Open Access Article
Open Access ArticlePoly(dimethylsiloxane) and oligo(dimethylsiloxane) solvent effects on aromatic donor–acceptor interactions†
Shogo
Amemori
 *abc,
Kyoka
Kikuchi
d and
Motohiro
Mizuno
*abc,
Kyoka
Kikuchi
d and
Motohiro
Mizuno
 *abc
*abc
aNanoMaterials Research Institute, Kanazawa University, Kanazawa 920-1192, Japan. E-mail: amemori@staff.kanazawa-u.ac.jp; mizuno@se.kanazawa-u.ac.jp
bGraduate School of Natural Science and Technology, Kanazawa University, Kanazawa 920-1192, Japan
cInstitute for Frontier Science Initiative, Kanazawa University, Kanazawa 920-1192, Japan
dSchool of Chemistry, College of Science and Engineering, Kanazawa University, Kanazawa 920-1192, Japan
First published on 7th January 2021
Abstract
Solvents with a wide range of polarities, including poly(dimethylsiloxane) and oligo(dimethylsiloxane), were used to evaluate aromatic donor–acceptor interactions between pyrene and pyromellitic diimide derivatives. The donor–acceptor interactions were stronger in siloxane solvents than in aliphatic solvents, possibly because of the poor solubility of the aromatics in siloxanes.
Aromatic donor–acceptor (D–A) interactions between electron-rich (donor) and electron-deficient (acceptor) aromatic molecules have been widely used as a driving force for the construction of supramolecular architectures1–5 such as capsules,2 liquid crystals,3 gels,4 and supramolecular polymers.5 The strength of the D–A interactions determines the properties of the supramolecular architecture, including stability, stimuli-sensitiveness, and self-repairing. Thus, it is crucial to understand and precisely control the structural and solvent dependence of D–A interactions.
Aromatic D–A interactions in the ground state and the π–π interactions between aromatic molecules are generally contributed by electrostatic, dispersion, and charge-transfer interactions, as well as solvophobic effects.6 Although the complex contributions make it difficult to strictly interpret the solvent effects on D–A interactions, the effects can be roughly divided by polar and non-polar solvent systems. In a polar solvent system such as water or methanol, the D–A interaction is mainly dominated by the hydrophobic effect, and thus more polar solvents give rise to higher association constants.7 Alternatively, in a non-polar solvent system, the lower polarity enables stronger D–A interactions.8 For example, the association constants between 1,3,5-trinitrobenzene and naphthalene in non-polar solvents are in the order n-heptane (9.58 M−1), CCl4 (5.16 M−1), CS2 (3.25 M−1), and CHCl3 (1.82 M−1).8a
Generally, the effects of low polarity solvents are interpreted as a result of their low dielectric constant facilitating electrostatic interaction between aromatic molecules.1a,8c In addition, the weak interactions6d between solvent molecules and solute molecules or the solvophobic effect8d in non-polar solvents contribute to an increase in the association constant between the solutes. However, the lowest polarity solvents to be evaluated for the solvent effect have been limited to aliphatic solvents such as n-hexane, n-heptane, and cyclohexane. It remains unclear whether the solvent effect is applicable to low-polarity solvents other than aliphatic solvents, such as poly(dimethylsiloxane) (PDMS) and oligo(dimethylsiloxane) (ODMS), nor is it clear what governs the D–A interactions in non-polar environments. Nevertheless, supramolecular architectures in non-polar solvents have been frequently constructed on the basis of D–A interactions.
