Dual enzyme responsive mannose-6-phosphate based vesicle for controlled lysosomal delivery†
Basudeb
Mondal
 *,
Tahiti
Dutta
*,
Tahiti
Dutta
 and
Sayam
Sen Gupta
and
Sayam
Sen Gupta
 *
*
Indian Institute of Science Education and Research Kolkata, Mohanpur Campus, Nadia-741246, West Bengal, India. E-mail: sayam.sengupta@iiserkol.ac.in; bm17rs053@iiserkol.ac.in
First published on 12th November 2020
Abstract
Dual enzyme responsive stable biomimetic vesicles composed of mannose-6-phosphate lipid can encapsulate and deliver dual dye/drug and protein/enzyme exclusively to the lysosome in HEK-293 cells. The release of the cargo from the vesicles can be temporally controlled due to the enzyme responsive morphology change of the M6P lipid assembly.
Lysosomes are membrane-bound organelles that contain high levels of hydrolytic enzymes to maintain metabolic homeostasis.1 Lysosomal functions and dysfunctions are associated with different types of human diseases.1,2 Recently, in cancer biology, lysosome has been identified as a promising target for effective cancer treatment.3 Due to a weakly acidic environment (pH 4.5–5.0) relative to the cytoplasm (pH 7.2), lysosomes are different from other organelles and involved in cell death through the release of cathepsins into the cytoplasm.1c,4 The release of cathepsins, a process associated with lysosome membrane permeabilization (LMP), can be induced by various stimuli, such as the generation of reactive oxygen species (ROS).2d,5 Lysosomotropic agents such as chloroquine can also induce lysosomal rupture via LMP, leading to cell death. Therefore, in vivo and in vitro lysosomal delivery of proteins and drugs (lysosomotropic agents) can serve as a suitable strategy for cancer therapy. One strategy that has been used recently is the use of macromolecular analogs of mannose-6-phosphate (M6P) labelled glycoproteins. These macromolecules, which include polymers and polypeptides, specifically bind to the M6P receptor (M6PR), and are endocytosed and then trafficked selectively to lysosomes. Hence these macromolecules can be used to specifically deliver cargo (drugs, proteins, etc.) into the lysosome.6 Additionally, these M6P receptors are overexpressed in some cancer cells (e.g., breast cancer) and thus could be used for targeted drug delivery during cancer therapy.7 However, the synthesis of M6P-containing macromolecules requires multiple steps, which prohibits their wide-spread use.6a,6d The synthesis of simple amphiphilic constructs that can similarly target lysosomes in addition to delivering multiple cargoes upon the application of stimuli is of interest.
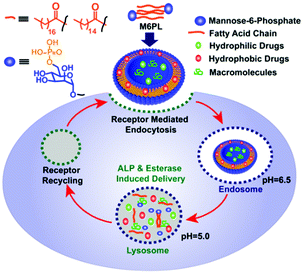
In this work, we report an M6P-containing lipid (M6PL: Fig. 1) that self-assembles into vesicles in water (Fig. S1, ESI†). These vesicles can concurrently encapsulate both hydrophobic (RBOE, anticancer drug doxorubicin) and hydrophilic dye/drug/protein (calcein or lysosomotropic agent chloroquine or enzyme galactosidase) (Fig. 2 and Fig. S1, ESI†). M6PL contains two functional groups (phosphate and ester) that can be cleaved by phosphatases and esterases, enzymes present in the lysosome. We show that the addition of these enzymes to the vesicle would release the encapsulated cargo, and the release profile can be temporally controlled. Finally, we also show that these M6PL vesicles are non-toxic to mammalian cells and are trafficked explicitly to the lysosome inside the mammalian cells (Fig. 1).
