Open Access Article
Open Access ArticleHighly convenient and highly specific-and-sensitive PCR using Se-atom modified dNTPs†
Bei
Hu‡
 a,
Yitao
Wang‡
a,
Na
Li
a,
Yitao
Wang‡
a,
Na
Li
 a,
Shun
Zhang
a,
Guangcheng
Luo
a,
Shun
Zhang
a,
Guangcheng
Luo
 a and
Zhen
Huang
a and
Zhen
Huang
 *ab
*ab
aKey Laboratory of Bio-Resource and Eco-environment of Ministry of Education, College of Life Sciences, Sichuan University, Chengdu, Sichuan 610064, P. R. China
bSzostak-CDHT Large Nucleic Acids Institute and SeNA Research Institute, Chengdu, Sichuan 610041, P. R. China. E-mail: huang@senaresearch.org
First published on 18th December 2020
Abstract
Primer design and condition optimization for PCR are tedious and labour-intensive. To conveniently achieve high selectivity, sensitivity and robustness, herein, we first report a new strategy with Se-dNTPs to enhance PCR specificity (over 240-fold) and sensitivity (up to single-digit), effectively eliminating non-specific products and simplifing PCR design and optimization.
The polymerase chain reaction (PCR) has become one of the most important techniques in modern life science.1–4 From a small amount of DNA template, PCR can exponentially generate a large number of DNA copies in a short period of time. However, nonspecific DNA fragments are often produced, especially in multiple-round PCR, decreasing its amplification efficiency. Therefore, many steps in PCR are essential to obtain a successful experiment, including primer design and condition optimization. In aspect of primer design, available primers are characterized according to the following parameters:5 (i) sufficient specificity for minimization of off-target annealing; (ii) appropriate melting-temperatures for balancing efficiency and specificity; (iii) a moderate GC content (40–60%) and absence of complementarity between primers, especially at their 3′ ends, for reducing nonspecific pairing between primers, which can significantly affect PCR efficiency and specificity; (iv) avoidance of a significant secondary structure, which can decrease amplification efficiency significantly. Despite availability of many free web-tools for primer design, their successful rate is not always satisfactory.6–8
The related verification and condition optimization are still necessary, including component quantities (such as DNA polymerase, primers, templates and dNTPs) and thermal conditions (such as annealing and extension temperatures). In addition, many other efforts have been used to increase PCR efficiency, specificity and robustness, including hot-start strategy with heat-activated polymerase or primer,9–11 directed-evolved DNA polymerase,12 chemical additives (including DMSO, betaine, metal nanoparticles, quantum dots and nano-polymers),13,14 supplementary recombinase and helicase,15,16 and modified or immobilized primers.17 These efforts are time-consuming and labour-intensive. Though many attentions have been paid to PCR, its non-specific amplification is often a challenge, especially when many primers are involved in an amplification, such as multiplex PCR.
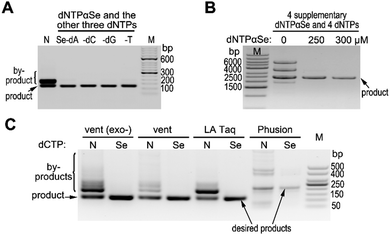
In our preliminary research, DNA polymerases can recognize dNTPαSe and incorporate it into DNA with high specificity and similar product yield.18 Based on these discoveries, we decided to explore Se-atom-assisted PCR (Se-PCR) for suppression of non-specific products, simplification of primer design, and ease of condition optimization. In order to investigate whether nonspecific products can be suppressed in PCR, we performed the reactions in the presence or absence of individual dNTPαSe replacing corresponding canonical dNTP (Fig. 1 and Table S1, ESI†). Excitingly, we found that even after 30 cycles of PCR reaction (using Taq DNA polymerase), nonspecific product was barely observed in the presence of any dNTPαSe, while nonspecific products were formed in the absence of dNTPαSe (Fig. 1A). Further, the PCR product yields were generally similar in the presence or absence of dNTPαSe. Furthermore, another strategy is to add four supplementary dNTPαSe analogues (as low as 1.0 equiv.) into PCR reactions. When PCR amplifying relatively long DNA sequences, the four supplementary analogues completely supressed nonspecific product formation as well (with similar yield, Fig. 1B). Excitingly, the specificity of PCR with high-fidelity DNA polymerases can be further improved (Fig. 1C), though they [vent (exo-), vent, LA Taq, Phusion polymerases] are of 2-, 5-, 6.5-, 52-fold higher fidelity than currently used Taq, respectively.19,20
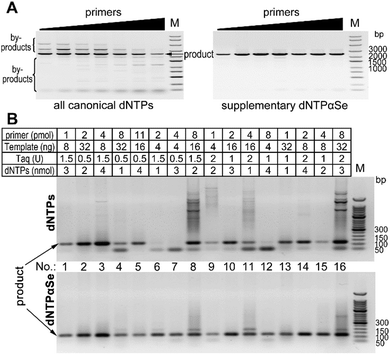
To study whether in the presence of dNTPαSe, the PCR reaction conditions can be easily optimized, we decided to explore a broad range of component concentrations. We carried out the gene cloning experiments by the primer concentration optimization, followed by 16 different sets of optimizing experiments using human genome. We found that at various concentrations of primers, all reactions with canonical dNTPs produced nonspecific products, while dNTPαSe produced barely any by-products (Fig. 2A). Further, when canonical dNTPs were used and primer concentration increased, more by-products were formed proportionally, while the reactions with supplementary dNTPαSe produced barely any by-products (Fig. 2A). Furthermore, we found that in the 16 different conditions for optimization, canonical dNTPs caused formation of many by-products, while dNTPαSe significantly reduced by-product formation (Fig. 2B). Clearly, dNTPαSe is better than the canonical dNTPs and can allow easy optimization.