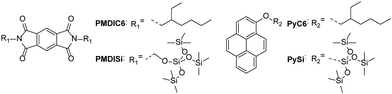
Here, we report the association constants between pyromellitic diimide (PMDI) derivatives (PMDIC6 and PMDISi) as acceptors and pyrene (Py) derivatives (PyC6 and PySi) as donors (Fig. 1) and determine the significant effect of the incompatibility between the solute and solvents on the stabilization of the D–A complexes by using PDMS and ODMS as solvents. PDMS and ODMS both have a flexible backbone of Si–O bonds, which impart ionic properties, covered entirely with non-polar methyl groups. Their particular structure results in a weak intermolecular force (dispersion force) among the PDMS and ODMS molecules, leading to their low surface energy, poor solvation ability, and low solubility parameters.9–12 The outstanding “non-polar” nature of PDMS and ODMS leads to their usefulness in applications such as crystallization solvents10 and building units that facilitate phase segregation for liquid crystals11 and block co-polymers.12 Determining the solvent effect of siloxanes will elucidate the complex nature of the aromatic D–A interactions in non-polar environments as well as aid in the rational design of supramolecular architectures using PDMS and ODMS.
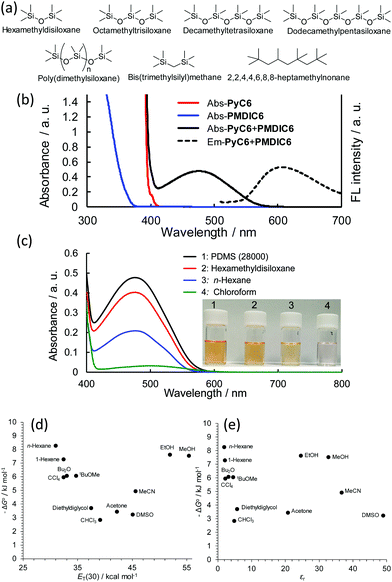
First, we prepared PMDIC6 and PyC6, which are PMDI and Py attached to alkyl chains as solubilizing groups, by means of one-step processes (see the ESI†). This resulted in adequate solubility of PMDIC6 and PyC6 in solvents having a wide range of polarities, such as PDMS and methanol. We used two PDMSs (PDMS(2000) with an average molecular weight of 2000 g mol−1 and PDMS(28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) with an average molecular weight of 28
000) with an average molecular weight of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and four ODMSs (Fig. 2a) to investigate the solvent effect. Although PMDIC6 and PyC6 exhibit considerably low absorption in the visible region, mixing the compounds induced a new absorption band originating from a charge-transfer absorption with a charge-transfer emission (Fig. 2b). The absorbance of the charge-transfer absorption strongly depended on the solvents (Fig. 2c). The time-dependence of the charge-transfer absorption of PyC6–PMDIC6 was measured in n-hexane and PDMS(28
000 g mol−1) and four ODMSs (Fig. 2a) to investigate the solvent effect. Although PMDIC6 and PyC6 exhibit considerably low absorption in the visible region, mixing the compounds induced a new absorption band originating from a charge-transfer absorption with a charge-transfer emission (Fig. 2b). The absorbance of the charge-transfer absorption strongly depended on the solvents (Fig. 2c). The time-dependence of the charge-transfer absorption of PyC6–PMDIC6 was measured in n-hexane and PDMS(28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) with varying temperatures. Both systems showed remarkable stability and a quick response (<5 min) of the charge-transfer absorption to the change in the temperature, indicating a rapid shift of equilibrium even in PDMS(28
000) with varying temperatures. Both systems showed remarkable stability and a quick response (<5 min) of the charge-transfer absorption to the change in the temperature, indicating a rapid shift of equilibrium even in PDMS(28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), which has a high viscosity (Fig. S1, ESI†). The association constants between PMDIC6 and PyC6 were evaluated from the charge-transfer absorption in the visible region by using nonlinear curve fitting with a 1
000), which has a high viscosity (Fig. S1, ESI†). The association constants between PMDIC6 and PyC6 were evaluated from the charge-transfer absorption in the visible region by using nonlinear curve fitting with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (eqn S1 and Fig. S4–S36, ESI†). The observed data in all solvents fit the 1
1 binding model (eqn S1 and Fig. S4–S36, ESI†). The observed data in all solvents fit the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model well within the concentration range that we used, supporting the premise that dimerization between the donors and acceptors predominated, although there might be minor polymeric species such as 2
1 model well within the concentration range that we used, supporting the premise that dimerization between the donors and acceptors predominated, although there might be minor polymeric species such as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes.