 | ||
| Fig. 1 Schematic representation of the lysosomal delivery of cargo molecules using a dual enzyme responsive M6PL vesicle. | ||
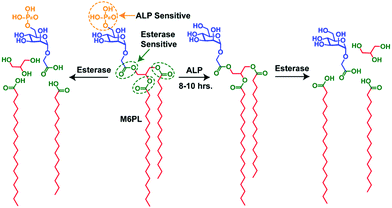 | ||
| Fig. 2 Schematic representation of the synthetic M6PL small molecule amphiphile and its enzyme (ALP and esterase) responsive properties. | ||
The synthesized small molecule (M6PL) has been characterized by 1H, 13C, and 31P NMR spectroscopy (ESI:† NMR section). Rehydration of M6PL leads to the formation of a vesicular structure to minimize the surface energy upon agitation with water (Fig. S1, ESI†). The formation of vesicular structures has been verified using dynamic light scattering (DLS: Fig. S3 and S13, ESI†), transmission electron microscopy (TEM: Fig. S2a, S4 and S12, ESI†), atomic force microscopy (AFM: Fig. S2b and S7, ESI†), scanning electron microscopy (SEM: Fig. S5, ESI†), and a dye loading study (UV-Vis: Fig. S2c, S8a and S9a, and fluorescence spectroscopy: Fig. S8b, S9 and S10, ESI†). The size distribution obtained from DLS corroborates well with TEM, SEM, and AFM imaging (Fig. S2a, S2b, S3, S13 and Fig. S4, S5, S7a, and S7c, ESI†). Dual dye encapsulation (calcein as a hydrophilic dye and RBOE as a hydrophobic dye) was performed to demonstrate the vesicular nature of the particles (Fig. S2c and S8a, ESI†). The hollow core of the vesicles was examined by encapsulating the hydrophilic dye calcein (termed as CLV). The UV-vis spectra of the dye-encapsulated vesicles (CLV) show a characteristic peak (λmax) at 495 nm (Fig. S2c and S8a, ESI†). The fluorescence emission spectra of the encapsulated calcein in vesicles (CLV) in the core-confined aqueous environment show an emission maximum (λmax) at 515 nm, with an emission intensity that is much lower than that observed in an aqueous solution of free dye (no amphiphile; Fig. S8b, ESI†). The encapsulation of the hydrophobic payload was evaluated by using the nonpolar dye RBOE, since the long octadecyl chain of the RBOE dye is suitable for its encapsulation within the hydrophobic bilayer membrane of the vesicles (termed as RLV). A clear blue shift in the UV-vis spectra of the RBOE-encapsulated vesicles (RLV) (λmax ≈ 560 nm; Fig. S2c and Fig. S9a, ESI:† blue) as compared to RBOE in water (λmax ≈ 575 nm; Fig. S9a, ESI:† black) indicates that the RBOE dye is located in a relatively nonpolar microenvironment inside the vesicles (Fig. S9a, ESI:† blue vs. red). Furthermore, the increase in the hydrodynamic diameter of RLV in DLS (Fig. S14, ESI†) and the TEM (Fig. S15, ESI†) measurements are well corroborated with the RBOE encapsulation in the vesicles. The fluorescence emission spectra of the dual dye encapsulated vesicles display appreciable FRET8 between the calcein and rhodamine (RBOE) pair which again proves the self-assembly of the M6PL amphiphile into vesicles8 and subsequent encapsulation of both dyes inside the vesicles (see Fig. S10 and S78 and the detailed discussion on FRET in the ESI†). We also successfully encapsulated anticancer drug doxorubicin (DLV) (UV-Vis and FL-spectra: Fig. S11, ESI†) and macromolecules such as the enzyme β-galactosidase (GLV) (Fig. S27, ESI†) or the protein BSA (BLV) (Fig. S23–S26, ESI†) inside the vesicles. UV-vis spectroscopy and fluorescence spectroscopy of fluorescently-labelled β-galactosidase (FL-β-gal) and BSA (FL-BSA), purified using size-exclusion chromatography, were used to determine the encapsulation efficiency (Fig. S23, ESI:† for BSA and Fig. S27b, ESI:† for β-gal). The activity of the encapsulated β-galactosidase inside the vesicles, evaluated using O-nitrophenyl-β-D-galactopyranoside (ONPG) activity assay (Fig. S27c and d, ESI†) in phosphate buffer pH 7.4, was found to be comparable to that of the free enzyme, which indicated that the encapsulated enzyme β-galactosidase retained its activity upon encapsulation (Fig. S27d, ESI†).
