEC, the [Mn3CaO4] cubane core is appended with a dangling Mn ion. The latter is commonly believed to play an important role in O–O bond formation by storing multiple electron–hole pairs and binding substrate water molecules. A similar phenomenon was also observed in carbon monoxide dehydrogenases (CODHs) from Carboxydothermus hydrogenoformans, which contain [Fe3NiS4] cubane cores with a dangling Fe2+ ion on the active site.7 Thus, the targeted synthesis of model compounds mimicking the “cubane-plus-dangler” motif is of great importance to understand the vital role of the dangling ion in biological multielectron redox reactions. However, such complexes are extremely rare with only four known examples (Scheme 1b–e).8–11 It should be noted that none of the reported compounds showed water binding ability neither at the cubane metal site nor at the dangling site. The absence of water binding sites and μ2-O bridges between the dangler and the parent cubane, both of which are vital in the native OEC water oxidation process, diminished the potential of these compounds as more accurate structural mimics. Here, we report a new “cubane-plus-dangler” compound with a Zn2+ ion appending the [Fe3Zn] motif. More importantly, substitution of Zn2+ with Ni2+ resulted in a new [Fe2Ni2]Fe complex, in which the coordination environment of the dangling ion as well as its connectivity with the parent cubane precisely resembles those of the native OEC.
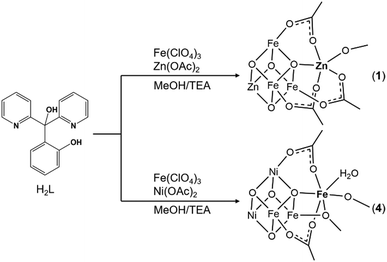
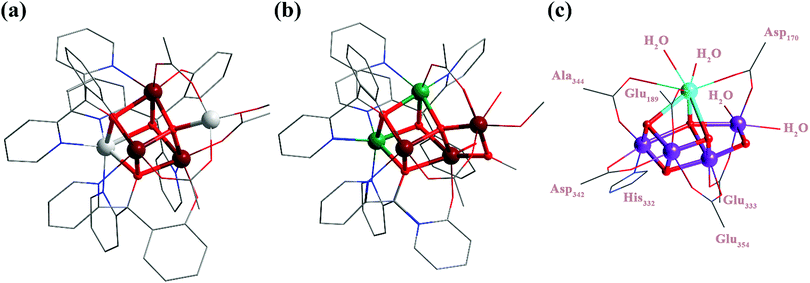
The trisubstituted methanol ligand scaffold has a strong tendency to form cubane-like metal clusters.12 We thus decided to explore their potential to stabilize the more complex “cubane-plus-dangler” structures. The reaction of Fe(ClO4)3·xH2O, Zn(OAc)2·2H2O, and 2-(hydroxy(bipyridin-2-yl)methyl)phenol (H2L) in anhydrous methanol gave a dark brown solution, from which dark brown crystals with the formula [Fe3Zn2L3O(OAc)3(CH3OH)](ClO4)2·(CH3OH)1.5 were subsequently isolated (Scheme 2). Single-crystal X-ray diffraction (XRD) studies revealed a [ZnFe3O4] cubane linked to a pentacoordinate “dangler” Zn2+via one μ4-O and three bridging acetate ligands (Fig. 1a). The three hexacoordinated Fe3+ ions on the cubane are symmetrically identical and each of them is coordinated with one pyridine, one phenoxide, and two μ3-alkoxide from L2− other than the bridging μ4-O and acetate ligand. The cubane Zn2+ is hexacoordinated with three pyridine and three μ3-alkoxide from three different L2−. Moreover, the dangling Zn2+ exhibits a trigonal bipyramidal coordination configuration with three acetates, one μ4-O, and one terminal CH3OH molecule. It should be noted that, regardless of the metal constitution and stoichiometrical deviation, the connecting mode of the dangling ion with the parent cubane through μ4-O and three acetate ligands is consistent with that of Zhang's Mn4Ca11 and Tilley's Co4Mn10 and is very similar to that of Christou's Mn3Ca2,8 in which one more bridging acetate is present. However, on the native OEC, other than μ4-O and two carboxylate groups, the dangling ion is also coordinated with the μ2-O and terminal water molecules, both of which were proposed to play vital roles during the water oxidation process.13 With the goal of producing a more accurate model compound, various metal ions other than zinc were introduced and their impact on the self-assembled products was systematically investigated.