2 complexes.
The evaluated association constant Ka and the standard Gibbs free energy of formation ΔG° between PMDIC6 and PyC6 in the solvents are listed in Table 1. In order to recognize the association behavior of the PMDIC6–PyC6 pair, the values of −ΔG° in the typically used solvents were plotted as a function of the relative permittivity εr13 and ET(30), which are representative empirical solvent polarity scales derived from the solvatochromism of pyridinium N-phenolate betaine dyes (Fig. 2d and e).14 The solvent effects were divided into non-polar and polar regions by using chloroform and acetone as the boundary, which is consistent with the reported propensity as discussed above.
| Solvent | K a (M−1)ab | −ΔG° (kJ mol−1) | ε r | E T(30) (kcal mol−1) |
|---|---|---|---|---|
a Association constants in all solvents were calculated by using non-linear least squares curve fitting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model.
b Standard error for non-linear least squares in parentheses.
c Data from ref. 13a.
d Data from ref. 13b.
e Data from ref. 13c.
f Data from ref. 13d.
g Data from ref. 13e.
h Data from ref. 14. 1 binding model.
b Standard error for non-linear least squares in parentheses.
c Data from ref. 13a.
d Data from ref. 13b.
e Data from ref. 13c.
f Data from ref. 13d.
g Data from ref. 13e.
h Data from ref. 14.
|
||||
| Methanol | 21 (0.2) | 7.5 | 33c | 55.4h |
| Ethanol | 22 (0.3) | 7.6 | 25c | 51.9h |
| Acetonitrile | 7.3 (0.1) | 4.9 | 37c | 45.6h |
| DMSO | 3.7 (0.2) | 3.2 | 47c | 45.1h |
| Acetone | 4.1 (0.2) | 3.5 | 21c | 42.2h |
| Chloroform | 3.2 (0.04) | 2.9 | 4.8c | 39.1h |
| Diethyldiglycol | 4.5 (0.06) | 3.7 | 5.7d | 37.5h |
| tert-Butylmethyl ether | 12 (0.2) | 6.1 | 4.5e | 34.7h |
| Di-n-butyl ether | 12 (0.1) | 6.1 | 3.1c | 33.0h |
| Tetrachloromethane | 11 (0.3) | 6.0 | 2.2c | 32.4h |
| 1-Hexene | 19 (0.4) | 7.3 | 2.1c | 32.4h |
| n-Hexane | 28 (0.4) | 8.3 | 1.9c | 31.0h |
| Hexamethyldisiloxane | 88 (1.1) | 11.1 | 2.2f | — |
| Octamethyltrisiloxane | 95 (1.3) | 11.3 | 2.3f | — |
| Decamethyltetrasiloxane | 110 (1.8) | 11.6 | 2.4f | — |
| Dodecamethylpentasiloxane | 110 (2.0) | 11.6 | 2.5f | — |
| Poly(dimethylsiloxane) (Mn 2000 g mol−1) | 130 (1.4) | 12.1 | 2.7f | — |
Poly(dimethylsiloxane) (Mn 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) 000 g mol−1) |
140 (3.5) | 12.2 | 2.8f | — |
| 2,2,4,4,6,8,8-Heptamethylnonane | 30 (0.3) | 8.4 | — | — |
| Bis(trimethylsilyl)methane | 45 (0.2) | 9.4 | 2.1g | — |
In the polar solvents, −ΔG° increased roughly with an increase in ET(30) (Fig. 2d), although there was no correlation of −ΔG° with the relative permittivity (Fig. 2e). These behaviors were interpreted as the hydrophobic effect playing a key role in the D–A aromatic interaction rather the electrostatic interaction.6c,7 If the electrostatic force dominates, in the solvents with a high dielectric constant, the binding energy should decrease as the electrostatic force decreases. In the non-polar solvent region, the archetypal trend observed was an increase in the association constants with both decreasing ET(30) and decreasing εr of the solvents (Fig. 2d and e).6d,8 The typical D–A interaction properties of the PMDIC6–PyC6 pair encouraged us to further investigate the solvent effects of PDMS and ODMS using this pair.