The M6P containing negatively charged amphiphilic glycolipid (M6PL) vesicles have both phosphate and esters groups, and thus are sensitive to the enzymes esterase and alkaline phosphatase (ALP). A complex cellular compartment such as a lysosome contains more than 60 hydrolytic enzymes (such as proteases, nucleases, glycosidases, lipases, phospholipases, phosphatases, esterases, and sulfatases).1a We hypothesized that the addition of alkaline phosphatase would hydrolyse the surface phosphate groups leading to a change in the ratio of the hydrophobicity and hydrophilicity of the M6PL amphiphile. This could further trigger a change in the morphology of the self-assembled M6PL in water. On the other hand, the addition of esterase to the vesicle would cleave the ester bond connecting the hydrophobic tail and hydrophilic head of the amphiphile leading to its complete dis-assembly. Both these processes would happen at different rates due to their relative ease of access to their respective functional group targets as well as the enzymatic activity. The different rates of hydrolysis described above would allow temporal control of the release of the loaded dyes/drugs/enzymes from the vesicle.
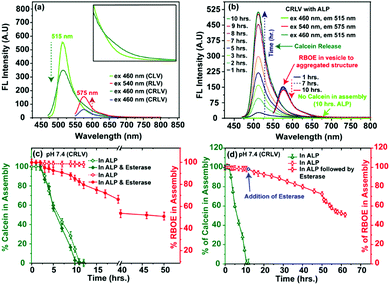
The ALP enzyme regulated dephosphorylation of M6PL was examined by treating 5 mg mL−1 (6.2 × 10−3 mmol) of M6PL molecule with ALP (2.5 × 10−6 nmol from 1 ng mL−1 stock of ALP) at physiological pH (pH = 7.4).9 Time-dependent 31P NMR spectroscopy suggests that incubation with ALP induces 50% and 90% dephosphorylation in 5 h and 10 h, respectively (Fig. S20, ESI†). Subsequently, the vesicle solution after incubation with ALP for 5 h and 10 h was investigated using TEM (Fig. S6b: for 5 h; Fig. S6c: for 10 h; and Fig. S2d, ESI†) and AFM (Fig. S2e and Fig. S7b, S7d: for 10 h, ESI†) to investigate the possible changes in the morphology. Dry state characterization of the ALP treated vesicles shows the presence of both vesicles and spherical aggregates in the TEM microscopy images (Fig. S6b, ESI†) after 5 h of incubation with ALP. In contrast, complete disappearance of vesicles and the appearance of spherical aggregates with a size of 75 nm were observed after 10 h of incubation with ALP (Fig. S2d and Fig. S6c, S19b, S21a: black, Fig. S22a, ESI†). The appearance of spherical aggregates was further confirmed by dry state microscopy imaging (TEM image: Fig. S2d, ESI;† AFM imaging: Fig. S2e, ESI†).
Transformation of vesicles to spherical aggregates in solution was investigated using absorption spectroscopy. A clear blue shift in the UV-vis spectra of the encapsulated RBOE dye from λmax ≈ 560 nm (RBOE in CRLV; Fig. S9a, ESI:† blue) to λmax ≈ 555 nm (Fig. S9a, ESI:† red) indicates that the hydrophobic RBOE dye is present in a relatively more compact non-polar microenvironment (as compared to CRLV; Fig. S2f, ESI† and Fig. S9a: blue; Fig. S8a: red, ESI†). The blue shift of the UV-vis absorption spectra for RBOE (λmax ≈ 560 nm to λmax ≈ 555 nm; Fig. S9a, ESI:† red) is a clear indication of the vesicle-to-spherical aggregate morphology transition, and this morphology transition is directly involved with incubation with the ALP enzyme (Fig. S2d, S2e, S2f, S6b, S6c, S19b, S21a and S22a, ESI†). Additionally, when the ALP incubated CRLV vesicle was dialyzed against water for 10 h (cut-off 10 kDa), the absorption spectral data indicated that the amount of encapsulated RBOE present in the vesicle was fully retained in the spherical aggregates formed (Fig. 3b and Fig. S9b, ESI:† RBOE). In contrast, no calcein absorption peak was observed. Fluorescence spectroscopy performed on these dialyzed spherical aggregates also show complete removal of the hydrophilic dye calcein (Fig. 3b and Fig. S18, ESI:† fluorescent), since upon excitation at 460 nm, no fluorescence emission was observed at both 515 nm (for calcein) and 575 nm (for RBOE) (Fig. 3b and Fig. S18, ESI:† fluorescent). In contrast, the fluorescence emission intensity at λmax ≈ 575 nm remains intact in the spherical aggregate upon excitation at 540 nm for RBOE (Fig. 3b and Fig. S17, S18 and S9b, ESI:† blue), indicating once again that RBOE was wholly retained in the spherical aggregate. The observations reported above confirm that dephosphorylation of M6PL upon addition of ALP leads to a change in the morphology from vesicles to spherical aggregates (Fig. S2d, S2e, S2f, S6c, S7b, S17b, S19b, S21a and S22a, ESI†), which is accompanied by the complete release of the hydrophilic encapsulate, while the hydrophobic molecule remains encapsulated. We believe that dephosphorylation of the phosphate group from mannose-6-phosphate leads to the formation of an amphiphile with much-reduced hydrophilicity, leading to a change in the morphology during re-assembly. Since the hydrophobic core remains intact in the re-assembled aggregate, RBOE is retained.
