Light-mediated chiroptical switching of an achiral foldamer host in presence of a carbohydrate guest†
Susnata
Pramanik
a,
Brice
Kauffmann
 b,
Stefan
Hecht
b,
Stefan
Hecht
 cde,
Yann
Ferrand
cde,
Yann
Ferrand
 f and
Ivan
Huc
f and
Ivan
Huc
 *a
*a
aDepartment Pharmazie and Center for Integrated Protein Science, Ludwig-Maximilians-Universität, Butenandtstr. 5-13, 81377 München, Germany. E-mail: ivan.huc@cup.lmu.de
bUniversité de Bordeaux CNRS, INSERM, UMS3033, Institut Européen de Chimie et Biologie (IECB), 2 rue Robert Escarpit, 33600 Pessac, France
cDepartment of Chemistry & IRIS Adlershof, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, 12489 Berlin, Germany
dDWI-Leibniz Institute for Interactive Materials, Forckenbeckstr. 50, 52074 Aachen, Germany
eInstitute of Technical and Macromolecular Chemistry, RWTH Aachen University, Worringer Weg 2, 52074 Aachen, Germany
fCBMN (UMR5248), Univ. Bordeaux—CNRS—IPB, Institut Européen de Chimie et Biologie, 2 rue Robert Escarpit, 33600 Pessac, France
First published on 8th December 2020
Abstract
A photoresponsive diarylethene was incorporated in an achiral helical foldamer container. A carbohydrate guest was found to induce opposite handedness upon binding to the open and closed forms of the diarylethene-containing foldamer, thus enabling chiroptical switching of an achiral host mediated by a chiral guest.
Chiroptical switches are molecules that can change their interaction with polarized light in response to an external reversible stimulus such as the interaction with a guest or a metal ion, a change in redox state, pH, solvent, temperature or pressure; a transition between solid and solution state; or the action of light itself.1 Helical chirality has often been exploited in chiroptical switches because molecular helicity tends to be strongly reflected in chiroptical properties. Thus, a helix may amplify the effect of a chiral stimulus that occurs at a single stereogenic centre acting as a strong helix handedness inducer.2 Conversely, when each monomer of a helical polymer bears a stereogenic center, helix handedness may be strongly biased by a cumulative effect even though the contribution of each chiral monomer is minute, and therefore responsive to minor changes of the environment.3 In light-controlled chiroptical switches, the light stimulus generally operates on the chiral entity itself, directly causing the appearance, disappearance, or inversion of a helix handedness bias.4 In contrast, we introduce here a chiroptical switch where a light trigger acts on an achiral helical receptor. The light-induced transition results in a relatively modest local conformational change. This subtle change, however, reverts the way the achiral foldamer receptor interacts with the same chiral guest, eventually leading to a considerable conformational and chiroptical response.
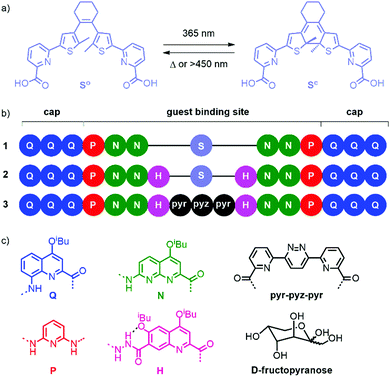
We5 and others6 have developed aromatic helical foldamers as potent receptors for saccharides in organic solvents. In the case of aromatic foldamer capsules,5,7i.e. helical containers that completely surround their substrate and seclude it from the solvent, high substrate selectivity has often been observed. Here, selectivity encompasses guest selectivity (among different saccharides), diastereoselectivity (with respect to helix handedness), and anomer and tautomer selectivity (among the different forms of a given saccharide). For example, the P helical conformer of achiral sequence 3 (Fig. 1b) has high affinity for β-D-fructo-pyranose.5a Thus, when D-fructose is added to 3, P helicity is induced almost quantitatively, resulting in an intense CD signal.