Substitution of Zn2+ ions by Co2+ and Mn2+ resulted in the isolation of compounds 2 and 3, respectively. XRD characterization revealed that compound 3 is isostructural with compound 1. The quality of the XRD data for compound 2 was low, but did allow determination of the unit cell and symmetry, both of which are consistent with those of compounds 1 and 3. Electrospray ionization mass spectrometry (ESI-MS) (vide infra) and ICP-OES (Fig. S2 and S3, ESI†) results also suggested that compounds 1, 2 and 3 are isostructural. Introduction of Ca2+ into the system failed and only resulted in a mixed-valent homometallic manganese cubane,12b likely due to the large ionic radius of calcium, which cannot fit the cubane vertices. When Ni2+ was introduced into the reaction, deep red block crystals of compound 4 with the formula [Fe3Ni2L3O(OAc)2(OCH3)(H2O)(CH3OH)](ClO4)2 were obtained (Scheme 2). XRD studies revealed an asymmetric [Fe2Ni2] cubane core linked to a hexacoordinate “dangler” Fe3+ (Fig. 1b). The Fe/Ni metal site assignment was based on the shorter average Fe–O/Fe–N bond distance and the longer Ni–O/Ni–N one.14 The ICP-OES results confirmed the Fe/Ni stoichiometry ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The most striking structural feature of 4 is the connectivity between the dangling Fe3+ and the cubane core, which is through one μ4-O, one lateral μ2-OR and two carboxylate groups. Furthermore, the implementation of an aqua ligand at the dangling site in 4 is unprecedented, and it is an important step towards O–O bond formation via water attack and exchange processes for a truly operational OEC mimic. Consequently, this compound strongly resembles the native OEC by mimicking the “cubane-plus-dangler” skeleton, the aqua binding site on the dangling metal ion, and the connectivity between the dangling ion and the parent cubane core. We tried to investigate the catalytic reactivity of 4 for oxygen evolution reaction (OER). Unfortunately, ESI-MS studies revealed no ion peaks corresponding to the [Fe3Ni2] motif, suggesting that 4 is unstable in solution (Fig. S4, ESI†) and diminishing its potential as a homogenous catalyst.
2. The most striking structural feature of 4 is the connectivity between the dangling Fe3+ and the cubane core, which is through one μ4-O, one lateral μ2-OR and two carboxylate groups. Furthermore, the implementation of an aqua ligand at the dangling site in 4 is unprecedented, and it is an important step towards O–O bond formation via water attack and exchange processes for a truly operational OEC mimic. Consequently, this compound strongly resembles the native OEC by mimicking the “cubane-plus-dangler” skeleton, the aqua binding site on the dangling metal ion, and the connectivity between the dangling ion and the parent cubane core. We tried to investigate the catalytic reactivity of 4 for oxygen evolution reaction (OER). Unfortunately, ESI-MS studies revealed no ion peaks corresponding to the [Fe3Ni2] motif, suggesting that 4 is unstable in solution (Fig. S4, ESI†) and diminishing its potential as a homogenous catalyst.
It is well accepted that the presence of redox-inactive metal ions has a great impact on the redox-active constituents in both biological and artificial catalysis systems.15 For example, Ca2+ is essential for the water oxidation activity in the native OEC of PSII.5 Substitution of the Ca2+ by other redox-inactive metal ions increases the redox potential of the OEC and decreases the activity.16 Such a heterometallic dependence on the redox potentials has also been extensively studied in iron compounds.17 Agapie et al. demonstrated that the reduction potentials of clusters are linearly correlated with the Lewis acidity of the redox-inactive metal. Although comparisons of related compounds have only been focused on their one-electron reduction potential, this heterometallic dependence has been evidenced by the systematic electrochemical study of a series of heterometallic Fe3M and Mn3M compounds.18 Therefore, it is of great interest to determine whether the effects could be applied in our system.