The esterase enzyme can act as another stimulus that can break the ester bond between the hydrophilic and hydrophobic parts of M6PL. In the absence of esterase enzyme, 7% release of dye/drug was observed from fluorescence spectroscopy after incubation for 50 h at two different pH values (PBS, pH = 7.4 and 5.0, 37 °C) (Fig. S28a and c, ESI†). In the presence of esterase, the release of more than 50% hydrophobic dye/drug (Fig. S28c: for RBOE and Fig. S28d, ESI:† for DOX) and 80% hydrophilic dye calcein (Fig. S28a, ESI†) was observed in 30 h from the CRLV vesicles at both pH values (PBS, pH = 7.4 and 5.0, 37 °C). The relatively slow degradation of ester bonds can explain the slow release of the dye/drug in the presence of esterase, since the ester groups are located deep inside the vesicles/spherical aggregates, unlike the phosphates which are present on the surface. 60% release of the anticancer drug DOX was observed (50 h) in the presence of esterase enzyme at pH 5.0 (Fig. S28d, ESI†).
The dual enzyme-responsive release can be used to an advantage where two enzymes can be applied synergistically in different sequences, such that two different types of drugs can be released sequentially depending upon the enzymatic concentration. Designing dual-enzyme-responsive systems, which show tunable drug release at the active site, is an important goal in the development of smart drug/enzyme delivery vehicles. To explore this possibility with dual-enzyme-responsive M6PL vesicles, the release behaviour was investigated by applying both the enzymes (ALP and esterase) together (Fig. 3c and Fig. S28b, S30, ESI†) as well as sequentially, such as the ALP enzyme followed by the esterase enzyme (Fig. 3d and Fig. S29b, ESI†). Initially, the dual dye/drug release from the vesicle was studied by the addition of both 10 μM ALP enzyme and esterase (combined effect) at two different pH values (PBS, pH = 7.4; Fig. 3c and Fig. S30 (ESI†): green for calcein and red for RBOE; Fig. S28b (ESI†): for calcein, and pH = 5.0, 7.4; 37 °C). In the presence of both ALP and esterase, 100% hydrophilic dye calcein and 20% hydrophobic dye RBOE were released in 8 h at pH = 7.4 (Fig. 3c and Fig. S29, ESI†), whereas 50% hydrophobic dye RBOE release was observed after 50 h of incubation at pH = 7.4 (Fig. 3c: red).
Next, we performed a sequential release study using ALP followed by esterase enzyme. In this case, we observed 100% release of calcein in 10 h after ALP (10 μM ALP enzyme) addition (Fig. 3c, 4d and Fig. S29, ESI:† green), followed by 50% release of RBOE/DOX (for RBOE; Fig. 3d: red and for DOX; Fig. S28d, ESI†) upon subsequent esterase addition in the next 30 h at two different pH values (PBS, pH = 7.4: Fig. 3d: red for RBOE and pH = 5.0; Fig. S28d, ESI,† for DOX, 37 °C). Control experiments performed in the absence of ALP and esterase treatment indicated 5% release of calcein (Fig. S28, ESI†). For β-galactosidase/BSA-protein loaded vesicles, we observed 80% release of β-galactosidase/BSA-protein in the presence of dual enzymes (ALP and esterase) at both pH values (PBS, pH = 7.4 and 5.0, 37 °C) (Fig. S32 and S33, ESI†). However, it should be noted that the synergistic and sequential release of the dual cargo molecules cannot be controlled inside the lysosome of the cell since both the enzymes are present.