We devised that this high selectivity offers opportunities for switching and endeavoured to introduce a photo-responsive unit in these capsule sequences. Our initial idea was not to implement a large photo-induced conformational change that could mediate guest capture and release. Instead, we initially thought that triggering a small structural change with light would alter selectivity in such a way that it may allow for the replacement of a saccharide guest by another. Diarylethenes are good examples of photoswitches where the photo-induced conformational change is both modest and well-defined.8 We therefore designed and synthesized unit “S” (Fig. 1a), a diarylethene functionalized with pyridinecarboxylic acid units for its incorporation in an oligoamide foldamer sequence (see ESI† for details on the synthesis).
Q3PN2 and Q3PN2H were chosen as common segments of foldamer sequences that selectively recognize organic acids, e.g.D-/L-tartaric acid,9 or saccharides,5a respectively. The UV-vis absorption spectra of these segments overlap with that of the dimethyl ester of S (Fig. S1, ESI†). At 365 nm, MeO-S-OMe (ε365 = 2700 M−1 cm−1) absorbs 6 to 8 times less than Q3PN2-Boc (ε365 = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1) and Q3PN2H-Boc (ε365 = 20
800 M−1 cm−1) and Q3PN2H-Boc (ε365 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1). Thus, a significant amounts of photons will be lost upon irradiating S within an aromatic foldamer sequence at this wavelength. Nevertheless, 1H NMR spectra of Q3PN2-Boc and Q3PN2 recorded after irradiation at 365 nm in CDCl3 (Fig. S2, ESI†) show no photodegradation, thus indicating their compatibility with the excitation of an S unit.
800 M−1 cm−1). Thus, a significant amounts of photons will be lost upon irradiating S within an aromatic foldamer sequence at this wavelength. Nevertheless, 1H NMR spectra of Q3PN2-Boc and Q3PN2 recorded after irradiation at 365 nm in CDCl3 (Fig. S2, ESI†) show no photodegradation, thus indicating their compatibility with the excitation of an S unit.
Sequence 1 was designed as a prototype. Energy minimized molecular models (MacroModel, MMFFs) of the (So) and (Sc) forms of 1 suggested that the overall capsule conformation would not change much upon ring closure of the diarylethene (Fig. 2a and b). In both forms, the methyl substituents of the thiophene rings protrude in the helix cavity, albeit at different orientations. Of note is the formation of two diasterogenic centers upon ring closure, whose stereochemistry may or may not relate to the handedness of the flanking helix segments.
Light-induced switching between 1o and 1c was probed by UV-vis spectroscopy. A 10 μM solution of 1o in 5/95 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 vol/vol was irradiated at 365 nm until no change in the absorption spectrum was observed. A new band characteristic of Sc emerged at 592 nm, which resulted in an intense colour change from light yellow to deep purple. The reverse toggling was achieved using light at λ > 450 nm. Nevertheless, successive switching cycles accomplished by repeating light irradiation led to some fatigue, i.e. a decrease of band intensity apparently due to intramolecular side reactions (Fig. S7, ESI†).
CHCl3 vol/vol was irradiated at 365 nm until no change in the absorption spectrum was observed. A new band characteristic of Sc emerged at 592 nm, which resulted in an intense colour change from light yellow to deep purple. The reverse toggling was achieved using light at λ > 450 nm. Nevertheless, successive switching cycles accomplished by repeating light irradiation led to some fatigue, i.e. a decrease of band intensity apparently due to intramolecular side reactions (Fig. S7, ESI†).
Crystal structures of 1 and 2 provided important structural information. Upon attempting to crystallize 1o, co-crystals in which 1c and 1o overlapped in a 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 ratio were eventually obtained.‡ The two structures are shown separately in Fig. 2c–g. Their overlapping in the crystal lattice is consistent with their similar shapes and with crystal-to-crystal transformations of other diarylethenes.§
70 ratio were eventually obtained.‡ The two structures are shown separately in Fig. 2c–g. Their overlapping in the crystal lattice is consistent with their similar shapes and with crystal-to-crystal transformations of other diarylethenes.§![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 However, the structures show not a capsule conformation but a central N2SN2 helix and two Q3P segments flipped outwards. Such flipping has been recently observed in related compounds11 and may reflect an imperfect fit of the S unit within the capsule shape. Furthermore, a crystal structure of 1o was obtained incidentally containing a Mg2+ ion (Fig. 2h and Fig. S24, ESI†).‡ Though the ion certainly biases the conformation, the fact that the S unit in this structure is found in the parallel conformation, unable to undergo ring closure (Fig. 2h), further suggests larger conformational freedom of 1 than we had anticipated.