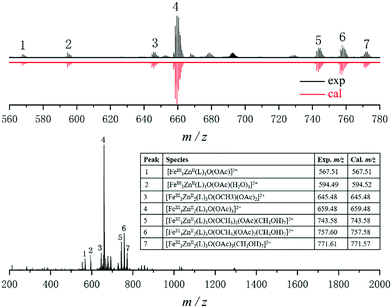
To probe the structural integrity of these compounds in the solution/gas phase, ESI-MS measurements were conducted on CH2Cl2/DMF (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions of crystals of 1, 2, and 3. For compound 1, a series of double charged ion peaks appeared, with mean peaks belonging to the same [FeIII3ZnII2(L)3O(OAcCH3)x]2+ motif (Fig. 2). Similar results were observed for compounds 2 and 3 (Fig. S2 and S3, ESI†), clearly demonstrating that the core structures of all compounds remain intact. The structural homogeneity of this series of compounds, together with their structural integrity in solution, allowed us to electrochemically investigate the effect of changing heterometal ions in the clusters on the iron reduction potentials. Cyclic voltammograms (CVs) in CH2Cl2/DMF (20
1) solutions of crystals of 1, 2, and 3. For compound 1, a series of double charged ion peaks appeared, with mean peaks belonging to the same [FeIII3ZnII2(L)3O(OAcCH3)x]2+ motif (Fig. 2). Similar results were observed for compounds 2 and 3 (Fig. S2 and S3, ESI†), clearly demonstrating that the core structures of all compounds remain intact. The structural homogeneity of this series of compounds, together with their structural integrity in solution, allowed us to electrochemically investigate the effect of changing heterometal ions in the clusters on the iron reduction potentials. Cyclic voltammograms (CVs) in CH2Cl2/DMF (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed quasireversible redox processes assigned as the [FeIII3MII2]/[FeIII2FeIIMII2] couple at −636 (MII = ZnII), −649 (MII = CoII) and −695 mV (MII = MnII) vs. the ferrocenium/ferrocene couple (Fc+/Fc) (Fig. S5, ESI†). The decrease in the reduction potential observed here correlates with the trend of increased Lewis acidity of the heterometal ions.19 Although the potential differences are not significant due to the small variations in their heterometal ion pKa values, such a Lewis acidity dependence is reproducible under identical conditions. Hence, the reduction potentials of the iron clusters can be tuned by incorporating heterometal ions with different Lewis acidity. Our results also suggested that this effect is not just limited to redox-inactive metals and can be extended to other 3d metals.
1) showed quasireversible redox processes assigned as the [FeIII3MII2]/[FeIII2FeIIMII2] couple at −636 (MII = ZnII), −649 (MII = CoII) and −695 mV (MII = MnII) vs. the ferrocenium/ferrocene couple (Fc+/Fc) (Fig. S5, ESI†). The decrease in the reduction potential observed here correlates with the trend of increased Lewis acidity of the heterometal ions.19 Although the potential differences are not significant due to the small variations in their heterometal ion pKa values, such a Lewis acidity dependence is reproducible under identical conditions. Hence, the reduction potentials of the iron clusters can be tuned by incorporating heterometal ions with different Lewis acidity. Our results also suggested that this effect is not just limited to redox-inactive metals and can be extended to other 3d metals.
In summary, a pentanuclear [Fe3Zn2] cluster was synthesized and structurally characterized, the structure of which exhibits high similarity to that of the native OEC. Substitution of Zn2+ with Co2+ and Mn2+ resulted in isostructural compounds. Electrochemical investigation revealed that the reduction potentials of the iron clusters can be tuned by using the Lewis acidity of the incorporated heterometal ions. Replacement of Zn2+ by Ni2+ produced a new model compound that perfectly mirrors the “cubane-plus-dangler” skeleton, the aqua binding site on the dangling metal ion, and the connectivity between the dangling ion and the parent cubane core of the native OEC. Our synthetic strategy that uses tri-substituted methanol as the “cubane-generator” and carboxylate as the pendant ligands provides a feasible approach for accessing model compounds of biological catalyst systems. Although these heterometallic clusters showed no catalytic activity for OER, they could be employed as single-source precursors for the synthesis of corresponding mixed-metal oxide materials for potential OER catalysts.20 This work is currently underway in our laboratory.
This work was supported by the National Natural Science Foundation of China (21471066 and 21871105). We would like to acknowledge the BL17B beamline of the National Center for Protein Sciences Shanghai (NFPS) at the Shanghai Synchrotron Radiation Facility (SSRF).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Yano and V. Yachandra, Chem. Rev., 2014, 114, 4175–4205 CrossRef CAS.