Further we have investigated the stability of RLV in the human plasma over a 50 h time interval. The DLS result showed that RLV is quite stable in the human plasma over a 50 h time period (Fig. S16, ESI†). Also, the dye release experiment of CRLV indicates that CRLV can circulate in the human plasma over the 50 h time period without much loss (7% for RBOE and 12% for calcein) of the cargo molecule (Fig. S31, ESI†).
Finally, the MTT assay of the vesicles showed very marginal toxicity (more than 80% viability: Fig. S34a, ESI†) on human kidney embryonic cell lines (HEK-293). The cell division study indicates that cell growth happens linearly in the presence of vesicles for HEK-293 (Fig. S34b, ESI†).
Lysosomes are the major intracellular compartments of the cell. Deficiency of lysosomal enzymes can lead to the accumulation of large molecules within the cell leading to cell death. Diseases associated with such enzyme deficiency are known as lysosomal storage diseases (LSDs). In addition, the acidity and enzyme activity could significantly increase in the cancer cells. To visualize the potential utility of M6PL vesicles for lysosomal targeting, the RBOE loaded vesicle (RLV) was incubated with HEK-293 cells at 37 °C with 5% CO2 for 4 h and then treated with LysoTracker green to specifically stain the acidic lysosomes. By fluorescence microscopy, a large number of highly green fluorescent punctate vesicle-like lysosomes were observed inside the cells (Fig. S35, ESI†). Further the locations of RLV inside the cells were detected from the red fluorescence distribution of RLVs and LysoTracker green. Fluorescence distribution inside the cells was observed from the co-localization of red fluorescence with the marker LysoTracker green dyes (Fig. 4 and Fig. S35, ESI†), which indicates that the location of RLV is inside the acidic lysosome. In addition, the colour intensity profile of the merged images from the bright area displays identical variation of the colour intensity for both green and red detection channels, which again supports the targeting of lysosomes (Fig. S36, ESI†). These colocalization experiments demonstrate that RBOE loaded M6PL vesicles were specifically trafficked to the lysosomal compartments. Similarly, a cellular internalization experiment has been demonstrated for CLV, CRLV, and GLV (Fig. 4 and Fig. S35, ESI†). The remarkable lysosomal selectivity of M6PL vesicles suggests that they can show more efficacious delivery of drugs or enzymes towards diseased cells (cancer and LSD).
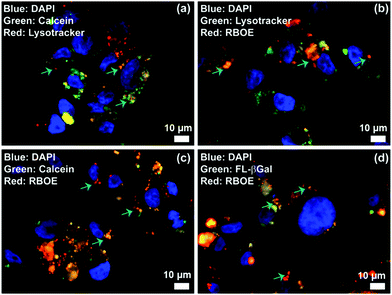 | ||
| Fig. 4 Lysosome delivery of cargo loaded M6PL vesicles: (a) calcein loaded vesicle (CLV), (b) RBOE loaded vesicle (RLV), (c) both calcein and RBOE loaded vesicle (CRLV), and (d) FL-β-galactosidase loaded vesicle (GLV). HEK-293 cells were cultured for 4 h with RLV, CLV, CRLV, and GLV (0.2 mg mL−1), respectively, in MEM containing 10% FBS, and then stained with LysoTracker Green/Red (50 nM) for 30 min. The cells were probed by epifluorescence microscopy (Fig. S35 and S36, ESI†). | ||
In summary, we have developed a simple dual enzyme responsive M6P-lipid based small molecule amphiphile (M6PL). M6PL self-assembles into stable vesicles (Fig. S12, ESI†) and can encapsulate both hydrophobic and hydrophilic dye/drug in aqueous medium. These vesicles can also encapsulate enzymes like β-galactosidase or protein like BSA. These vesicles were responsive to the enzymes alkaline phosphatase or esterase, which allowed the release of the encapsulated dual cargoes whose release profile could be controlled temporally. They show minimal toxicity to HEK-293 cells in vitro and were selectively trafficked into the lysosome. M6PL could be potentially used as a carrier for the delivery of drugs or enzymes to cells affected by diseases such as LSD.
B. M acknowledges CSIR, New Delhi for fellowship. T. D acknowledges KVPY for scholarship. We acknowledge IISER Kolkata for infrastructure. We acknowledge Prof. Guruswamy Kumaraswamy and Prof. Arindam Chowdhury from IITB for valuable discussion. Dr Sayam Sen Gupta acknowledges SERB (DST Nano-mission) grant number DST/NM/NB/2018/16 for funding.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. deDuve, Eur. J. Biochem., 1983, 137, 391–397 CrossRef CAS; (b) B. A. Schröder, C. Wrocklage, A. Hasilik and P. Saftig, Proteomics, 2010, 10, 4053–4076 CrossRef; (c) J. Luzio, P. Pryor and N. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622–632 CrossRef CAS.