10 However, the structures show not a capsule conformation but a central N2SN2 helix and two Q3P segments flipped outwards. Such flipping has been recently observed in related compounds11 and may reflect an imperfect fit of the S unit within the capsule shape. Furthermore, a crystal structure of 1o was obtained incidentally containing a Mg2+ ion (Fig. 2h and Fig. S24, ESI†).‡ Though the ion certainly biases the conformation, the fact that the S unit in this structure is found in the parallel conformation, unable to undergo ring closure (Fig. 2h), further suggests larger conformational freedom of 1 than we had anticipated.
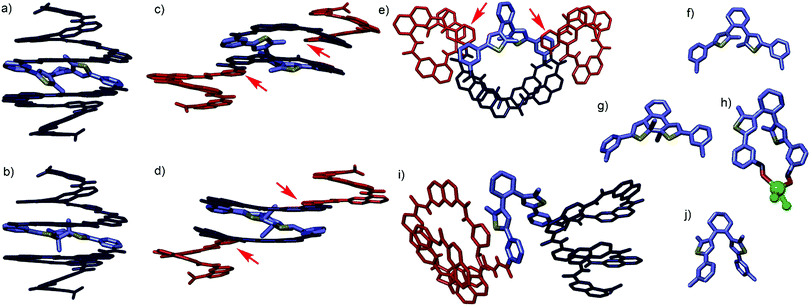 | ||
| Fig. 2 Energy minimised capsule conformations of (a) 1o and (b) 1c. Single crystal structures of 1 (c–h) and 2 (i and j). (c) Side view, (e) top view and (f) isolated S unit of the structure of 1o. (d) Side view and (g) isolated S unit of the structure of 1c. Note that the stereochemistry of Sc is opposite in (d) and in (b). (h) Isolated S unit complexed to MgCl2 in a crystal structure of 1o⊃MgCl2 (Fig. S24, ESI†). The Mg2+ ion coordinates the carbonyl oxygen atoms of S unit. (i) Side view and (j) isolated S unit of the crystal structure of 2o. Red and dark blue segments indicate M and P helices, respectively. S unit are shown in blue-grey, sulphur atoms are shown in yellow. Red arrows indicate monomers where helix handedness inversion occurs. | ||
Circular dichroism (CD) and NMR titrations of 1 with tartaric acid in 5/95 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCl3 vol/vol revealed no binding, perhaps because the methyl groups of S interfere with the accessibility of the Q3PN2 segments for hydrogen bonding. We thus turned to longer sequence 2 which we expected to possess a larger inner cavity (see ESI† for detailed synthesis). Single crystals of 2o suitable for X-ray diffraction were obtained‡ and revealed yet another conformation of the S unit (Fig. 2j), with the two Q3PN2H segments positioned as two independent hemi-capsules (Fig. 2i). In the crystal lattice, one of these segments is intertwined in a double helix with a neighbouring molecule (Fig. S25, ESI†). Such aggregation appears not to be significant in solution (Fig. S3 and S4, ESI†). Nevertheless, the multiple conformations observed in the solid state and the sharp NMR spectra together point to a significant flexibility of the S unit and to rapid dynamics in solution.