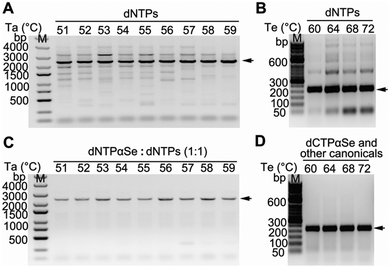
To investigate whether PCR thermal conditions can be easily optimized,21,22 in the presence of dNTPαSe, we performed a series of PCRs at various annealing temperatures (Ta) and extension temperatures (Te). We found that the reactions with canonical dNTPs produced many by-products under various Ta and Te combinations (Fig. 3A and B), while supplementary dNTPαSe offered clean products under the same conditions (Fig. 3C and D). These results indicated that the supplementary dNTPαSe can effectively improve the specificity of PCR, provide a broader range of thermal conditions, and simplify the optimization steps for highly specific PCR amplification.
Based on our results above, we decided to explore whether we can choose primers randomly for a PCR reaction, namely simplifying primer design. With 20 different primer pairs chosen arbitrarily, we performed PCR reactions on the same template. We found that only using canonical dNTPs, 6 out of 20 primer pairs were satisfactory (successful rate 30%), while in the presence of dCTPαSe, 19 out of 20 were successful (rate 95%, Fig. 4 and Table S2, ESI†).
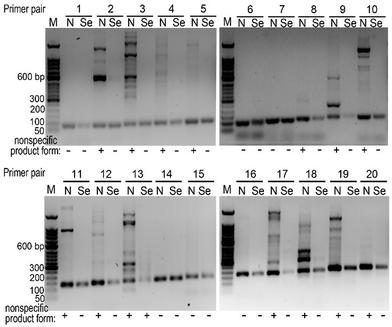 | ||
| Fig. 4 The Se-PCR specificity with randomly selected primers. “N” indicates the reactions with all canonical dNTPs, while “Se” indicates reactions with dCTPαSe and other three canonical dNTPs. | ||
Encouraged by the high specificity on simplex PCR with dNTPαSe, we explored multiplex PCR (MP-PCR), a powerful tool for simultaneously detecting many target sequences in one tube.23–25 Our results indicated that using canonical dNTPs, the conventional MP-PCRs still formed many by-products even after the time-consuming optimization (Fig. 5), while in the presence of dCTPαSe, MP-PCRs conveniently offered clean reactions without significant by-products, indicating the suppression of nonspecific amplifications. Further, we found that the Se-modified products moved faster than the corresponding canonical ones on native PAGE (Fig. 5), which is probably caused by the hydrophobic nature of the Se-modifications.
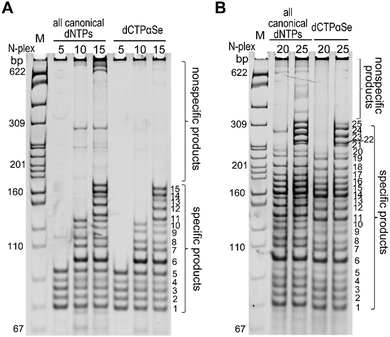 | ||
| Fig. 5 Multiplex PCR with dCTPαSe and the other three canonicals. (A) 5-, 10- and 15-plex PCRs. (B) 20- and 25-plex PCRs. | ||
Effective suppression of nonspecific amplification with dNTPαSe is necessary, as multiplex PCR can amplify multiple target sequences by using multiple primer pairs simultaneously and the complexity of primers can trigger formation of primer-dimers and other nonspecific products. In order to understand the nonspecific suppression, we performed the melting-temperature (Tm) study with the Se-PCR products, namely Se-DNAs. Interestingly, we have found that the Se-DNAs have lower Tm values, compared to the corresponding unmodified DNAs (Table S3, ESI†). The lower Tm can discourage the mis-annealing of the primers and mis-extension of dNTPs.