- (a) A. Zouni, H.-T. Witt, J. Kern, P. Fromme, N. Krauss, W. Saenger and P. Orth, Nature, 2001, 409, 739–743 CrossRef CAS; (b) K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Science, 2004, 303, 1831–1838 CrossRef CAS; (c) Y. Umena, K. Kawakami, J.-R. Shen and N. Kamiya, Nature, 2011, 473, 55–60 CrossRef CAS; (d) M. Suga, F. Akita, K. Hirata, G. Ueno, H. Murakami, Y. Nakajima, T. Shimizu, K. Yamashita, M. Yamamoto, H. Ago and J.-R. Shen, Nature, 2015, 517, 99–103 CrossRef CAS; (e) J. Kern, R. Chatterjee, I. D. Young, F. D. Fuller, L. Lassalle, M. Ibrahim, S. Gul, T. Fransson, A. S. Brewster, R. Alonso-Mori, R. Hussein, M. Zhang, L. Douthit, C. de Lichtenberg, M. H. Cheah, D. Shevela, J. Wersig, I. Seuffert, D. Sokaras, E. Pastor, C. Weninger, T. Kroll, R. G. Sierra, P. Aller, A. Butryn, A. M. Orville, M. Liang, A. Batyuk, J. E. Koglin, S. Carbajo, S. Boutet, N. W. Moriarty, J. M. Holton, H. Dobbek, P. D. Adams, U. Bergmann, N. K. Sauter, A. Zouni, J. Messinger, J. Yano and V. K. Yachandra, Nature, 2018, 563, 421–425 CrossRef CAS.
- (a) J. Limburg, J. S. Vrettos, L. M. Liable-Sands, A. L. Rheingold, R. H. Crabtree and G. W. Brudvig, Science, 1999, 283, 1524–1527 CrossRef CAS; (b) S. Mukhopadhyay, S. K. Mandal, S. Bhaduri and W. H. Armstrong, Chem. Rev., 2004, 104, 3981–4026 CrossRef CAS; (c) C. S. Mullins and V. L. Pecoraro, Coord. Chem. Rev., 2008, 252, 416–443 CrossRef CAS; (d) A. Sartorel, M. Bonchio, S. Campagna and F. Scandola, Chem. Soc. Rev., 2013, 42, 2262–2280 RSC; (e) M. M. Najafpour, G. Renger, M. Hołyńska, A. N. Moghaddam, E.-M. Aro, R. Carpentier, H. Nishihara, J. J. Eaton-Rye, J.-R. Shen and S. I. Allakhverdiev, Chem. Rev., 2016, 116, 2886–2936 CrossRef CAS.
- (a) F. Song, R. Moré, M. Schilling, G. Smolentsev, N. Azzaroli, T. Fox, S. Luber and G. R. Patzke, J. Am. Chem. Soc., 2017, 139, 14198–14208 CrossRef CAS; (b) F. Song, K. Al-Ameed, M. Schilling, T. Fox, S. Luber and G. R. Patzke, J. Am. Chem. Soc., 2019, 141, 8846–8857 CrossRef CAS; (c) A. I. Nguyen, D. L. M. Suess, L. E. Darago, P. H. Oyala, D. S. Levine, M. S. Ziegler, R. D. Britt and T. D. Tilley, J. Am. Chem. Soc., 2017, 139, 5579–5587 CrossRef CAS; (d) X. Jiang, J. Li, B. Yang, X.-Z. Wei, B.-W. Dong, Y. Kao, M.-Y. Huang, C.-H. Tung and L.-Z. Wu, Angew. Chem., Int. Ed., 2018, 57, 7850–7854 CrossRef CAS; (e) N. S. McCool, D. M. Robinson, J. E. Sheats and G. C. Dismukes, J. Am. Chem. Soc., 2011, 133, 11446–11449 CrossRef CAS; (f) Y.-P. Wu, J.-W. Tian, S. Liu, B. Li, J. Zhao, L.-F. Ma, D.-S. Li, Y.-Q. Lan and X. Bu, Angew. Chem., Int. Ed., 2019, 58, 12185–12189 CrossRef CAS.
- (a) C. F. Yocum, Coord. Chem. Rev., 2008, 252, 296–305 CrossRef CAS; (b) M. M. Najafpour, I. Zaharieva, Z. Zand, S. Maedeh Hosseini, M. Kouzmanova, M. Hołyńska, I. Tranca, A. W. Larkum, J.-R. Shen and S. I. Allakhverdiev, Coord. Chem. Rev., 2020, 409, 213183 CrossRef CAS.