CDCl3 vol/vol revealed no binding, perhaps because the methyl groups of S interfere with the accessibility of the Q3PN2 segments for hydrogen bonding. We thus turned to longer sequence 2 which we expected to possess a larger inner cavity (see ESI† for detailed synthesis). Single crystals of 2o suitable for X-ray diffraction were obtained‡ and revealed yet another conformation of the S unit (Fig. 2j), with the two Q3PN2H segments positioned as two independent hemi-capsules (Fig. 2i). In the crystal lattice, one of these segments is intertwined in a double helix with a neighbouring molecule (Fig. S25, ESI†). Such aggregation appears not to be significant in solution (Fig. S3 and S4, ESI†). Nevertheless, the multiple conformations observed in the solid state and the sharp NMR spectra together point to a significant flexibility of the S unit and to rapid dynamics in solution.
Reversible light-induced switching between 2o and 2c was similar to that observed with 1 (Fig. S8, ESI†). Monitoring ring closure by 1H NMR revealed a photostationary state (PSS) at 365 nm consisting of a 25/75 2o![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c ratio. Upon irradiation with wavelengths λ > 450 nm, the PSS reverted to a 98/2 2o
2c ratio. Upon irradiation with wavelengths λ > 450 nm, the PSS reverted to a 98/2 2o![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
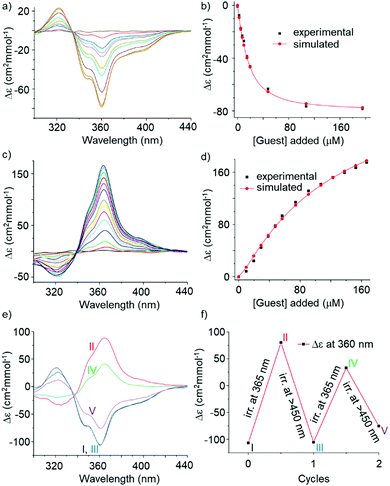
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c ratio within minutes (Fig. S5, ESI†). Due to the analogy between sequences 2 and 3, we assessed binding of fructose to 2. As an initial test, 25 equiv. of D-fructose were added to a solution of 2o in 5% DMSO in CHCl3. The CD spectra showed the slow emergence of an intense negative band at 360 nm, i.e. the induction of an M helix opposite to earlier observations with 3.5a Handedness induction was shown to plateau after 4.5 h (Fig. S9, ESI†). The titration data could be fitted to a 1
2c ratio within minutes (Fig. S5, ESI†). Due to the analogy between sequences 2 and 3, we assessed binding of fructose to 2. As an initial test, 25 equiv. of D-fructose were added to a solution of 2o in 5% DMSO in CHCl3. The CD spectra showed the slow emergence of an intense negative band at 360 nm, i.e. the induction of an M helix opposite to earlier observations with 3.5a Handedness induction was shown to plateau after 4.5 h (Fig. S9, ESI†). The titration data could be fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm (Ka = 7.7 × 104 M−1, Fig. 3a, b and Fig. S10, ESI†) and molecular modelling suggested that fructose could fit in the capsule-like conformation of 2 (Fig. S22, ESI†). 1H NMR titrations revealed that host–guest binding is fast on the NMR timescale (Fig. S12 and S13, ESI†), even at low temperature (Fig. S14 and S15, ESI†), and causes modest (<0.2 ppm) chemical shifts variations. This result contrasts with earlier observations, e.g. with 3, which showed slow exchange of the binding of organic guests in foldamer capsules.5,9 Fast exchange with 2 may result from the flexibility of the central S unit revealed by solid state investigations (Fig. 2). It is remarkable that the flexibility of the receptor does not hamper the diastereoselectivity highlighted by the high Δε values.
1 binding isotherm (Ka = 7.7 × 104 M−1, Fig. 3a, b and Fig. S10, ESI†) and molecular modelling suggested that fructose could fit in the capsule-like conformation of 2 (Fig. S22, ESI†). 1H NMR titrations revealed that host–guest binding is fast on the NMR timescale (Fig. S12 and S13, ESI†), even at low temperature (Fig. S14 and S15, ESI†), and causes modest (<0.2 ppm) chemical shifts variations. This result contrasts with earlier observations, e.g. with 3, which showed slow exchange of the binding of organic guests in foldamer capsules.5,9 Fast exchange with 2 may result from the flexibility of the central S unit revealed by solid state investigations (Fig. 2). It is remarkable that the flexibility of the receptor does not hamper the diastereoselectivity highlighted by the high Δε values.
