Assembly of four modules onto a tetraazide platform by consecutive 1,2,3-triazole formations†
Abstract
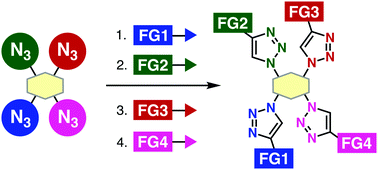
Efficient consecutive 1,2,3-triazole formations using multiazide platforms are disclosed. On the basis of unique clickability of the 1-adamantyl azido group, a four-step synthesis of tetrakis(triazole)s was achieved from a tetraazide platform molecule. This method was applied to a convergent synthesis of tetrafunctionalized probes in a modular synthetic manner.


 Please wait while we load your content...
Please wait while we load your content...