C-Terminal lactamization of peptides†
Abstract
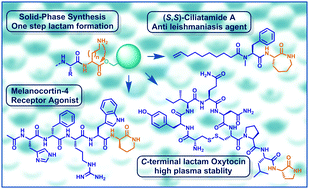
Solid-phase synthesis of peptides (SPPS) with release through formation of C-terminal γ-, δ-, or ε-lactams is presented. The natural products ciliatamide A and C were synthesized in up to 90% yield. Peptides carrying C-terminal lactams were shown to possess increased bio-stability and comparable biological activity as compared to the parent non-lactamized peptide amides.
- This article is part of the themed collection: Bioorthogonal and click chemistry: Celebrating the 2022 Nobel Prize in Chemistry


 Please wait while we load your content...
Please wait while we load your content...