- (a) N. Fehrenbacher and M. Jäättelä, Cancer Res., 2005, 65, 2993–2995 CrossRef CAS; (b) C. Fennelly and R. K. Amaravadi, Methods Mol. Biol., 2017, 1594, 293–308 CrossRef CAS; (c) F. M. Platt, A. d’Azzo and B. L. Davidson, Nat. Rev. Dis. Primers, 2018, 4, 27 CrossRef; (d) M. E. Guicciardi, M. Leist and G. J. Gores, Oncogene, 2004, 23, 2881–2890 CrossRef CAS.
- (a) Y. H. Hung, L. M. Chen, J. Y. Yang and W. Y. Yang, Nat. Commun., 2013, 4, 2111 CrossRef; (b) N. H. Petersen, O. D. Olsen, L. Groth-Pedersen, A. M. Ellegaard, M. Bilgin, S. Redmer, M. S. Ostenfeld, D. Ulanet, T. H. Dovmark, A. Lønborg, S. D. Vindeløv, D. Hanahan, C. Arenz, C. S. Ejsing, T. Kirkegaard, M. Rohde, J. Nylandsted and M. Jttel, Cancer Cell, 2013, 24, 379–393 CrossRef CAS.
- (a) J. R. Casey, S. Grinstein and J. Orlowski, Nat. Rev. Mol. Cell Biol., 2010, 11, 50–61 CrossRef CAS; (b) P. Boya and G. Kroemer, Oncogene, 2008, 27, 6434–6451 CrossRef CAS.
- (a) G. Kroemer and M. Jttel, Nat. Rev. Cancer, 2005, 5, 886–897 CrossRef CAS; (b) T. Yamashima and S. Oikawa, Prog. Neurobiol., 2009, 89, 343–358 CrossRef CAS; (c) J. Tian, L. Ding, H. J. Xu, Z. Shen, H. Ju, L. Jia, L. Bao and J. S. Yu, J. Am. Chem. Soc., 2013, 135, 18850–18858 CrossRef CAS.
- (a) S. M. Banik, K. Pedram, S. Wisnovsky, G. Ahn, N. M. Riley and C. R. Bertozzi, Nature, 2020, 584, 291–297 CrossRef CAS; (b) E. D. Vedove, G. Costabile and O. M. Merkel, Adv. Healthcare Mater., 2018, 7, 1701398 CrossRef; (c) L. M. A. Ali, M. Simon, K. El Cheikh, J. A. Kondrotas, A. Godefroy, C. Nguyen, M. Garcia, A. Morère, M. G. Bobo and L. Maillard, Bioconjugate Chem., 2019, 30, 2533–2538 CrossRef CAS; (d) S. Das, N. Parekh, B. Mondal and S. Sen Gupta, ACS Macro Lett., 2016, 5, 809–813 CrossRef CAS; (e) B. Mondal, B. Pandey, N. Parekh, S. Panda, T. Dutta, A. Padhy and S. S. Gupta, Biomater. Sci., 2020, 8, 6322–6336 RSC.
- (a) A. T. De Souza, G. R. Hankins, M. K. Washington, T. C. Orton and R. L. Jirtle, Nat. Genet., 1995, 11, 447–449 CrossRef CAS; (b) B. D. O’gorman, J. Weiss, A. Hettiaratchi, M. S. Firth and D. C. Scott, Endocrinology, 2002, 143, 4287–4294 CrossRef.
- (a) L. B. A. Johansson and A. Niemi, J. Phys. Chem., 1987, 91(11), 3020–3023 CrossRef CAS; (b) S. Das, D. K. Sharma, S. Chakrabarty, A. Chowdhury and S. S. Gupta, Langmuir, 2015, 31, 3402–3412 CrossRef CAS.
- B. Mondal, S. Das, S. Panda, T. Dutta and S. S. Gupta, ChemPlusChem, 2020, 85(5), 1053–1064 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc06169g |
| This journal is © The Royal Society of Chemistry 2021 |