Although empirical ET(30) solvent scales for PDMSs and ODMSs have not been reported, their solvent polarity is similar to that derived from other empirical solvent polarity parameters such as the Kamlet–Taft polarity parameters for alkanes.10b,15 Bis(trimethylsilyl)methane and the branched alkane heptamethylnonane were used to evaluate the structural effects of the solvents on the D–A interaction (Fig. 2a). The association constants and −ΔG° of the siloxanes gradually increased as their molecular weight increased, although there appeared to be a plateau. The association constant for PDMS(28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was 5 times higher than that of n-hexane and 40 times higher than that of chloroform, which had the lowest association constant in this experiment. This is the first report on the solvent effects of PDMS and ODMS promoting the formation of aromatic D–A complexes. Because the association constant of 2,2,4,4,6,8,8-heptamethylnonane with branched methyl moieties was comparable to that of n-hexane, the higher association constants of the ODMSs were not attributable to the differences in the molecular size and the methyl moieties between n-hexane and the ODMSs.
000) was 5 times higher than that of n-hexane and 40 times higher than that of chloroform, which had the lowest association constant in this experiment. This is the first report on the solvent effects of PDMS and ODMS promoting the formation of aromatic D–A complexes. Because the association constant of 2,2,4,4,6,8,8-heptamethylnonane with branched methyl moieties was comparable to that of n-hexane, the higher association constants of the ODMSs were not attributable to the differences in the molecular size and the methyl moieties between n-hexane and the ODMSs.
Considering that the relative permittivity and empirical solvent polarity parameters of the siloxanes and n-hexane are much the same, electrostatic forces are not sufficient to explain the high association constants of the siloxane solvents. To obtain further information, we evaluated the solubility of PyC6 and PMDIC6 in n-hexane, PDMSs, and ODMSs (Table S1, ESI†). The higher molecular weight siloxanes showed lower solubility of PyC6 and PMDIC6, and all the siloxanes were poor solvents for the dyes compared with n-hexane. The intermolecular forces of PDMS and ODMS, which are mainly governed by the dispersion force, are weaker than those of alkanes.9b Although consideration of the interaction between the aromatic molecules and the solvent molecules from the solubility test is difficult because of their different molecular sizes and entropy of mixing, the low solubility of PyC6 and PMDIC6 in the siloxanes suggests that PDMSs and ODMSs could not inhibit the self-aggregation of the aromatics that was driven by the dispersion force, indicating an incompatibility between the aromatic molecules and the siloxane solvents. In fact, block co-polymers of poly(dimethylsiloxane) and polystyrene, which have an aromatic moiety, are well-known to phase separate into nanoscale morphologies owing to their incompatibility.12a,12d As the solubility trend was consistent with that of the association constants between PyC6 and PMDIC6, the incompatibility of the siloxanes to the aromatics could accelerate the D–A interactions as well as the self-aggregation.