The 2c/2o mixture at the PSS was then titrated with D-fructose. CD demonstrated an even more intense, but positive, band at 364 nm (Fig. 3c), highlighting the induction of P handedness. Thus, 2c binds fructose as well but with a diastereoselectivity inverse to that of 2o. The CD band is intense despite the fact that part of it is cancelled by the presence of 25% 2o at the PSS. Molecular models suggest that fructose does not fit as well in the cavity of the capsule form of 2c (Fig. S22, ESI†) since ring closure orients the methyl groups such that they occupy part of the space. Thus, binding geometries of 2o and 2c differ. One should consider that, in these experiments, 2c was produced in the absence of any chiral material and thus itself exists as a racemic mixture of Sc enantiomers, regardless of the handedness of the flanking helices. The titration data show a slightly sigmoidal curve that could be fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm (Ka = 8.7 × 103 M−1, Fig. 3d and Fig. S11, ESI†) taking into consideration the presence of 2o. Note that the model used did not consider the possibility that the two diastereomers of 2c may not bind the same to fructose. CD titrations sum up all binding events and may not allow for this level of accuracy. 1H NMR titrations of 2c were hampered by broad signals (Fig. S19, ESI†). The reciprocal effect of complex formation on the fructose anomeric and furanose/pyranose equilibria was not investigated.
1 binding isotherm (Ka = 8.7 × 103 M−1, Fig. 3d and Fig. S11, ESI†) taking into consideration the presence of 2o. Note that the model used did not consider the possibility that the two diastereomers of 2c may not bind the same to fructose. CD titrations sum up all binding events and may not allow for this level of accuracy. 1H NMR titrations of 2c were hampered by broad signals (Fig. S19, ESI†). The reciprocal effect of complex formation on the fructose anomeric and furanose/pyranose equilibria was not investigated.
The opposite binding diastereoselectivity of achiral 2o and 2c, and the intense CD bands, suggest that their light-induced interconversion in presence of fructose may work as a chiroptical switch having the intriguing and novel feature that 2o is achiral. A 10 μM solution of 2o in 5% DMSO in CHCl3 was treated with 25 equiv. of D-fructose to reach saturation and induce M handedness, and then irradiated with 365 nm light. The inversion of the CD band was observed (Fig. S17, ESI†) albeit with a reduction of intensity of 47% compared to that obtained when irradiating first and subsequently adding the sugar (Fig. S16, ESI†). The reason for this behaviour is not fully understood yet. Perhaps the presence of the sugar induces conformations less favourable to cyclization, eventually leading to a PSS where the proportion of Sc is smaller. The larger proportion of 2o would then partly cancel the induced CD resulting from 2c. Broad NMR spectra did not allow to ascertain this (Fig. S19, ESI†). In these experiments, irradiation is performed after fructose addition and induction of helix handedness. A chirality transfer from the helices to an axial chirality of So that would result in an enrichment of one enantiomer of Sc is in principle possible. Such transfer has been observed in other systems.12 However, we found no trace of such a transfer, i.e. no CD band above 500 nm that would surely belong to Sc (Fig. S18, ESI†).
When trying to switch the 2c⊃fructose complex back to the 2o⊃fructose complex using visible light, very slow kinetics of handedness reversal were observed. These experiments were then repeated in 10% DMSO (instead of 5%) in CHCl3 and excess fructose. DMSO was expected to accelerate helix inversion kinetics and to also alter sugar binding. Indeed, in this case, shining alternatively 365 nm light and >450 nm light allowed for a back and forth switching between a prevalence of P helices and a prevalence of M helices (Fig. 3e and f). The system nevertheless showed some fatigue, possibly due to the photo-instability of the capsule itself after prolonged light exposure.
Despite some obvious imperfections such as slow kinetics and fatigue, achiral oligomer 2 demonstrates chiroptical switching behaviour in the presence of fructose. This behaviour can be accounted for by the fact that small structural changes in the foldamer structure lead to drastic changes in sugar binding selectivity. Other sequences that show a similar behaviour have already been found in our laboratories and will be reported elsewhere. The photo-induced reversal of guest enantiomer binding opens up capabilities in various areas such as switchable asymmetric catalysis and chiral guest swapping. For example, upon locking the handedness of Q3PN2H segments with chiral groups,5a successively shining UV and visible light in presence of racemic fructose would lead to the capture of one enantiomer and the release of the other. Upon shining light on part of a solution, one might then produce a 2c/2o gradient that would generate a gradient of enantiomers, i.e. a physical separation of enantiomers by light.
We thank Dr Pedro Mateus for assistance with the fitting of titration data and Dr Arundhati Roy for assistance with irradiation experiments.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. Goto and E. Yashima, J. Am. Chem. Soc., 2002, 124, 7943–7949 CrossRef CAS PubMed; (b) T. Muraoka, K. Kinbara and T. Aida, Nature, 2006, 440, 512–515 CrossRef CAS PubMed; (c) B. L. Feringa, R. A. van Delden, N. Koumura and E. M. Geertsema, Chem. Rev., 2000, 100, 1789–1816 CrossRef CAS PubMed; (d) K. Kinbara, T. Muraokaa and T. Aida, Org. Biomol. Chem., 2008, 6, 1871–1876 RSC; (e) J. W. Canary, Chem. Soc. Rev., 2009, 38, 747–756 RSC; (f) H. Iida, T. Mizoguchi, S.-D. Oh and E. Yashima, Polym. Chem., 2010, 1, 841–848 RSC; (g) J. W. Canary, S. Mortezaei and J. Liang, Coord. Chem. Rev., 2010, 254, 2249–2266 CrossRef CAS; (h) B. L. Feringa and W. R. Browne, Molecular Switches, Wiley-VCH, Weinheim, 2nd edn, 2011 CrossRef; (i) Z. Dai, J. Lee and W. Zhang, Molecules, 2012, 17, 1247–1277 CrossRef CAS PubMed; (j) H. Isla and J. Crassous, C. R. Chim., 2016, 19, 39–49 CrossRef CAS; (k) S. Kawabata, N. Ousaka and E. Yashima, Chem. Commun., 2018, 54, 2417–2420 RSC; (l) J. Zhang, Y. Zhou, Y. Yao, Z. Cheng, T. Gao, H. Li and P. Yan, J. Mater. Chem. C, 2020, 8, 6788–6796 RSC.
- (a) C. Dolain, V. Maurizot and I. Huc, Angew. Chem., Int. Ed., 2003, 42, 2737–2740 CrossRef PubMed; (b) H. Jiang, C. Dolain, J.-M. Léger, H. Gornitzka and I. Huc, J. Am. Chem. Soc., 2004, 126, 1034–1035 CrossRef CAS PubMed.
- (a) T. Nakano and Y. Okamoto, Chem. Rev., 2001, 101, 4013–4038 CrossRef CAS PubMed; (b) J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte and N. A. J. M. Sommerdijk, Chem. Rev., 2001, 101, 4039–4070 CrossRef CAS PubMed; (c) E. Yashima, K. Maeda and Y. Furusho, Acc. Chem. Res., 2008, 41, 1166–1180 CrossRef CAS PubMed; (d) K. Akagi, Chem. Rev., 2009, 109, 5354–5401 CrossRef CAS PubMed; (e) E. Yashima, K. Maeda, H. Iida, Y. Furusho and K. Nagai, Chem. Rev., 2009, 109, 6102–6211 CrossRef CAS PubMed.
- (a) K. Uchida, M. Walko, J. J. D. de Jong, S. Sukata, S. Kobatake, A. Meetsma, J. van Esch and B. L. Feringa, Org. Biomol. Chem., 2006, 4, 1002–1006 RSC; (b) Y. Tani, T. Ubukata, Y. Yokoyama and Y. Yokoyama, J. Org. Chem., 2007, 72, 1639–1644 CrossRef CAS PubMed; (c) T. Hirose, M. Irie and K. Matsuda, New J. Chem., 2009, 33, 1332–1334 RSC; (d) W. Li, X. Li, Y. Xie, Y. Wu, M. Li, X.-Y. Wu, W.-H. Zhu and H. Tian, Sci. Rep., 2015, 5, 9186 CrossRef CAS PubMed; (e) T. Nakagawa, T. Ubukata and Y. Yokoyama, J. Photochem. Photobiol., C, 2018, 34, 152–191 CrossRef CAS; (f) D. Roke, S. J. Wezenberg and B. L. Feringa, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9423–9431 CrossRef CAS PubMed; (g) C. Jurissek, F. Berger, F. Eisenreich, M. Kathan and S. Hecht, Angew. Chem., Int. Ed., 2019, 58, 1945–1949 CrossRef CAS PubMed.
- (a) N. Chandramouli, Y. Ferrand, G. Lautrette, B. Kauffmann, C. D. Mackereth, M. Laguerre, D. Dubreuil and I. Huc, Nat. Chem., 2015, 7, 334–341 CrossRef CAS PubMed; (b) P. Mateus, B. Wicher, Y. Ferrand and I. Huc, Chem. Commun., 2018, 54, 5078–5081 RSC; (c) S. Saha, B. Kauffmann, Y. Ferrand and I. Huc, Angew. Chem., Int. Ed., 2018, 57, 13542–13546 CrossRef CAS PubMed; (d) P. Mateus, N. Chandramouli, C. D. Mackereth, B. Kauffmann, Y. Ferrand and I. Huc, Angew. Chem., Int. Ed., 2020, 59, 5797–5805 CrossRef CAS PubMed.
- (a) M. Waki, H. Abe and M. Inouye, Angew. Chem., Int. Ed., 2007, 46, 3059–3061 CrossRef CAS PubMed; (b) H. Abe, H. Machiguchi, S. Matsumoto and M. Inouye, J. Org. Chem., 2008, 73, 4650–4661 CrossRef CAS PubMed; (c) J. Y. Hwang, H.-G. Jeon, Y. R. Choi, J. Kim, P. Kang, S. Lee and K.-S. Jeong, Org. Lett., 2017, 19, 5625–5628 CrossRef CAS PubMed.
- Y. Ferrand and I. Huc, Acc. Chem. Res., 2018, 51, 970–977 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- Y. Ferrand, A. M. Kendhale, B. Kauffmann, A. Grélard, C. Marie, V. Blot, M. Pipelier, D. Dubreuil and I. Huc, J. Am. Chem. Soc., 2010, 132, 7858–7859 CrossRef CAS PubMed.
- M. Morimoto and M. Irie, Chem. Commun., 2005, 3895–3905 RSC.
- B. Gole, B. Kauffmann, V. Maurizot, I. Huc and Y. Ferrand, Angew. Chem., Int. Ed., 2019, 58, 8063–8067 CrossRef CAS PubMed.
- (a) T. Yamaguchi, K. Uchida and M. Irie, J. Am. Chem. Soc., 1997, 119, 6066–6071 CrossRef CAS; (b) M. Fredersdorf, R. Göstl, A. Kolmer, V. Schmidts, P. Monecke, S. Hecht and C. M. Thiele, Chem. – Eur. J., 2015, 21, 14545–14554 CrossRef CAS PubMed; (c) C. Jurissek, F. Berger, F. Eisenreich, M. Kathan and S. Hecht, Angew. Chem., Int. Ed., 2019, 58, 1945–1949 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2032748–2032750. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc06484j |
| ‡ Single co-crystals suitable for X-ray diffraction analysis were obtained by slow liquid–liquid diffusion. 1c/1o (CCDC 2032748); 1o⊃MgCl2 (CCDC 2032749), 2o (CCDC 2032750). See the ESI† for details about crystallographic methods and data. |
| § Because of the notorious fragility of foldamer crystals, crystal-to-crystal transformations with 1 or 2 were not attempted. |
| This journal is © The Royal Society of Chemistry 2021 |