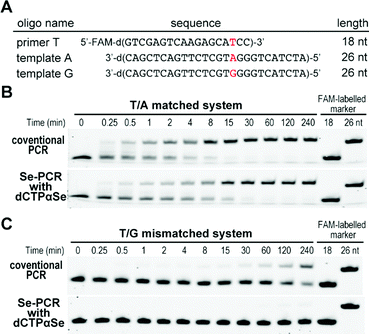
Further, with dNTPαSe, we performed the extension on a mis-matched template, where the mis-matched site (T/G) is four nucleotides away from the elongation site (Fig. 6A). Our study has revealed that compared with canonical dCTP, dCTPαSe enhanced the mis-match discrimination of DNA polymerase. Furthermore, our steady-state kinetic study has indicated that dCTPαSe is over 240-fold more discriminative than the canonical (Fig. 6B, C and Fig. S1, ESI†), thereby revealing its PCR specificity enhancement on a mis-paired template. As the incorporation of dNTPαSe is slower than that of the canonical one,18 the step of dNTPαSe incorporation can offer sufficient time to DNA polymerase for discriminating against mismatched primer/template (such as T/G wobble pair) and enhancing PCR polymerization specificity.
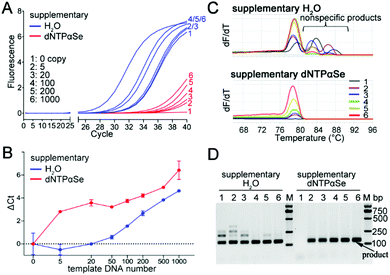
In addition, we explored the effect of dNTPαSe on sensitivity, and we found that compared with canonical PCR strategy, efficiency and yield of Se-PCR (two strategies) might vary (Fig. 1–4), and sensitivity differences among these three strategies were small in the absence of background DNAs (Fig. S2, ESI†). However, in the presence of background and low number of copies of target molecules, Se-PCR is more specific and sensitive than the canonical, because of its suppression on nonspecific products (Fig. 7), which cause the consumption of the substrates and primers, therefore reducing the sensitivity and specificity. We performed qPCR for detecting human papilloma virus type 16 (HPV-16), in the presence of human genome as background DNA (Fig. 7). Further, we measured the high-resolution melting (Fig. 7C) and carried out agarose gel electrophoresis (Fig. 7D). We found that using the supplement strategy, dNTPαSe could largely increase specificity by eliminating by-products formed with the commercial kits (Fig. 7C and D). Furthermore, we discovered that being consistent with the suppression of nonspecific products and background noise, the dNTPαSe-supplemented system allowed the detection up to 5 copies of targets (Fig. 7B), approximately 20-fold more sensitive than the original kit, in the presence of human DNA.
In conclusion, we have discovered that dNTPαSe can significantly reduce the off-target amplification in PCR. We have established two strategies for highly-specific DNA polymerization and synthesis: (i) replacement of canonical dNTP(s) with dNTPαSe and (ii) supplement with four dNTPαSe analogues. In addition, we have found that dNTPαSe can effectively improve the specificity (over 240 folds) and in turn enhance sensitivity (up to single-digit copies of targets) via inhibiting nonspecific polymerization. Moreover, dNTPαSe can usefully simplify design and optimization of highly-specific PCR and MP-PCR.
Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Chang, J. Li and L. Wang, Anal. Chim. Acta, 2016, 910, 12–24 CrossRef CAS PubMed.
- Y. Zhang and H.-R. Jiang, Anal. Chim. Acta, 2016, 914, 7–16 CrossRef CAS PubMed.
- H. Tian, Y. Sun, C. Liu, X. Duan, W. Tang and Z. Li, Anal. Chem., 2016, 88, 11384–11389 CrossRef CAS PubMed.
- D. K. W. Chu, Y. Pan, S. M. S. Cheng, K. P. Y. Hui, P. Krishnan, Y. Liu, D. Y. M. Ng, C. K. C. Wan, P. Yang, Q. Wang, M. Peiris and L. L. M. Poon, Clin. Chem., 2020, 66, hvaa029 CrossRef PubMed.