- (a) B. Gerey, E. Gouré, J. Fortage, J. Pécaut and M.-N. Collomb, Coord. Chem. Rev., 2016, 319, 1–24 CrossRef CAS; (b) H. B. Lee, E. Y. Tsui and T. Agapie, Chem. Commun., 2017, 53, 6832–6835 RSC; (c) A. A. Alaimo, E. S. Koumousi, L. Cunha-Silva, L. J. McCormick, S. J. Teat, V. Psycharis, C. P. Raptopoulou, S. Mukherjee, C. Li, S. D. Gupta, A. Escuer, G. Christou and T. C. Stamatatos, Inorg. Chem., 2017, 56, 10760–10774 CrossRef CAS.
- H. Dobbek, V. Svetlitchnyi, L. Gremer, R. Huber and O. Meyer, Science, 2001, 293, 1281–1285 CrossRef CAS.
- S. Mukherjee, J. A. Stull, J. Yano, T. C. Stamatatos, K. Pringouri, T. A. Stich, K. A. Abboud, R. D. Britt, V. K. Yachandra and G. Christou, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2257–2262 CrossRef CAS.
- J. S. Kanady, P.-H. Lin, K. M. Carsch, R. J. Nielsen, M. K. Takase, W. A. Goddard and T. Agapie, J. Am. Chem. Soc., 2014, 136, 14373–14376 CrossRef CAS.
- A. I. Nguyen, L. E. Darago, D. Balcells and T. D. Tilley, J. Am. Chem. Soc., 2018, 140, 9030–9033 CrossRef CAS.
- C. Zhang, C. Chen, H. Dong, J.-R. Shen, H. Dau and J. Zhao, Science, 2015, 348, 690–693 CrossRef CAS.
- (a) B. F. Abrahams, T. A. Hudson and R. Robson, Chem. – Eur. J., 2006, 12, 7095–7102 CrossRef CAS; (b) Y. Zhang, C. Yang, F. Sun, G. Wu and S. Qiu, Inorg. Chem. Commun., 2019, 106, 6–10 CrossRef CAS.
- (a) N. Cox, D. A. Pantazis, F. Neese and W. Lubitz, Acc. Chem. Res., 2013, 46, 1588–1596 CrossRef CAS; (b) B. Zhang and L. Sun, Chem. Soc. Rev., 2019, 48, 2216–2264 RSC.
- I. A. Lutsenko, M. A. Kiskin, Y. V. Nelyubina, N. N. Efimov, Y. V. Maksimov, V. K. Imshennik, E. M. Zueva, A. S. Goloveshkin, A. V. Khoroshilov, E. Rentschler, A. A. Sidorov and I. L. Eremenko, Polyhedron, 2019, 159, 426–435 CrossRef CAS.
- J. S. Vrettos, D. A. Stone and G. W. Brudvig, Biochemistry, 2001, 40, 7937–7945 CrossRef CAS.
- (a) N. Ishida, M. Sugiura, F. Rappaport, T.-L. Lai, A. W. Rutherford and A. Boussac, J. Biol. Chem., 2008, 283, 13330–13340 CrossRef CAS; (b) N. Cox, L. Rapatskiy, J.-H. Su, D. A. Pantazis, M. Sugiura, L. Kulik, P. Dorlet, A. W. Rutherford, F. Neese, A. Boussac, W. Lubitz and J. Messinger, J. Am. Chem. Soc., 2011, 133, 3635–3648 CrossRef CAS.
- (a) M. G. Finnegan, R. C. Conover, J.-B. Park, Z. H. Zhou, M. W. W. Adams and M. K. Johnson, Inorg. Chem., 1995, 34, 5358–5369 CrossRef CAS; (b) S. Bang, Y.-M. Lee, S. Hong, K.-B. Cho, Y. Nishida, M. S. Seo, R. Sarangi, S. Fukuzumi and W. Nam, Nat. Chem., 2014, 6, 934–940 CrossRef CAS.
- (a) E. Y. Tsui, R. Tran, J. Yano and T. Agapie, Nat. Chem., 2013, 5, 293–299 CrossRef CAS; (b) D. E. Herbert, D. Lionetti, J. Rittle and T. Agapie, J. Am. Chem. Soc., 2013, 135, 19075–19078 CrossRef CAS.
- D. D. Perrin, Ionisation Constants Of Inorganic Acids And Bases In Aqueous Solution, Pergamon Press, New York, 1982 Search PubMed.
- (a) M. W. Louie and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 12329–12337 CrossRef CAS; (b) Z. Wei, H. Han, A. S. Filatov and E. V. Dikarev, Chem. Sci., 2014, 5, 813–818 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2033749–2033751. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc07050e |
| This journal is © The Royal Society of Chemistry 2021 |