Bis(trimethylsilyl)methane has a higher surface tension (19.1 mN m−1) than hexamethyldisiloxane (16.2 mN m−1), implying that an increase in the intermolecular force is observed upon replacing the Si–O–Si bond with a Si–C–Si bond.16 As expected, a lower association constant between PyC6 and PMDIC6 was observed in bis(trimethylsilyl)methane (45 M−1) compared to that in hexamethyldisiloxane (88 M−1). In addition, bis(trimethylsilyl)methane was a good solvent for PyC6 and PMDIC6 compared with hexamethyldisiloxane (Table S1, ESI†). This result supports the hypothesis that the incompatibility between the aromatic and siloxane molecules induces higher association constants. It can be noted that the surface tension is not directly correlated with the association constant owing to the molecular weight dependence of the surface tension.9b
To evaluate the effect of the side chains, PMDISi and PySi with a tris(trimethylsilyloxy)silyl moiety were synthesized (Fig. 1). The charge-transfer absorption of the PMDISi–PySi pair showed significant stability and depended on the solvents similar to the PMDIC6–PyC6 pair (Fig. S2 and S3, ESI†). The solubility test clearly showed higher solubility of PMDISi and PySi compared with PMDIC6 and PyC6, indicating the high compatibility of the tris(trimethylsilyloxy)silyl moiety with PDMSs and ODMSs (Table S1, ESI†). Nevertheless, the association constants between PMDISi and PySi in siloxanes were three to five times higher than those in n-hexane (Table 2). This difference is consistent with the solvent effect of the media for the PMDIC6–PyC6 pairs. Remarkably, the solvent effects for the association constants of the PMDIC6–PySi and PMDISi–PyC6 pairs were almost identical to those of the PMDIC6–PyC6 and PMDISi–PySi pairs, although the association constants in the same solvents depended on the combination of the donor and the acceptor (Table S2, ESI†). The dependence of the association constant on the D–A combination may be attributed to the geometric preference for stacking between the aromatic cores as a result of the steric hindrance of the side chains, or to the difference in the electrostatic properties of the aromatic cores by the side chains. It is significant that the side chains affected the association constants but not the solvent effect of the siloxanes. These findings show the generality of the solvent effect and the crucial role of the incompatibility between the solvent and the aromatic core rather than the side chains for the solvent effect.
| Solvent | K a (M−1)ab | −ΔG° (kJ mol−1) |
|---|---|---|
a Association constants were calculated by using non-linear least squares curve fitting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model.
b Standard error for non-linear least squares in parentheses. 1 binding model.
b Standard error for non-linear least squares in parentheses.
|
||
| n-Hexane | 23 (0.3) | 7.8 |
| Hexamethyldisiloxane | 72 (0.9) | 10.6 |
| Octamethyltrisiloxane | 81 (1.8) | 10.9 |
| Decamethyltetrasiloxane | 91 (0.8) | 11.2 |
| Dodecamethylpentasiloxane | 96 (0.9) | 11.3 |
| Poly(dimethylsiloxane) (Mn 2000 g mol−1) | 130 (1.4) | 12.1 |
Poly(dimethylsiloxane) (Mn 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) 000 g mol−1) |
140 (3.9) | 12.3 |
In conclusion, we demonstrated the solvent effects of PDMS and ODMS on aromatic D–A interactions by evaluating the association constants between PMDI and Py derivatives in various solvents. The siloxanes induced higher association constants in non-polar environments. The solvent effect may originate from the incompatibility between the aromatic cores and the siloxane molecules owing to the weak dispersion force. Recently, there has been a debate on the exact mechanism of the interaction between aromatic molecules.6f These findings may help elucidate the origin of aromatic D–A interactions and π–π interactions. The cause of the solvent effect of the siloxanes on the D–A interaction requires further investigation using various donor and acceptor molecules.
This work was supported by JSPS KAKENHI Grants 18K14187 (Early-Career Scientists).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2038–2054 CrossRef CAS; (b) M. Kumar, K. V. Rao and S. J. George, Phys. Chem. Chem. Phys., 2014, 16, 1300–1313 RSC; (c) H. Kar and S. Ghosh, Isr. J. Chem., 2019, 59, 881–891 CrossRef CAS.
- (a) C. Wang, S. Yin, S. Chen, H. Xu, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2008, 47, 9049–9052 CrossRef CAS; (b) C. Wang, Y. Guo, Y. Wang, H. Xu and X. Zhang, Chem. Commun., 2009, 5380–5382 RSC; (c) S. Chakraborty, D. Ray, V. K. Aswal and S. Ghosh, Chem. – Eur. J., 2018, 24, 16379–16387 CrossRef CAS.
- (a) V. Percec, M. Glodde, T. K. Bera, Y. Miura, I. Shiyanovskaya, K. D. Singer, V. S. K. Balagurusamy, P. A. Heiney, I. Schnell, A. Rapp, H. W. Spiess, S. D. Hudsonk and H. Duan, Nature, 2002, 417, 384–387 CrossRef; (b) K. R. Leight, B. E. Esarey, A. E. Murray and J. J. Reczek, Chem. Mater., 2012, 24, 3318–3328 CrossRef CAS; (c) M. V. Winkle, D. A. Scrymgeour, B. Kaehr and J. J. Reczek, Adv. Mater., 2018, 30, 1706787 CrossRef.
- (a) T. Yuan, M. Vazquez, A. N. Goldner, Y. Xu, R. Contrucci, M. A. Firestone, M. A. Olson and L. Fang, Adv. Funct. Mater., 2016, 26, 8604–8612 CrossRef CAS; (b) C. Li, C. Shen, J. Nie and H. Qiu, Chem. – Asian J., 2018, 13, 1678–1682 CrossRef CAS; (c) J. Han, D. Yang, X. Jin, Y. Jiang, M. Liu and P. Duan, Angew. Chem., Int. Ed., 2019, 58, 7013–7019 CrossRef CAS.
- (a) S. Burattini, B. W. Greenland, D. H. Merino, W. Weng, J. Seppala, H. M. Colquhoun, W. Hayes, M. E. MacKay, I. W. Hamley and S. J. Rowan, J. Am. Chem. Soc., 2010, 132, 12051–12058 CrossRef CAS; (b) T. Haino, A. Watanabe, T. Hirao and T. Ikeda, Angew. Chem., Int. Ed., 2012, 51, 1473–1476 CrossRef CAS; (c) Y. Han, Y. Tian, Z. Li and F. Wang, Chem. Soc. Rev., 2018, 47, 5165–5176 RSC; (d) K. Jalani, A. D. Das, R. Sasmal, S. S. Agasti and S. J. George, Nat. Commun., 2020, 11, 1–9 Search PubMed.
- (a) C. A. Hunter and J. K. M. Sanders, J. Am. Chem. Soc., 1990, 112, 5525–5534 CrossRef CAS; (b) Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564–584 RSC; (c) C. R. Martinez and B. L. Iverson, Chem. Sci., 2012, 3, 2191–2201 RSC; (d) Z. Chen, B. Fimmel and F. Würthner, Org. Biomol. Chem., 2012, 10, 5845–5855 RSC; (e) R. Thakuria, N. K. Nath and B. K. Saha, Cryst. Growth Des., 2019, 19, 523–528 CrossRef CAS; (f) K. Carter-Fenk and J. M. Herbert, Chem. Sci., 2020, 6758–6765 RSC.
- M. S. Cubberley and B. L. Iverson, J. Am. Chem. Soc., 2001, 123, 7560–7563 CrossRef CAS.
- (a) C. C. Thompson and P. A. D. de Maine, J. Am. Chem. Soc., 1963, 85, 3096–3101 CrossRef CAS; (b) C. C. Thompson and P. A. D. De Maine, J. Phys. Chem., 1965, 69, 2766–2771 CrossRef CAS; (c) R. Foster, Organic Charge-Transfer Complexes, Academic Press Inc., London, 1969 Search PubMed; (d) G. M. Prentice, S. I. Pascu, S. V. Filip, K. R. West and G. D. Pantoş, Chem. Commun., 2015, 51, 8265–8268 RSC; (e) V. C. Wakchaure, L. V. Pillai, G. Goudappagouda, K. C. Ranjeesh, S. Chakrabarty, S. Ravindranathan, P. R. Rajamohanan and S. S. Babu, Chem. Commun., 2019, 55, 9371–9374 RSC.
- (a) E. L. Warrick, M. J. Hunter and A. J. Barry, Ind. Eng. Chem., 1952, 44, 2196–2202 CrossRef CAS; (b) M. J. Owen, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19, 97–103 CrossRef CAS; (c) C. Rücker and K. Kümmerer, Chem. Rev., 2015, 115, 466–524 CrossRef.
- (a) W. H. Monillas, J. F. Young, G. P. A. Yap and K. H. Theopold, Dalton Trans., 2013, 42, 9198–9210 RSC; (b) M. A. Ab Rani, N. Borduas, V. Colquhoun, R. Hanley, H. Johnson, S. Larger, P. D. Lickiss, V. Llopis-Mestre, S. Luu, M. Mogstad, P. Oczipka, J. R. Sherwood, T. Welton and J. Y. Xing, Green Chem., 2014, 16, 1282–1296 RSC; (c) K. E. Aldrich and A. L. Odom, Chem. Commun., 2019, 55, 4403–4406 RSC.
- (a) C. A. Vieth, E. T. Samulski and N. Sanjeeva Murthy, Liq. Cryst., 1995, 19, 557–563 CrossRef CAS; (b) R. H. Zha, B. F. M. De Waal, M. Lutz, A. J. P. Teunissen and E. W. Meijer, J. Am. Chem. Soc., 2016, 138, 5693–5698 CrossRef CAS; (c) A. Kawafuchi, S. Kutsumizu, Y. Kawase, I. Tokiwa, T. Udagawa and Y. Miwa, Phys. Chem. Chem. Phys., 2020, 22, 10132–10141 RSC.
- (a) Y. S. Jung and C. A. Ross, Nano Lett., 2007, 7, 2046–2050 CrossRef CAS; (b) M. D. Rodwogin, C. S. Spanjers, C. Leighton and M. A. Hillmyer, ACS Nano, 2010, 4, 725–732 CrossRef CAS; (c) B. Van Genabeek, B. F. M. De Waal, M. M. J. Gosens, L. M. Pitet, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2016, 138, 4210–4218 CrossRef CAS; (d) J. Garnier, J. Arias-Zapata, O. Marconot, S. Arnaud, S. Böhme, C. Girardot, D. Buttard and M. Zelsmann, ACS Appl. Mater. Interfaces, 2016, 8, 9954–9960 CrossRef CAS; (e) J. A. Berrocal, R. H. Zha, B. F. M. D. Waal, J. A. M. Lugger, M. Lutz and E. W. Meijer, ACS Nano, 2017, 11, 3733–3741 CrossRef CAS.
- (a) D. R. Lide, CRC Handbook of Chemistry And Physics: A Ready-Reference Book of Chemical And Physical Data, CRC Press, Boca Raton, FL, 90th edn, 2014 Search PubMed; (b) F. Calderazzo and F. A. Cotton, Inorg. Chem., 1962, 1, 30–36 CrossRef CAS; (c) I. M. Smallwood, Handbook of Organic Solvent Properties, Elsevier Ltd, 1996 Search PubMed; (d) M. S. Beevers, S. J. Mumby, S. J. Clarson and J. A. Semlyen, Polymer, 1983, 24, 1565–1570 CrossRef CAS; (e) R. A. Holroyd, K. Itoh and M. Nishikawa, Nucl. Instrum. Methods Phys. Res., Sect. A, 1997, 390, 233–236 CrossRef CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- J. E. Brady, D. Bjorkman, C. D. Herter and P. W. Carr, Anal. Chem., 1984, 56, 278–283 CrossRef CAS.
- (a) R. Wagner, L. Richter, Y. Wu, J. Weißmüller, J. Reiners, E. Hengge, A. Kleewein and K. Hassler, Appl. Organomet. Chem., 1997, 11, 645–657 CrossRef CAS; (b) J. Tan, Y. Liu and Z. Ye, J. Mol. Liq., 2019, 279, 657–661 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H 13C NMR HRMS data, curve fitting data for association constant, solubility test. See DOI: 10.1039/d0cc06638a |
| This journal is © The Royal Society of Chemistry 2021 |