- S. A. Bustin, R. Mueller and T. Nolan, Methods Mol. Biol., 2020, 2065, 5–22 CrossRef CAS PubMed.
- K. Wang, H. Li, Y. Xu, Q. Shao, J. Yi, R. Wang, W. Cai, X. Hang, C. Zhang, H. Cai and W. Qu, Nucleic Acids Res., 2019, 47, W610–W613 CrossRef CAS PubMed.
- J. Ye, G. Coulouris, I. Zaretskaya, I. Cutcutache, S. Rozen and T. L. Madden, BMC Bioinf., 2012, 13, 134 CrossRef CAS PubMed.
- D. Sun, M. K. Ostermaier, F. M. Heydenreich, D. Mayer, R. Jaussi, J. Standfuss and D. B. Veprintsev, PLoS One, 2013, 8, e78878 CrossRef CAS PubMed.
- D. E. Birch, Nature, 1996, 381, 445–446 CrossRef CAS PubMed.
- A. V. Lebedev, N. Paul, J. Yee, V. A. Timoshchuk, J. Shum, K. Miyagi, J. Kellum, R. I. Hogrefe and G. Zon, Nucleic Acids Res., 2008, 36, e131 CrossRef PubMed.
- M. R. Green and J. Sambrook, Cold Spring Harbor Protoc., 2018, 2018, 346–349 Search PubMed.
- T. A. Coulther, H. R. Stern and P. J. Beuning, Trends Biotechnol., 2019, 37, 1091–1103 CrossRef CAS PubMed.
- P. Chen, D. Pan, C. Fan, J. Chen, K. Huang, D. Wang, H. Zhang, Y. Li, G. Feng, P. Liang, L. He and Y. Shi, Nat. Nanotechnol., 2011, 6, 639–644 CrossRef CAS PubMed.
- Y. Zhong, L. Huang, Z. Zhang, Y. Xiong, L. Sun and J. Weng, Int. J. Nanomed., 2016, 11, 5989–6002 CrossRef CAS PubMed.
- M. Vincent, Y. Xu and H. Kong, EMBO Rep., 2004, 5, 795–800 CrossRef CAS PubMed.
- Y. Shigemori, T. Mikawa, T. Shibata and M. Oishi, Nucleic Acids Res., 2005, 33, e126 CrossRef PubMed.
- L. S. Meuzelaar, O. Lancaster, J. P. Pasche, G. Kopal and A. J. Brookes, Nat. Methods, 2007, 4, 835–837 CrossRef CAS PubMed.
- B. Hu, Y. Wang, S. Sun, W. Yan, C. Zhang, D. Luo, H. Deng, L. R. Hu and Z. Huang, Angew. Chem., Int. Ed., 2019, 58, 7835–7839 CrossRef CAS PubMed.
- K. Böhlke, F. M. Pisani, C. E. Vorgias, B. Frey, H. Sobek, M. Rossi and G. Antranikian, Nucleic Acids Res., 2000, 28, 3910–3917 CrossRef PubMed.
- P. Mattila, J. Korpela, T. Tenkanen and K. Pitkänen, Nucleic Acids Res., 1991, 19, 4967–4973 CrossRef CAS PubMed.
- O. Henegariu, N. A. Heerema, S. R. Dlouhy, G. H. Vance and P. H. Vogt, Biotechniques, 1997, 23, 504–511 CrossRef CAS PubMed.
- M. R. Green and J. Sambrook, Cold Spring Harb. Protoc., 2019, 2019, pdb.top095109 CrossRef PubMed.
- W. K. Kim, J. S. No, S. H. Lee, D. H. Song, D. Lee, J. A. Kim, S. H. Gu, S. Park, S. T. Jeong, H. C. Kim, T. A. Klein, M. R. Wiley, G. Palacios and J. W. Song, Emerging Infect. Dis., 2018, 24, 249–257 CrossRef CAS PubMed.
- J. Li, J. Li, S. Xu, S. Xiong, J. Yang, X. Chen, S. Wang, X. Qiao and T. Zhou, Food Chem., 2019, 295, 395–402 CrossRef CAS PubMed.
- Y. Peng, S. Zhao, K. Wang, J. Song, Y. Yan, Y. Zhou, K. Shi, F. Jian, R. Wang, L. Zhang and C. Ning, Front. Microbiol., 2020, 11, 606 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and optimization of the experimental conditions. See DOI: 10.1039/d0cc06172g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |