Influence of nanoparticles on the haemostatic balance: between thrombosis and haemorrhage
Huong D. N.
Tran
ab,
Shehzahdi Shebbrin
Moonshi
a,
Zhi Ping
Xu
b and
Hang Thu
Ta
 *abc
*abc
aQueensland Micro- and Nanotechnology, Griffith University, Nathan, Queensland 4111, Australia. E-mail: h.ta@griffith.edu.au
bAustralian Institute for Bioengineering and Nanotechnology, University of Queensland, St Lucia, Queensland 4072, Australia
cSchool of Environment and Science, Griffith University, Nathan, Queensland 4111, Australia
First published on 14th October 2021
Abstract
Maintenance of a delicate haemostatic balance or a balance between clotting and bleeding is critical to human health. Irrespective of administration route, nanoparticles can reach the bloodstream and might interrupt the haemostatic balance by interfering with one or more components of the coagulation, anticoagulation, and fibrinolytic systems, which potentially lead to thrombosis or haemorrhage. However, inadequate understanding of their effects on the haemostatic balance, along with the fact that most studies mainly focus on the functionality of nanoparticles while forgetting or leaving behind their risk to the body's haemostatic balance, is a major concern. Hence, our review aims to provide a comprehensive depiction of nanoparticle-haemostatic balance interactions, which has not yet been covered. The synergistic roles of cells and plasma factors participating in haemostatic balance are presented. Possible interactions and interference of each type of nanoparticle with the haemostatic balance are comprehensively discussed, particularly focusing on the underlying mechanisms. Interactions of nanoparticles with innate immunity potentially linked to haemostasis are mentioned. Various physicochemical characteristics that influence the nanoparticle-haemostatic balance are detailed. Challenges and future directions are also proposed. This insight would be valuable for the establishment of nanoparticles that can either avoid unintended interference with the haemostatic balance or purposely downregulate/upregulate its key components in a controlled manner.
1. Introduction
Nanoparticles are a fundamental building block of nanotechnology, referring to particles with three dimensions at the nanoscale (approximately 1–1000 nm).1 Nanoparticles possess unique physiochemical properties owing to the large surface to volume ratio. They can be potentially employed in diverse fields including biosensors, biotechnology, the food industry, agriculture, waste management, energy, cosmetics, and especially biomedicine.2–8 The pivotal role of nanoparticles in biomedicine has been affirmed with the continuously increasing number of their applications in molecular imaging, image-guided therapy and therapeutic treatment of various diseases.7,9–23 However, very few can successfully progress to clinical translation and commercialization in spite of positive preclinical data that has been reported. One of the remaining challenges is the lack of a full assessment of their health risks as there are always cell–nanoparticle or blood–nanoparticle interactions when they enter the body via any route.2,24Maintenance of the haemostatic balance is critical to human body health.25 The term “haemostasis” is defined as a natural response process of the body to stop bleeding from damaged blood vessels.26 In 1958, the prevalent notion “haemostatic balance” was first elaborated by Astrup, describing the balance between the tendency of blood to clot and for such clots to lyse.27 This is the delicate equilibrium between procoagulant and anticoagulant factors that interact with each other to ensure effective haemostasis at the sites of vascular injury. The notion has now been broadened to the concept that blood has a strong tendency to clot when tissue is injured, and the intact vasculature requires major anticoagulant systems to prevent clots adhering to and stabilising in the vasculature.28 As a result, the delicate thrombo-haemorrhagic balance, in other words the balance between clotting and bleeding, is always maintained under normal physiological conditions. Any interruption in the haemostatic balance might lead to either excess bleeding (haemorrhage) or abnormal clot formation in the absence of bleeding (thrombosis).2,25,29,30
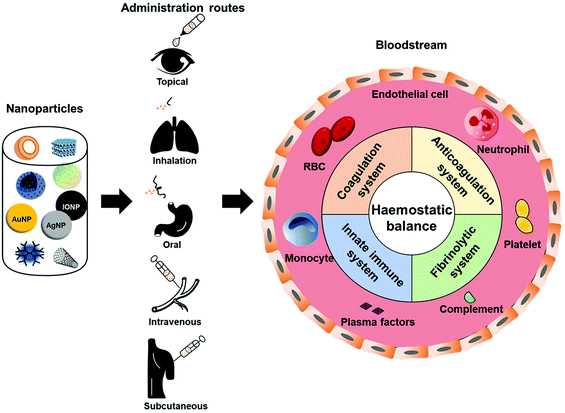
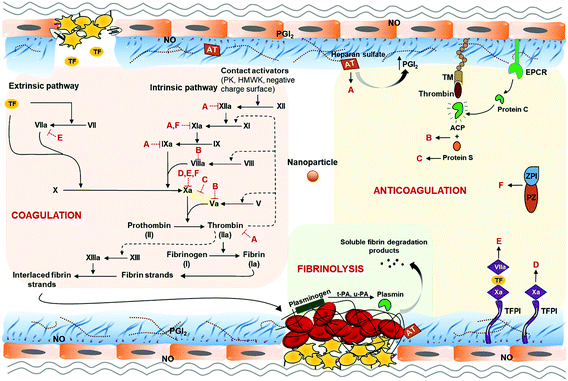
Regardless of the administration route and the intended target, nanoparticles can reach the circulatory system due to their ability to permeate epithelium after dermal penetration, oral ingestion, or inhalation.2,25,31 Once inside the blood stream, they can potentially interfere with the haemostatic balance in unintended ways29,32,33 (Fig. 1), causing haemorrhage or lethal coagulation disorders (i.e. disseminated intravascular coagulation and deep vein thrombosis), and thus raising concerns regarding the safety of these nanoparticles.31,33 To date, thrombosis and related complications are the greatest hurdles involved in the clinical translation of many nanoparticles.30 Different types of nanoparticles will affect the haemostatic balance in a different manner. Changes in one or more physicochemical characteristics of a specific type of nanoparticle (i.e. size, shape, surface charge, stabilizing/coating material) could significantly alter its effect on haemostatic balance. However, inadequate understanding of nanoparticles’ effects on the haemostatic balance, which is the root of their toxicity in the blood system, is a major concern. Most studies usually focus on the functionality of the nanoparticle systems while forgetting or leaving behind their risks to the body's haemostatic balance. Therefore, cautious design of nanoparticles based on in-depth knowledge of their behaviours toward the blood haemostasis would be beneficial in tackling the complications accompanied with their use, thereby improving their haemocompatibility and speeding up their translation to the clinic and market.
All previous reviews with similar topics mainly focused on the nanomaterials with coagulation effects and left out those with anticoagulant or thrombolytic effects. Moreover, the underlying mechanisms were often not discussed. With that consideration in mind, this review aims to provide a complete depiction of the interactions between nanoparticles and the haemostatic balance (thrombosis/haemorrhage), which has yet to be thoroughly discussed to date. The roles of cells and plasma factors participating in coagulation and anticoagulation systems along with fibrinolytic systems to maintain the thrombo-haemorrhagic balance will be presented. Importantly, nanoparticle interactions with each component of the haemostatic balance are discussed comprehensively with a focus on the underlying mechanisms. The interaction of nanoparticles with innate immunity, which could potentially interfere with the haemostatic balance concerning the intrinsic link between the innate immune system and haemostasis, will be discussed. Moreover, various physicochemical characteristics of nanoparticles that influence the nanoparticle-haemostatic balance will be detailed. Challenges and future directions will also be proposed.
In comparison with previous reviews with similar topics, our paper (1) is the first review discussing the influence of nanoparticles on the whole haemostatic balance through their interaction with the haemostasis components and the innate immune system, which is potentially linked to haemostasis, (2) categorises and discusses all possible effects, along with the underlying mechanisms of each type of nanoparticle on each component of the haemostatic balance, (3) provides a comprehensive conclusion for the effects of each type of nanoparticle (along with its specified physicochemical characteristics) on the haemostatic balance, which would be beneficial for the design of nanoparticles.
2. The role of blood coagulation, anticoagulation, and fibrinolytic systems in haemostatic balance
A haemostatic balance under normal physiological conditions is achieved through the clotting and anticlotting effects, in equilibrium with each other, controlled by blood coagulation, anticoagulation, and fibrinolytic systems.34,35 The blood coagulation system mediates haemostasis at the vascular injury sites.36 Upon injury, damaged endothelial cells expose sub-endothelial collagens for the initiation of primary haemostasis, where platelets aggregate and form a temporary platelet plug. Subsequently, secondary haemostasis is initiated with the involvement of a coagulation cascade, which results in a fibrin mesh that entraps the platelet plug and red blood cells (RBCs) to form a blood clot and stops the bleeding.37,38 In contrast, the anticoagulation system prevents clots from forming (prevents primary and/or secondary haemostasis) while the fibrinolytic system breaks down clots that have already been formed.39 As a result, clot formation is restricted to the injury site, thus preventing haemostasis at the wrong place, which inadvertently results in thrombosis. Nevertheless, induced anticoagulation and fibrinolysis could lead to prolonged bleeding or haemorrhage.Vascular endothelial cells, platelets, red blood cells, along with plasma coagulation factors, anticoagulation factors, and fibrinolytic enzymes and activators are components of blood coagulation, anticoagulation, and fibrinolytic systems. The roles of each component and their association with others in order to maintain the haemostatic balance will be discussed in the following subsections.
2.1 Vascular endothelium
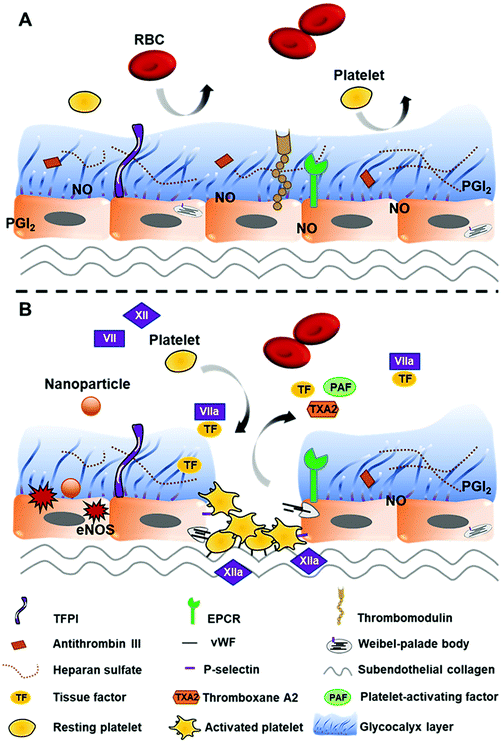
Vascular endothelial cells play an important role in the regulation of platelet adhesion, thrombosis, and fibrinolysis.31 Healthy endothelial cells are protected by a glycocalyx layer consisting of heparan sulfate that has an affinity for anticoagulant proteins such as antithrombin III (AT III or AT) and tissue factor pathway inhibitor (TFPI) (Fig. 2A). These proteins (AT and TFPI) and anticoagulant mediators (heparin cofactor II, endothelial protein C receptor (EPCR), and thrombomodulin (TM)) expressed on the endothelium surface, together with platelet adhesion and aggregation inhibitors (nitric oxide (NO), prostacyclin (PGI2), and CD39/NTPDase1), are secreted by the endothelium to maintain the thrombo-resistant or anticoagulant nature of intact vascular endothelial cells.40–43 In addition, AT also further stimulates PGI2 production which results in the inhibition of platelet aggregation and vasodilation.39Interaction with nanoparticles can cause endothelium dysfunction. Damage to endothelial cells not only leads to the exposure of tissue factors (TFs) (CD142 or FIII), which activates the extrinsic pathway of haemostasis, but also exposes subendothelial collagens that bind FXII to initiate the intrinsic pathway. Moreover, von Willebrand factor (vWF), thromboxane A2 (TXA2), P-selectin (CD62P/GMP-140/PADGEM), and platelet-activating factors (PAFs) released by injured endothelial cells along with the exposed collagens are associated with platelet recruitment, adhesion, and activation31,36 (Fig. 2B).
2.2 Platelets
Platelets (thrombocytes) are a crucial cellular component that is involved in the regulation of haemostatic balance.3 They originate from megakaryocytes and are anucleate, discoid in shape, and around 2–4 μm in diameter. Around 33% of all platelets are stored in the spleen, while the rest circulate in the circulatory system (∼150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–450
000–450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 platelets per mm3) without adhering to the intact vascular endothelium.31 Upon injury, damaged endothelium exposes TF, collagen, and other thrombogenic factors such as vWF, TXA2, and PAFs for the initiation of primary haemostasis. Platelets become activated once they come in contact with vWF and sub-endothelial collagens and adhere to the injured endothelium and vessel wall.37,44–46 The platelet activation process is characterised by a drastic increase in cytosolic Ca2+, which elicits the reorganisation of the platelet cytoskeleton, resulting in a change in shape (from disc to sphere shape), pseudopodia formation, aggregation, and exocytosis of contents stored inside the platelet's granules47 (Table 1). Adhesive glycoproteins (vWF, fibrinogen, P-selectin, thrombospondin, and vitronectin), coagulation factors (plasminogen, kininogen, factor V, XI, XIII), plasminogen activator inhibitor-1 (PAI-1), TXA2, PAFs, adenosine diphosphate (ADP), and serotonin secreted by activated platelets mediate vasoconstriction and platelet aggregation, activate more platelets and attract them to come to form a weak platelet plug that temporarily seals the injured area.37,41,47,48 There is a certain number of glycoprotein IIb/IIIa (GpIIb/IIIa) receptors presented on the surface of resting platelets (approximately 50
000 platelets per mm3) without adhering to the intact vascular endothelium.31 Upon injury, damaged endothelium exposes TF, collagen, and other thrombogenic factors such as vWF, TXA2, and PAFs for the initiation of primary haemostasis. Platelets become activated once they come in contact with vWF and sub-endothelial collagens and adhere to the injured endothelium and vessel wall.37,44–46 The platelet activation process is characterised by a drastic increase in cytosolic Ca2+, which elicits the reorganisation of the platelet cytoskeleton, resulting in a change in shape (from disc to sphere shape), pseudopodia formation, aggregation, and exocytosis of contents stored inside the platelet's granules47 (Table 1). Adhesive glycoproteins (vWF, fibrinogen, P-selectin, thrombospondin, and vitronectin), coagulation factors (plasminogen, kininogen, factor V, XI, XIII), plasminogen activator inhibitor-1 (PAI-1), TXA2, PAFs, adenosine diphosphate (ADP), and serotonin secreted by activated platelets mediate vasoconstriction and platelet aggregation, activate more platelets and attract them to come to form a weak platelet plug that temporarily seals the injured area.37,41,47,48 There is a certain number of glycoprotein IIb/IIIa (GpIIb/IIIa) receptors presented on the surface of resting platelets (approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per platelet).49 Upon activation, GpIIb/IIIa stored in the internal pool of platelets will move to their surface, thereby increasing the number of expressed GpIIb/IIIa. These receptors, both the newly expressed and the previously presented ones, undergo a conformation change process, which is related to extracellular ionised calcium and the expression of ligand-induced binding sites to develop a high-affinity for fibrinogen.49–51 Fibrin forms the bridge between platelets and entraps the platelet plug and other surrounding blood cells to form a stable clot.37,41,47,48
000 per platelet).49 Upon activation, GpIIb/IIIa stored in the internal pool of platelets will move to their surface, thereby increasing the number of expressed GpIIb/IIIa. These receptors, both the newly expressed and the previously presented ones, undergo a conformation change process, which is related to extracellular ionised calcium and the expression of ligand-induced binding sites to develop a high-affinity for fibrinogen.49–51 Fibrin forms the bridge between platelets and entraps the platelet plug and other surrounding blood cells to form a stable clot.37,41,47,48
| Granules | Content class | Factors released |
|---|---|---|
| vWF, von Willebrand factor; IGF, insulin-like growth factor; EGF, epidermal growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor β; PF4, platelet factor 4; VEGF, vascular endothelial growth factor; PAI-1, plasminogen activator inhibitor-1; TFPI, tissue factor pathway inhibitor; MMP, matrix metalloproteinase; ADP, adenosine diphosphate; ATP, adenosine triphosphate; GDP, guanosine diphosphate; GTP, guanosine triphosphate; NO, nitric oxide; TXA2, thromboxane A2; PAF, platelet-activating factor. | ||
| Alpha granules | Adhesive glycoproteins | vWF, thrombospondin, P-selectin, fibrinogen, fibronectin, vitronectin |
| Coagulation factors | Plasminogen, kininogens, protein S, factor V, factor XI, factor XIII | |
| Growth factors | IGF, EGF, PDGF, TGF-β | |
| Angiogenic factors | PF4 inhibitor, VEGF | |
| Protease inhibitors | C1-inhibitor, PAI-1, TFPI, α2-antiplasmin, α2-antitripsin, α2-macroglobulin | |
| Immunoglobulins-chemokines | IL8, IL1β, CD40, CXCL4 (platelet basic protein/NAP-2), CXCL (PF4), CXCL1, CXCL5, CCL5 (RANTES), CCL (MIP-1α) | |
| Proteases | MMP2, MMP9 | |
| Dense granules (or delta granules) | Amines | Serotonin, histamine |
| Bivalent cations | Ca2+, Mg2+ | |
| Polyphosphates | ADP, ATP, GDP, GTP | |
| Lysosome granules | Enzymes | Acid proteases, glycohydrolases |
| Other soluble mediators | NO, TXA2, defensins, PAF | |
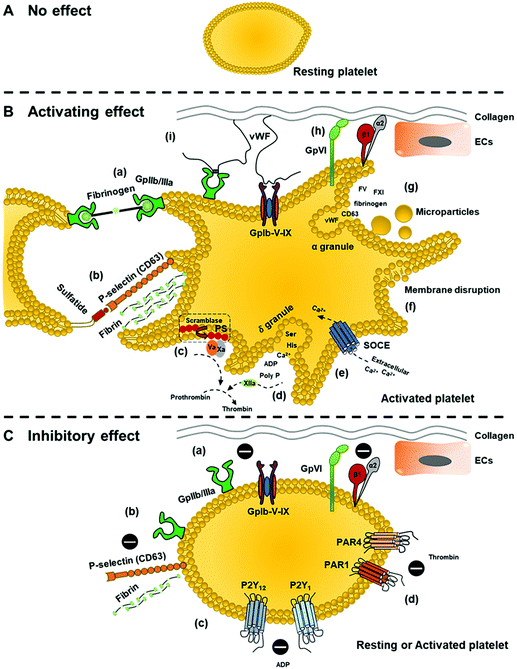
Generally, the interactions of nanoparticles with platelets can affect platelet functions. Different types of nanoparticles with varied size, charge, coating materials, and composition may lead to different outcomes, including activating effect, inhibitory effect, or no effect on platelets (Fig. 3). Excess activation effects on platelets without the presence of injury would lead to a hypercoagulable state (thrombophilia) and increase the risk of thrombosis. Meanwhile, excessive inhibitory effects of platelets would lead to prolonged and uncontrolled bleeding when the injury occurs.
2.3 Red blood cells (RBCs)
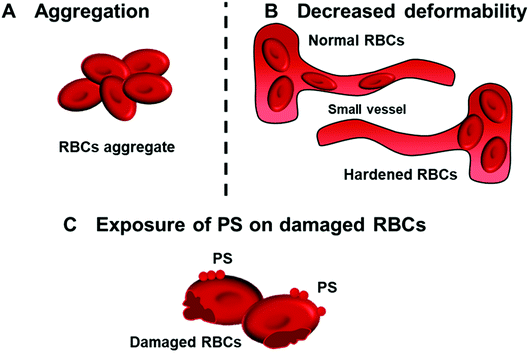
RBCs (erythrocytes) are a cellular component that also takes part in haemostatic balance control and has been underestimated in the past. Detailed mechanisms on how RBCs perform their roles in haemostasis have been reviewed in-depth previously.52 Briefly, RBCs attribute to haemostatic balance through hemorheological properties owing to their abundance and large size.53 The influence of hemorheology, which can be defined as the flow property of blood, and its elements on haemostasis and thrombosis are dependent on the blood shear rates and viscosity where RBCs are a main contributor.54,55 The blood viscosity affects platelet distribution within vessels based on the axial margination phenomenon in which RBCs tend to move to the centre of vessels and push platelets towards the periphery, facilitating their collision with the vasculature for haemostatic events.56Interactions of nanoparticles with RBCs can cause RBC aggregation57 (Fig. 4A). Aggregation of RBCs, especially in small vessels, normally increases the blood viscosity in the centre of vessels and platelet margination, resulting in induced endothelium activation and platelet aggregation.54 Furthermore, nanoparticle interactions with RBCs can also alter the deformability of RBCs, which is the ability of RBCs to change their shape in response to applied stress without resulting in haemolysis58 (Fig. 4B). A decrease in RBC deformability is related to higher risk of thrombosis since rigid RBCs can block small vessels easily, alter the blood flow, and provoke platelet activation.59 In addition to hemorheology, RBC–nanoparticle interactions can lead to the exposure of phosphatidylserine (PS) on the RBCs surface, contributing to blood coagulation60 (Fig. 4C). PS is a key phospholipid localised within the plasma membrane. Upon the high shear stress, oxidative stress, or complement attack, damaged RBCs expose PS on the membrane surface, providing a procoagulant surface for the accumulation of coagulation complexes such as prothrombinase and intrinsic tenase that facilitate thrombus formation.53
2.4 Plasma factors
Besides cellular components, the haemostatic balance is mediated by plasma factors which function as components of blood coagulation, anticoagulation, and fibrinolytic systems. The majority of circulating plasma coagulation factors are zymogens, precursors of enzymes, which will be converted into the active form once the coagulation cascade is initiated. The other plasma coagulation factors are non-enzymatic and act as either a cofactor (e.g., TF (or factor III or FIII), FV and FVIII, high-molecular-weight kininogen (HMWK or HK)) or substrate (e.g., fibrinogen). These factors form a coagulation cascade in secondary haemostasis, which can be divided into extrinsic and intrinsic pathways (Fig. 5). Both pathways lead to thrombin generation and ultimately fibrin formation to create a stable blood clot at the injury site. The extrinsic pathway is activated by TFs exposed on damaged endothelial cells or tissues, initiating the coagulation cascade. TFs then form a TF-FVIIa complex through the direct capture of TFs with free FVIIa circulated in plasma and/or the binding of TFs with VII, followed by the proteolytic conversion of FVII to FVIIa due to the exposed TFs.61 In a parallel manner, the intrinsic pathway begins with FXII, prekallikrein (PK), and HMWK.44 FXII can be activated via the contact with negatively charged molecules and nanoparticle surfaces such as dextran sulfate, glass, kaolin, Celite, and silica.62–65 It can also be autoactivated by the membrane of activated platelets,66 resulting in the activation of the kallikrein-kinin system and FXI, as well as other downstream zymogens in the intrinsic pathway.33 It is important to note that, apart from activated FXII (FXIIa), a small amount of thrombin generated by the extrinsic pathway can in turn activate FXI and thus facilitate the activation of the intrinsic pathway and amplification of thrombin generation.In different circumstances, plasma factors of the anticoagulation system and fibrinolytic system function in the opposite manner to the coagulation system to downregulate and balance the haemostasis. The anticoagulation system regulates the haemostatic balance by preventing clot formation via four pathways, including the AT glycosaminoglycan pathway, protein C pathway, TFPI pathway, and protein Z dependent inhibitor pathway.39 AT is a small protein in the bloodstream that has anticoagulant activity. It binds to heparan sulfate expressed on vascular endothelial cell surfaces and then exerts inhibitory effects on thrombin, FXa, FIXa, FXIa, and FXIIa.39,67 In addition, it also inhibits platelet aggregation by triggering the production of PGI2.39 Protein C and protein S are the main elements of the protein C pathway. Protein C is presented to a TM-thrombin complex by EPCR on endothelial cells for the conversion to its activated form, activated protein C (APC). APC degrades two coagulation factors, FVa and FVIIIa, with assistance from protein S as a cofactor. Moreover, protein S can independently and reversibly inhibit prothrombinase complex (FXa-FVa) in the intrinsic pathway of the coagulation cascade. TFPI is another anticoagulant factor which targets the extrinsic pathway. TFPI binds to FXa to form TFPI-FXa complex and inactivates FXa. TFPI can also form a quaternary complex of TFPI-FXa-TF-FVIIa to inhibit both FXa and FVIIa. Lastly, another group of anticoagulant plasma factors are protein Z-dependent protease inhibitor (ZPI) and protein Z (PZ) as a cofactor of ZPI. ZPI not only independently inhibits FXIa but also inactivates FXa in the presence of PZ.39
In addition to the anticoagulation system, the haemostatic balance is also regulated by the fibrinolytic system to break down clots that have been formed.39 Plasmin is a key enzyme of the fibrinolytic system, which is converted from clot-bound plasminogen by two distinct plasminogen activators named t-PA (tissue-type plasminogen activator) and u-PA (urokinase-type plasminogen activator) synthesised by endothelial cells.39 As a proteolytic enzyme, plasmin can cleave cross-linked fibrin of the clot to soluble fibrin degradation products which will be cleared away by flowing blood, thus dissolving blood clots.68 Furthermore, plasmin can also upregulate the production of itself by making more active forms of u-PA and t-PA.39
Since nanoparticle surfaces can activate coagulation factor XII to initiate the intrinsic pathway of coagulation or possibly affect plasminogen activation, it is reasonable to anticipate that nanoparticles might unintentionally interfere with the coagulation cascade, fibrinolytic system, and overall haemostasis.
3. Effects of nanoparticles on haemostatic balance – the underlying mechanisms
Upon reaching the bloodstream, nanoparticles encounter many blood components and biological systems, including the blood coagulation, anticoagulation, and fibrinolytic systems. Unintended interactions of nanoparticles with these systems can result in a dysregulation of the haemostatic balance.2,29 The possible effects of various types of nanoparticles on haemostatic balance, along with the underlying molecular mechanisms, will be discussed in the following subsections (Fig. 2–5).Nanoparticles can be purposefully engineered to interact with these systems, thus intentionally affecting the haemostatic balance by either inducing or preventing coagulation in order to avoid bleeding or prevent thrombosis, respectively. They can be synthesised from the haemostasis-induced naturally-derived materials or can be engineered (i.e. be loaded with drugs and/or decorated with peptides, antibodies, recombinant factors, or markers on the surface) to obtain the desirable effect on the haemostatic balance, as reviewed elsewhere.36,69 Nanoparticles intentionally engineered to affect the haemostatic balance are outside the scope of this review and are excluded.
3.1 Inorganic nanoparticles
All nanomaterials discussed in this section have no specific coating unless specified otherwise. Specific characteristics of each nanoparticle are detailed in Table 2.| Nanoparticle | Shape | Size | Charge | Coating/stabiliser | Concentration tested | Main finding | Ref. |
|---|---|---|---|---|---|---|---|
| Carbon nanotube | Tube | SWCNTs: 1–2 nm in outer diameter, 5–30 μm in length (TEM) | Not specified | None | 100 and 200 μg mL−1in vitro | Induced platelet activation and aggregation in vitro | 71 and 72 |
| MWCNTs: 60–100 nm or 30 ± 15 nm in outer diameter, 1–5 μm in length (TEM) | Systemic levels of 5 μg mL−1 in rat | Caused vascular thrombosis in vivo | |||||
| Carbon nanotube | Tube | 60 nm (TEM) | Neutral | None | 100 μg mL−1 | Induced platelet aggregation in vitro | 73 |
| Carbon nano-diamond | Tetragonal | 4–10 nm (TEM) | Negative | None | 1 μg mL−1in vitro | Evoked platelet activation in vitro | 78 |
| 250 μg kg−1 of mice (IV route) | Caused pulmonary thromboembolism in vivo | ||||||
| Nano-diamond | Not specified | 100 nm (SEM) | Negative | Carboxylate groups | 0.1 mg mL−1 | Caused abnormal RBC aggregates | 79 |
| Carbon nanotube (pristine, amine- and carboxyl-modified) | Tube | Diameter: 26–31 nm; length: 490–580 nm (TEM) | Not specified | DSPE-PEG or pluronic F127 | 100 μg mL−1in vitro | Except for pluronic-coated pristine carbon nanotubes exhibiting no effect, all nanotubes triggered intrinsic cascade via interaction with FIXa and promote its enzyme activity in vitro | 74 |
| 250 μg per mice (∼100 μg mL−1 as in vitro) (IV route) | Functionalization mitigated procoagulant effect in vivo | ||||||
| Carbon nanotube | Tube | Diameter: 4–5 nm; length: 500–1000 nm (TEM) | Not specified | Albumin | 30–150 μg mL−1 | Pre-treatment with albumin lessens thrombogenic effect of carbon nanotubes in vitro | 84 |
| Carbon nanotube | Tube | Diameter: 6–20 nm; length: 700–4000 nm (TEM) | Not specified | None | Cumulative dose: 32 or 128 μg per mice (oropharyngeal aspiration) | Induced fibrinogen and factor VII levels, reduced TT, showed procoagulant activity in vitro | 75 |
| Carbon nanotube (long and short carboxyl-modified; long and short amine-modified) | Tube | Length: 926 and 223 nm (long and short carboxyl-modified nanotube, respectively) | Negative and positive | None | 0.005–0.16 mg mL−1 | Induced platelet activation and RBC damage by altering the cell's integrity | 254 |
| 945 and 266 nm (long and short amine-modified nanotube, respectively) | |||||||
| Carbon dots synthesized from Cirsium setosum Carbonisata extract | Nearly spherical | 2.6 ± 0.7 nm (TEM) | Not specified | None | High dose: 8.33 mg kg−1; medium dose: 3.33 mg kg−1; low dose: 1.67 mg kg−1 of mice (intraperitoneal injection) | Reduced bleeding time in vivo by activating fibrinogen and triggering the extrinsic pathway | 76 |
| Carbon dots | Round | 3 nm (TEM) | Not specified | None | 25–120 μM in vitro | Inhibited platelet activation and aggregation in vitro | 77 |
| 1 mg kg−1 of mice in vivo (IV route) | Decreased death rate of pulmonary thromboembolism-induced mice in vivo | ||||||
| Fullerenol C60(OH)24 | Spherical | 4.3 ± 0.2 (DLS) | Not specified | None | 100 μg mL−1 | Triggered TF expression on HUVECs in vitro | 80 |
| Fullerenol C60(OH)24 | Spherical | ∼1.3 nm in outer diameter (TEM) | Not specified | None | 100 μg mL−1 | No effect on platelets in vitro | 72 |
| Fullerenol | Not specified | 1.13 ± 0.32 nm (AFM) | Negative | None | 0.1, 0.5 and 1.0 mM in vitro and in vivo in rat (IV route) | Affected both extrinsic and intrinsic pathway, inhibited Xa and thrombin activity in vitro | 81 |
| Prolonged bleeding time and inhibited thrombosis in vivo | |||||||
| Silver | Spherical | ∼20 nm (TEM, DLS) | Not specified | Citrate | 2 and 4 mg L−1 | HUVECs increased permeability which is a main factor leading to endothelial dysfunction in vitro | 85 |
| Silver | Spherical | ∼20 nm (TEM, DLS) | Not specified | PEG | 125–625 μM | Reduced platelet adhesion and inhibited platelet aggregation in vitro | 90 |
| Silver | Spherical | 10–100 nm | Not specified | None | 10–250 μg mL−1in vitro | Induced platelet activation and aggregation in vitro | 88 |
| 0.05–0.1 mg kg−1 (IV route) or 5–10 mg kg−1 of rat (intratracheal instillation route) | Enhanced venous thrombus formation, platelet aggregation, and PS externalization in vivo | ||||||
| Silver | Spherical | 10–15 nm (TEM) | Not specified | Sodium polyacrylate | 30 mg L−1 | Triggered platelet activation, induced kallikrein-like, FXIIa-like, and thrombin-antithrombin III complex in vitro | 89 |
| 12 (DLS) | |||||||
| Silver | Spheroid | 16 (DLS) | Not specified | Polyvinyl pyrolidone (PVP) | 50 μg mL−1 | Promoted platelet adhesion and procoagulant effect in vitro | 91 |
| Silver | Spherical | ∼20 nm (TEM) | Negative | PVP or citrate | ∼500 μg mL−1 | No effect of platelet aggregation and coagulation in vitro | 92 |
| AgNP-PVP: 58.6 ± 2.4 nm (DLS) | At 530 μg mL−1, citrate-AgNPs showed prolonged coagulation time | ||||||
| AgNP-citrate: 26.6 ± 1.89 nm (DLS) | |||||||
| Silver | Spherical | ∼10–15 nm (TEM, SEM) | Not specified | Lignin | 0–60 μg mL−1 | Reduced platelet aggregation of PRP at 15 μg per 0.25 mL reaction in vitro | 93 |
| Silver | Spherical or nanowire | Spherical nanoparticles: 30 or 100 nm | Nanoparticles: negative | PVP | 50 and 150 μg mL−1 | Reduced RBC deformability by all AgNPs and silver nanowires. 30 nm-NPs reduced RBC deformability the most compared with 100 nm-NPs and nanowires in vitro | 94 |
| Nanowires: diameter of 40 nm; length of 1–2 μm | Nanowires: not specified | All silver nanomaterials reduced RBC aggregation at 150 μg mL−1. At 50 μg mL−1, 30 nm-NPs did not in vitro | |||||
| Silver | Spherical | 10–15 nm (TEM) | Not specified | Citrate | 0.5–50 μM in vitro | Antiplatelet property in vitro and in vivo | 87 |
| 2–8 mg kg−1 of mice (IV route) | |||||||
| Silver | Spherical | 13–45 nm | Not specified | D-Glucose | 0.05–5 μM | Prevented platelet adhesion and integrin-mediated platelet responses in vitro | 95 |
| Silver | Spherical | 2–3.7 nm (TEM) | Not specified | Reduced glutathione (GSH), polyethylene glycol (PEG) and lipoic acid (LA) | 12.5–100 μg mL−1 | Decreased the level of P-selectin, GPIIb/IIIa, TXB2, and the release of MMP-1, MMP-2 by AgNPs-LA at 100 μg mL−1 and AgNPs-GSH and AgNPs-PEG at 50 and 100 μg mL−1in vitro | 97 |
| No effect on endothelial cells and platelet viability in vitro | |||||||
| Silver synthesized from leaf and seed extracts of Synsepalum dulcificum | Fairly spherical | 5–26 nm (TEM) | Not specified | None | Use 0.5 mL of 150 μg mL−1 nanoparticles in 5 mL of blood | Caused dispersion of RBCs of the clot | 107 |
| Exerted anticoagulant and thrombolysis activities in vitro | |||||||
| Silver synthesized from nest extract of paper wasp (Polistes sp.) | Sphere, triangle, hexagon, rod, and rhombus | 12.5–95.55 nm (TEM) | Not specified | None | Use 0.5 mL of 150 μg mL−1 nanoparticles in 5 mL of blood | Exerted anticoagulant activities in vitro | 106 |
| Silver synthesized from extract of spider cobweb (CB), pod (KP), seed (KS) and seed shell (KSS) of kolanut (Cola nitida) | Nearly spherical | 3–80 nm (SAED) | Not specified | None | 100 μg mL−1 | Exerted anticoagulant activity in vitro | 105 |
| Silver synthesized from leaf extract of Petiveria alliacea (PA) | Nearly spherical | 16.70–33.74 nm (TEM) | Not specified | None | ∼167 μg mL−1 | Exhibited anticoagulant property similar to EDTA in vitro | 104 |
| Preserved RBC structure in vitro | |||||||
| Silver synthesized from Euphorbia acruensis | Closely spherical | 10–40 nm (TEM) | Not specified | None | 50 μg mL−1 | Showed thrombolytic activity in vitro | 103 |
| Silver synthesized from Pseudomonas aeruginosa | Spherical | 80 nm (DLS) | Not specified | None | 0.5% (v/v) | Displayed excellent anticoagulant activity in vitro | 102 |
| Silver synthesized from Gluconobacter roseus | Irregular shape | 10 nm (TEM) | Negative | None | 0.9–3.5 nM | Reduced platelet aggregation and showed anticoagulant effect in vitro | 101 |
| 68 nm (DLS) | |||||||
| Silver | Spherical | 6–16 nm (TEM) | Negative | Low-molecular-weight sulfoethyl chitosan | 0.1 mg mL−1 | Inhibited the activity of Xa in vitro | 100 |
| Silver | Triangular, truncated triangular, hexagon/elongated hexagon and spherical | 148 ± 9 nm to 610 ± 112 nm (DLS) | Negative | Heparin | 10 μM | Delayed coagulation time with the longest time caused by hexagonal nanoparticles in vitro | 109 |
| Silver and gold | Not specified | AgNPs: 10 nm | Not specified | Citrate | Low dose: 10 μg kg−1 day−1; high dose: 100 μg kg−1 day−1 of rat in vivo (IV route) | No effect on APTT and PT compared with blood only control | 96 |
| AuNPs: 12.8 nm (TEM) | |||||||
| Gold | Spherical | ∼20–70 nm (TEM) | Not specified | Citrate | 0–50 μM | 68 nm-nanoparticles were inert to platelets while ∼20 nm-nanoparticles exerted platelet activation in vitro | 111 |
| Gold | Nearly spherical | ∼30 nm (TEM) | Positive and negative | Citrate, 11-mercaptoundecanoic acid, or 11-mercaptoundecylamine | 50 μg mL−1 | No effect on platelets in vitro | 112 |
| Gold | Spherical | 20–50 nm (DLS) | Not specified | PEI or PVP | 1–10% | Induced platelet aggregation in vitro | 118 |
| Gold | Spherical, oval | 12–85 nm (TEM) | Negative | Citrate, PEG-thiol, protein corona (HFib), clopidogrel, or RGD | 1.2–5 nM | Nanoparticles with RGD coating exhibited procoagulant effect while those with PEG-thiol, clopidogrel, and HFib affected platelet adhesion, fibrin build-up, and finally prevented clot formation in vitro | 116 |
| Citrate-AuNPs had no effect at 1.2 nM while demonstrating pro-thrombogenic effect at 5 nM in vitro | |||||||
| Gold | Spherical | ∼18 nm (DLS) | Negative | Citrate | 5 μg mL−1 | No effect on platelet aggregation when measured by light aggregometry method but induced aggregation detected by QCM-D method in vitro | 120 |
| 16.5 ± 2 nm (TEM) | |||||||
| Gold | Spherical | 5–60 nm | Not specified | Citrate | 5–40 μM | No effect of platelet aggregation with nanoparticles <30 nm while inhibited platelet aggregation with nanoparticles >60 nm in vitro | 119 |
| Gold synthesized from earthworm extract | Spherical | 6.13 ± 2.13 nm (TEM) | Not specified | None | Involve 0.03% extract and 60 μM HAuCl4·3H2O | Reinforced the anticoagulant activity when combining with heparin (0.02 U mL−1) in vitro | 268 |
| Gold | Spherical, rodlike, hollow, core/shell silica/gold | ∼25–51 nm (not specified for rodlike) (DLS) | Negative | Monocarboxy (1-mercaptoundec-11-yl) hexaethylene glycol (OEG) | 0.8–3.3 nM | No significant effect on HUVECs | 117 |
| Iron carbide | Not specified | ∼30 nm (TEM) | Not specified | Carbon and/or PEG with different end groups including –CH3, –NH2, –COOH, -IgG, and -ProteinA-protected-IgG | 0.5–2 mg mL−1 | Platelet activation and reduced blood clotting time in vitro | 121 |
| PEGylation attenuated the observed effect on coagulation | |||||||
| Iron oxide | Not specified | Not specified | Not specified | PAA | 1–62 μg mL−1 | No effect on platelet activation and aggregation in vitro | 124 |
| Iron oxide | Spheroid-like | 72.6 ± 0.57 nm (TEM) | Not specified | None | 25–200 μg mL−1in vitro | Induced RBC aggregation and altered RBC rigidity by PS externalisation in vitro | 122 |
| 88.78 nm (DLS) | 12 mg Fe per kg of rat (IV route) | Caused RBC apoptosis in vivo | |||||
| Iron oxide | Spheroid-like | 5–6 nm (TEM) | Negative or positive | Hyaluronic acid, chitosan, or PAA | 4–1000 μg mL−1 | Iron oxide nanoparticles with chitosan and hyaluronic coating showed least effect on platelets, RBCs, and coagulation in vitro | 130 |
| ∼30 nm (DLS) | |||||||
| Iron oxide | Spherical | 60–70 nm (DLS) | Negative | Amorphous silica | 0.025–0.1 mg mL−1 | Induced platelet aggregation at dose >0.05 mg mL−1in vitro | 123 |
| Iron oxide | Irregular | 9.08 ± 1.48 nm (TEM) | Negative | Dextran | 0.008–1 mg mL−1 | No effect on platelets in vitro | 129 |
| 25.3 ± 0.97 nm (DLS) | |||||||
| Iron oxide | Not specified | Starch-iron oxide NP: 45 nm (DLS) | Not specified | Starch or citrate | 64–256 μM | Starch-iron oxide nanoparticles had no effect on platelets while those coated with citrate had antiplatelet effect in vitro | 125 |
| Citrate-iron oxide NP: 35 nm (DLS) | |||||||
| Iron oxide | Not specified | 68 ± 22 nm to 88 ± 30 nm (DLS) | Positive, negative, neutral | PVA (12 or 31 kDa) | 50–500 μg mL−1 | Inhibitory effect on platelet aggregation regardless of PVA charge and molecular weight in vitro | 126 |
| Caused fibrinogen conformation change in vitro | |||||||
| Iron oxide | Spherical | 57–62 nm (DLS) | Negative | Citrate | 75, 150, and 300 μM | Suppressed platelet aggregation in vitro | 127 |
| Iron oxide | Irregular shape | 150 nm (DLS) | Negative | Sodium alginate sulfate (SAS) | 0.01–10 mg mL−1 | Reduced PF4 concentration, and platelet activation, fibrinogen solidification. Prolonged coagulation time in vitro | 128 |
| Silica | Spherical | 58 nm (TEM) | Negative | None | 50 and 100 μg mL−1 | NO imbalance, HUVECs dysfunction in vitro | 137 |
| 106.33 ± 1.23 nm (DLS) | |||||||
| Silica | Near spherical | 58.11 ± 7.30 nm (TEM) | Negative | None | 1.8–16.2 mg kg−1 of rat (tracheal instillation route) | Increased CD31 expression, NO imbalance, increased coagulant factors (TF, vWF, FXa) and decreased anticoagulant factors (TFPI, antithrombin, t-PA) in vitro | 134 |
| Silica | Not specified | 16–310 nm | Not specified | None | 1000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nanoparticles per cell 000 nanoparticles per cell |
Exhibited procoagulatory effect on HUVECs in vitro | 252 |
| Silica | Not specified | 10–40 nm | Not specified | None | 0.001, 0.01, 0.2, 0.4 mg mL−1 | Enhanced FX activation and shortened coagulation time in vitro | 269 |
| Organically (organosilane derivatives) modified silica | Not specified | Non-PEGylated: 51 nm (DLS) | Negative | None or PEG | 50–350 μg mL−1 | Significant procoagulant effect in vitro except for highly PEGylated nanoparticles with poor procoagulant effect in vitro | 142 |
| Synthetic amorphous silica | PEGylated: 45 nm (DLS) | None | |||||
| 35 nm (DLS) | |||||||
| Amorphous silica | Spherical | 10–500 nm (TEM) | Negative | None | 10–200 μg mL−1 | Induced platelet activation and aggregation in vitro | 139 |
| Silica | Nearly spherical | 58.11 ± 7.30 nm (DLS) | Negative | None | 1.8–16.2 mg per kg bw of rat (intratracheal instillation route) | Increased endothelial dysfunction and pre-thrombotic state in vivo | 134 |
| Dye-labelled core/shell silica | Spherical | 245 ± 10.82 nm (TEM) | Negative | None | 10–250 μg mL−1 | Promoted platelet adhesion to endothelial cells in vitro | 140 |
| Silica | Spherical | 47.9 ± 7.1 nm (TEM) | Not specified | PEG | 20–1000 μg mL−1 | Slightly reduced platelet adhesion to endothelial cells and no effect to platelet aggregation at low dose (20–200 μg mL−1) in vitro | 141 |
| 66.8 ± 0.3 nm (DLS) | Significantly induced platelet adhesion and aggregation at high dose (1000 μg mL−1) in vitro | ||||||
| Silica | Spherical | 50 and 500 nm (TEM and DLS) | Negative | None | 0.2–5 μg mL−1in vitro | Induced platelet aggregation in vitro | 253 |
| 0.5 mg kg−1 of mice (intraperitoneal route) | Caused systemic coagulation events in vivo | ||||||
| Silica | Spherical | 70–1000 nm (DLS) | Not specified | None | 0.02 mg mL−1in vitro | 70 nm-NPs activated intrinsic pathway via the interaction with FXII in vitro | 48 |
| 100 mg kg−1 of mice (IV route) | 70 nm-NPs caused consumptive coagulopathy in vivo | ||||||
| Silica | Spherical | 30–1000 nm (DLS) | Not specified | None | 500 μg per mice (intranasal exposure) | 30 and 70 nm-NPs promoted abnormal activation of intrinsic coagulation in vivo | 132 |
| Silica | Spherical | 4–85 nm | Negative | None | 0.01–100 nM | NPs with 12–85 nm in size exhibited coagulant effect in vitro via the FXII distortion | 133 |
| Silica | Spherical | 53.79 ± 1.75 nm (DLS) | Negative | Polyphosphate (polyP) | 0.05–0.5 mg mL−1 | Induced thrombin generation and triggered contact pathway of coagulation cascade in vitro | 135 |
| Silica | Spherical or multi-facetted | 20 nm (TEM, SEM) | Negative in 0.9% saline | None | 20 mg kg−1 of rat (IV route) | Initiated extrinsic pathway, induced TF level, and might cause endothelial cells dysfunction in vivo | 136 |
| Silica | Spherical | 52.05 ± 8.38 nm (TEM) | Negative | None | 20 mg kg−1 of rat (IV route) | Caused prethrombotic and hypercoagulable state via induced platelet aggregation, platelet activation, hyperactivity of coagulation and resistance of fibrinolysis in vivo | 138 |
| Silica | Spherical | ∼80 nm except RMSN with 62 ± 12 nm (TEM) | Negative charge except A-MSN with positive charge | Functionalized with PEG, aminopropyl (A-MSN), methylphosphonate propyl (P-MSN), methyl (M-MSN), phenyl (Ph-MSN), mercaptopropyl (T-MSN), and Rhodamine B-propyl (R-MSN) | 0.1 and 1.0 mg mL−1 | Prolonged coagulation time by PEG-MSN, R-MSN, P-MSN, and bare MSN at 1.0 mg mL−1in vitro | 143 |
| Rutile titanium oxide | Rod | 4–6 nm (TEM) | Not specified | None | 0.4–10 μg mL−1in vitro | Triggered platelet aggregation in vitro and in vivo | 144 |
| 1 or 5 mg kg−1 of rat (intratracheal instillation route) | |||||||
| Rutile titanium oxide | Not specified | 67 nm (SEM) | Not specified | None | 1 mg kg−1 of mice (arterial catherization route) | No effect on platelets and hemodynamic parameters in vivo | 145 |
| 309 ± 38 nm (DLS) | |||||||
| Rutile titanium oxide | Needle-like | 10 × 40 nm (TEM) | Not specified | None | 0.1 mg mL−1in vitro | No effect on platelets in vitro and did not exert prothrombotic effect in vivo | 146 |
| 1 mg kg−1 of mice (arterial catherization route) | |||||||
| Titanium oxide synthesized from Alternaria solani | Irregular shapes (SEM) | ∼15 nm crystallite size (XRD) | Not specified | None | 50–100 μg mL−1 | Inhibited platelet aggregation and exhibited super antiplatelet and anticoagulant activities in vitro | 148 |
| Titanium oxide synthesized from extract of Cola nitida | Nearly spherical | 25.00–191.41 nm (TEM) | Not specified | None | 80 μg mL−1 | Prevented coagulation in vitro | 147 |
| Nickel | Spherical | 62 nm (SEM) | Not specified | None | 0.05 mg mL−1 | Changed platelet shape in vitro | 270 |
| Zinc oxide | Rectangular | 431 nm in 0.3 M glucose (DLS) | Negative | None | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio of platelet rich plasma 1 v/v ratio of platelet rich plasma |
Promoted platelet activation in vitro | 149 |
| Strong agglomeration and fast sedimentation in PBS-citrate | |||||||
| Zinc oxide | Spherical | 20 and 100 nm (DLS) | Positive and negative | Bare, citrate, and L-serine | 0.01–0.5 mg mL−1 | Increased APTT and PT regardless of size or surface coating of the nanoparticles in vitro | 150 |
| Zinc oxide | Spherical | Diameter: 20–250/50–350 nm (TEM) | Not specified | None | Cumulative dose: 32 or 64 μg per mice (oropharyngeal aspiration) | Induced factor VIII level and showed procoagulant activity in vitro | 75 |
| Hydroxyapatite | Rod-like (HAp1) and needle-shape (HAp2) | Rod-like: width ∼15–30 nm, length ∼40–70 nm | Not specified | None | 10 μg mL−1–10 mg mL−1 | No effect on platelet adhesion, aggregation, activation as well as both intrinsic and extrinsic pathway of coagulation system at 1–10 mg mL−1 | 271 |
| Needle-shape: 30–60 nm, length ∼200–500 nm (TEM) | Exhibited slight thrombogenic activity at 10 mg mL−1 by HAp2 | ||||||
| Interfered with vWF and CD31 expression in endothelial cells by HAp2 at 10 and 50 μg mL−1in vitro | |||||||
| Tungsten | Mostly spherical | 20 nm (TEM) | Not specified | None | 10–100 μg mL−1 | Prolonged clotting time at all concentration with the maximum effect at 40 μg mL−1in vitro | 272 |
| EMT-type zeolite | Cage-like | 10–20 nm (DLS) | Negative | None | 100 and 200 μg mL−1 | Exhibited high selective affinity to fibrinogen and inhibited the interaction between fibrinogen and β-amyloid (Aβ), decreasing the delay in clot dissolution in vitro in the presence of Aβ | 273 |
| Calcium carbonate (CaCO3) | Nearly spherical | 100 nm | Not specified | None | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio between NPs and whole blood in vitro 1 volume ratio between NPs and whole blood in vitro |
Caused rapid coagulation at pH 5.0 but no thrombus at pH 7.4 in vitro | 274 |
| 500 mg and 1000 mg per mice every three days for up to 15 days in vivo (topical) | Induced thrombus and fibrin clots in vivo | ||||||
| Cerium | Cubic crystallite structure | 5 and 40 nm (TEM) | Not specified | None | 0–50 μg mL−1 | No significant effect on platelet aggregation and coagulation | 275 |
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) SWCNT > MWCNT > SRM1648. The platelet aggregation induced by these carbon nanoparticles correlated to the activation of the GpIIb/IIIa receptors, platelet degranulation, translocation of P-selectin to the platelet surface, and the tendency to mimic molecular bridges in platelet–platelet interactions. The prothrombotic effect of carbon nanotubes regarding platelet activation and aggregation was further explored in studies by Simak's group.72 Their results were consistent with the previous study in which SWCNTs (outer diameter <2 nm, 5–15 μm in length for S1 SWCNT and 1–2 nm of outer diameter, 5–30 μm in length for S2 SWCNT) had higher platelet aggregation (34 ± 5% for S1 and 32 ± 6% for S2) than MWCNTs (outer diameter was 60–100 nm, 1–2 μm in length for M60 and 30 ± 15 nm of outer diameter, 1–5 μm in length for M30) with platelet aggregation of 27 ± 3% (M60) and 38 ± 9% (M30). Amorphous carbon nanopowder (ACN) (outer diameter was ∼30 nm) showed a weak effect on platelet aggregation (15 ± 2%). It was reported that the effects of carbon nanotubes on platelet activation, degranulation, and aggregation were accompanied by elevated intracellular [Ca2+] in platelets, which is the second key messenger-mediating platelet activation. Platelets raised intracellular [Ca2+] by either releasing it from intracellular stores or the entering of extracellular Ca2+ through plasma membrane channels, including store-operated Ca2+ entry (SOCE), second messenger-operated Ca2+ entry (SMOC), and receptor-operated Ca2+ entry (ROC).72 As the carbon nanotube-facilitated extracellular Ca2+ influx was sensitive to calcium entry blockers 2-APB and SKF 96365, SOCE was proved to be involved in platelet activation induced by carbon nanotubes.72,73 It was proposed that MWCNTs ruptured the dense tubular system, a Ca2+ pool after penetrating the instantly resealed platelet membrane, leading to intracellular Ca2+ depletion and activating SOCE.73
SWCNT > MWCNT > SRM1648. The platelet aggregation induced by these carbon nanoparticles correlated to the activation of the GpIIb/IIIa receptors, platelet degranulation, translocation of P-selectin to the platelet surface, and the tendency to mimic molecular bridges in platelet–platelet interactions. The prothrombotic effect of carbon nanotubes regarding platelet activation and aggregation was further explored in studies by Simak's group.72 Their results were consistent with the previous study in which SWCNTs (outer diameter <2 nm, 5–15 μm in length for S1 SWCNT and 1–2 nm of outer diameter, 5–30 μm in length for S2 SWCNT) had higher platelet aggregation (34 ± 5% for S1 and 32 ± 6% for S2) than MWCNTs (outer diameter was 60–100 nm, 1–2 μm in length for M60 and 30 ± 15 nm of outer diameter, 1–5 μm in length for M30) with platelet aggregation of 27 ± 3% (M60) and 38 ± 9% (M30). Amorphous carbon nanopowder (ACN) (outer diameter was ∼30 nm) showed a weak effect on platelet aggregation (15 ± 2%). It was reported that the effects of carbon nanotubes on platelet activation, degranulation, and aggregation were accompanied by elevated intracellular [Ca2+] in platelets, which is the second key messenger-mediating platelet activation. Platelets raised intracellular [Ca2+] by either releasing it from intracellular stores or the entering of extracellular Ca2+ through plasma membrane channels, including store-operated Ca2+ entry (SOCE), second messenger-operated Ca2+ entry (SMOC), and receptor-operated Ca2+ entry (ROC).72 As the carbon nanotube-facilitated extracellular Ca2+ influx was sensitive to calcium entry blockers 2-APB and SKF 96365, SOCE was proved to be involved in platelet activation induced by carbon nanotubes.72,73 It was proposed that MWCNTs ruptured the dense tubular system, a Ca2+ pool after penetrating the instantly resealed platelet membrane, leading to intracellular Ca2+ depletion and activating SOCE.73
Besides affecting platelets, carbon nanotubes and carbon dots also interfere with plasma factors of the coagulation system. By evaluating activated partial thromboplastin time (APTT) or partial thromboplastin time (PTT), Burke et al. concluded that, except for pluronic-coated pristine MWCNTs, all MWCNTs (pristine, carboxylated, or amidated; coated with pluronic F127 or distearoylphosphoethanolamine-(polyethylene glycol)-5000 (DSPE-PEG); range of diameter was 26–31 nm, median length was 490–580 nm) at the concentration of 100 μg mL−1 triggered the intrinsic pathway by preferentially interacting with FIXa and acting as a platform to promote its enzyme activity.74 It was revealed that the levels of fibrinogen and FVII were increased in vivo by carbon nanotubes (diameter was 6–20 nm; length: 700–4000 nm), demonstrating procoagulant activity.75 As reported by Luo et al., carbon dots synthesized from Cirsium setosum Carbonisata extract (∼2.6 nm) reduced the bleeding time in mice by triggering the extrinsic pathway and activating fibrinogen.76 However, there was a study presenting the anticoagulant activity of carbon dots synthesized from garlic (Allium sativum) extract through the reduced death rate of pulmonary thromboembolism-induced mice models.77 This was due to the ability to inhibit platelet activation by decreasing the phospholipase C/PKC and mitogen activated protein kinase (MAPK) activation.
Carbon nano-diamonds (CNDs) with the size range of 4–10 nm can evoke platelet activation at low concentration (1 μg ml−1).78 Kumari et al. demonstrated that CNDs elevated the intracellular Ca2+ level in platelets and increased the expression of phosphatidylserine on the platelet membrane. CND-treated platelets showed reduced viability and altered morphology with developed lamellipodia or filopodia. In vivo results evidenced extensive pulmonary thromboembolism in mice after intravenous (IV) injection of CNDs.78 Furthermore, nanodiamonds (100 nm) were found to greatly increase attraction forces between RBC membranes, leading to the formation of large and abnormal RBC aggregates79 (Fig. 6).
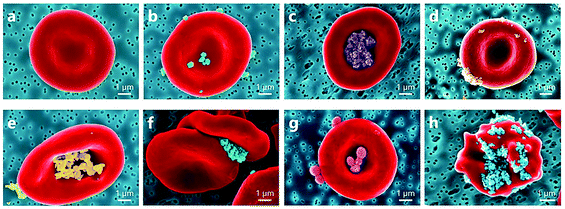 | ||
| Fig. 6 Coloured SEM images presenting a diversity of observed NP localizations on the RBC surface: (a) normal conditions; RBC incubated with (b) rutile-coated TiO2 nanoparticles, (c) alumina-polyol-coated TiO2 nanoparticles, (d) uncoated TiO2 nanoparticles (15 nm), (e) uncoated ZnO nanoparticles, (f) carboxylate nanodiamonds (100 nm), and (g) polymeric nanoparticles; (h) echinocyte form of RBC due to adhesion of carboxylate nanodiamonds. Among the interactions with inorganic nanoparticles, RBC incubation with nanodiamonds results in stronger RBC aggregation forces and influences the shape of RBCs.79 | ||
Gelderman et al. reported that fullerenol C60(OH)24 nanoparticles (∼4.3 nm) at 100 μg mL−1 significantly triggered the expression of TF (CD142) on human umbilical vein endothelial cells (HUVECs) for the extrinsic coagulation pathway after 24 h of in vitro culture (4 ± 2% CD142+ cells in control vs. 54 ± 20% CD142+ cells in treatment group).80 In contrast, fullerenol nanoparticles (∼1.13 ± 0.32 nm), at 0.5 and 1.0 mM, inhibited thrombin and FXa, thus delaying bleeding time in rats.81 At 0.1 mM concentration, the fullerenol nanoparticles had no effect. In another study, fullerenol C60 (∼1.3 nm) and fullerene C60 (∼0.7 nm) had no effect on platelets at the concentration of 100 μg mL−1.72
An in vivo study carried out by Singh et al. depicted an extreme thrombotic effect in mice after IV injection of atomically thin graphene oxide sheets (GO).82 As explored in in vitro tests, GO sheets triggered platelet aggregation through the intracellular release of Ca2+ and the activation of Src kinases. At the concentration of 2 μg mL−1, this effect of GO sheets was higher than that induced by 1 U mL−1 of thrombin. Continuing this study, Singh et al. discovered that amine-modified GO sheets (GO-NH2) (2 and 10 μg mL−1) did not show any induced or inhibitory effect on platelets, without noticeable change in the ROS level.83 There was no in vivo pulmonary thromboembolism after GO-NH2 exposure.
In summary, most carbon-based nanoparticles discussed in this section (Table 2) had either negative charge or charge not specified. Regardless of size and shape, all of them exhibited thrombogenic effects except pristine carbon nanotubes coated with pluronic F12774 or pre-treated with albumin,84 carbon dots synthesized from garlic (Allium sativum) extract,77 and most of the investigated fullerenol.72,81 Coating the nanoparticles with pluronic or albumin meant they were able to prevent or lessen the thrombogenic effect of carbon-based nanoparticles.74,84
Furthermore, various studies demonstrated the procoagulant and prothrombotic properties of AgNPs exhibited via the interaction with platelets. The accumulation of AgNPs within platelets can interfere with intra-platelet activities regardless of surface coating87–90 (Fig. 7). AgNPs, 10–100 nm in diameter, induced intracellular [Ca2+] (250 μg mL−1 of AgNPs), which upregulated GpIIb/IIIa (100 μg mL−1 of AgNPs) and P-selectin expression (100 μg mL−1 of AgNPs), and serotonin secretion (250 μg mL−1 of AgNPs).88 Enhanced thrombin and phosphatidylserine generation (250 μg mL−1 of AgNPs) were observed in fresh human platelets as evidence for platelet aggregation induced by AgNPs. Accumulated AgNPs (stabilized with sodium polyacrylate, 30 mg L−1, 10–15 nm) triggered α-granule secretion and induced kallikrein-like, FXIIa-like, and thrombin-antithrombin III complex.89 Further exposure of AgNPs in rats (0.05–0.1 mg kg−1 intravenous or 5–10 mg kg−1 intratracheal instillation) induced platelet aggregation, phosphatidylserine externalization, and vascular thrombus formation ex vivo.88,89 In another study, AgNPs (16 nm, coated with polyvinylpyrrolidone (PVP)) only promoted platelet adhesion but not platelet aggregation at the concentration of 50 μg mL−1 as compared with the control91 (Fig. 8). However, AgNPs (20 nm) with neither PVP coating nor citrate coating exerted any effect on platelet aggregation and coagulation at a concentration of up to ∼500 μg mL−1.92 The lignin capped AgNPs (∼10–15 nm) significantly reduced platelet aggregation of platelet rich plasma (PRP) at 15 μg per 0.25 mL.93 In addition to platelets, a study investigated the effect of AgNPs on RBCs and established that after 4 h of incubation, all silver nanomaterials functionalised with PVP (30 nm AgNPs, 100 nm AgNPs, and silver nanowires) exhibited a significant reduction in RBCs deformability at both 50 and 150 μg mL−1.94 AgNPs (30 nm) reduced RBC deformability the most compared with 100 nm AgNPs and silver nanowires. Unlike RBC deformability, all PVP coated silver nanomaterials decreased RBC aggregation at high concentration (150 μg mL−1) whilst 30 nm AgNPs had no effect on RBC aggregation at low concentration (50 μg mL−1).
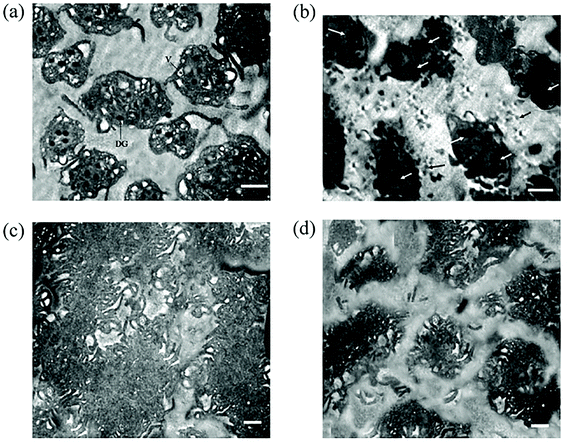 | ||
| Fig. 7 Electron micrographs through sections of activated and aggregated platelets with and without pre-treatment of AgNPs. (a) Intact platelets showing hyaloplasmic processes (pseudopods), dense granules with eccentric opacity, and vacuoles with limiting membrane. (b) Platelets pretreated with silver nanoparticles showed accumulation of AgNPs in vacuolar spaces (white arrow) with the absence of hyaloplasmic processes. Nanoparticle clusters (black arrow) are also seen in the surrounding microenvironment. (c) Electron micrograph demonstrating intimate adherence between the platelets during thrombus formation. Only occasional narrow spaces are visible between some cells. (d) AgNPs-pretreated platelets failed to aggregate and could only manage to form small, diffuse, and loosely packed clumps separated by wide distances.87 | ||
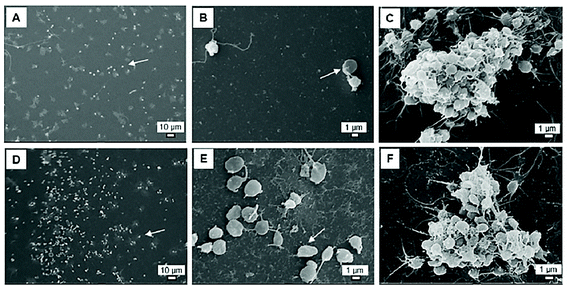 | ||
| Fig. 8 SEM pictures of platelet adhesion without (A–C) or with PVP-AgNPs at a final concentration of 50 μg mL−1 (D–F). Platelet aggregation was induced by arachidonic acid (C, F). Arrows indicate adherent platelets.91 | ||
By contrast, some other studies reported the antiplatelet properties of AgNPs (stabilised with either citrate or D-glucose).87,95,96 Accumulative AgNPs within platelet granules impeded integrin-mediated platelet responses such as adhesion to immobilized fibrinogen and platelet conformation change, namely retraction of a fibrin clot, in a concentration-dependent manner in vitro and in vivo, regardless of agonists used.87,95 AgNPs (stabilised with D-glucose) also inhibited platelet aggregation induced by either ADP, thrombin, or collagen in vitro and in mouse whole blood in a dose-dependent manner.95 AgNPs functionalized with lipoic acid, reduced glutathione (GSH) and polyethylene glycol (PEG) decreased aggregation of platelets by reducing the level of P-selectin, GPIIb/IIIa, TXB2, and the release of MMP-1, MMP-2.97 It was stated that platelet aggregation can be promoted by MMP-1 and MMP-2. While the mechanism of action of MMP-2 remained unclear, MMP-1 activates PAR1 on platelets.98 The MMP1-PAR1 obstruction can curtail thrombogenesis.98
Aside from platelets, plasma factors are also the target of AgNP interactions that leads to anticoagulant and antifibrinolytic effects. Several studies described the conformational change of fibrin through its interactions with AgNPs (either being coated with PEG or stabilised with citrate),87,90,99 which leads to the inhibition of fibrin polymerization and thrombus formation in vitro.99 Nevertheless, it is worth noting that this effect is less pronounced in plasma than in a purified system due to nonspecific interactions of AgNPs with other plasma proteins such as globulin and albumin. In another study, chitosan coated AgNPs showed inhibitory effects on FXa.100 Interestingly, almost all biogenic or green AgNPs exerted thrombolysis activity101–107 (Table 2). The proposed mechanisms are (1) green AgNPs may activate the conversion of plasminogen to plasmin which then dissolves the blood clot, or (2) directly targeting fibrin causes fibrin degradation as reported by Harish et al.108
To sum up, all reported biogenic AgNPs exhibited anticoagulant effects regardless of their physicochemical characteristics such as size, shape, and charge.101–107 Coating or stabilizing the non-biogenic AgNPs with PEG,90,97 citrate,87,92,96D-glucose,95 lignin,93 reduced glutathione (GSH),97 lipoic acid (LA),97 heparin,109 and low molecular weight sulfoethyl chitosan100 can prevent their procoagulant effects or even promote the anticoagulant activity (i.e. those with citrate coating). Coating of AgNPs with PVP exhibited inconsistent results. In some studies, PVP-AgNPs were shown to be compatible with the haemostatic balance with no effect on platelet aggregation and coagulation92 and decreased RBC aggregation.94 In contrast, PVP-AgNPs in other studies exhibited procoagulant, promoted platelet adhesion91 and reduced RBC deformability.94 These inconsistencies were not related to PVP-AgNPs size, shape, and concentration. Pristine non-biogenic AgNPs88 and AgNPs coated with sodium polyacrylate89 interfered and shifted the haemostatic balance to the thrombogenic side. In contrast, AgNPs coated with heparin shifted the haemostatic balance to the anticoagulant side, regardless of their shape.109
Fibrinogen can strongly bind to gold nanoparticles (stabilised with citrate) due to the presence of cysteine residues presented in alpha, beta, and gamma chains of fibrinogen, which allows Au–S bond formation and could induce blood clots.113 However, another study reported that fibrinogen bound on the surface of gold nanoparticles which were stabilised with citrate only increased the nanoparticle size but did not cause blood coagulation as in the above study.114 Deng et al. demonstrated that poly(acrylic acid) (PAA) conjugated on the surface of gold nanoparticles binds to fibrinogen and unfolds its conformation.115 Unexpectedly, gold nanoparticles functionalized with human fibrinogen (HFib), PEG-thiol, or clopidogrel on the surface, prevented fibrin build-up as well as cross-linking with platelets, thus disrupting clot formation.116
All AuNPs reviewed in this paper are provided in Table 2. Taken together, AuNPs coated with 11-mercaptoundecanoic acid,112 11-mercaptoundecylamine,112 PEG-thiol,116 HFib,116 clopidogrel,116 and monocarboxy (1-mercaptoundec-11-yl) hexaethylene glycol (OEG)117 had no effect on platelets,112,116 HUVECs,117 or coagulation,116 while those with polyethylenimine (PEI) or polyvinylpyrrolidone (PVP) coating induced platelet aggregation.118 Although there was a study reporting that citrate-coated gold nanoparticles could interact with fibrinogen and induce blood clot,113 most citrate-coated AuNPs reviewed in this section had no thrombotic effect,96,111,112,114,116,119 except for 20 nm-citrate coated AuNPs111 and 12 to 85 nm-citrate coated AuNPs at high dose (5 nM).116 These results implied that the size and dose of nanoparticles influence the effect of AuNPs on the haemostatic balance in addition to surface functionalisation. Analysis methods also had impact as well. In Santos-Martinez et al.'s study, induced platelet aggregation by citrate coated AuNPs was detected by quartz crystal microbalance with dissipation (QCM-D), while no aggregation was observed by the light aggregometry method.120 Indeed, most investigated AuNPs were spherical in shape. Hence, further investigations for the effect of other shapes of gold nanoparticles, such as rod, cage, star, triangle, hexagonal, were highly needed.
In contrast, PEGylation of iron carbide nanoparticles attenuated the influence of the nano-magnets on haemostatic components. No significant effect was observed at a concentration of 0.5 mg mL−1.121 In other comparable studies, starch-coated IONPs (45 nm, 128–256 μM)125 and dextran-stabilised IONPs (25.3 ± 0.97 nm, 0.008–1 mg mL−1)129 did not exert any effect on platelet function.
However, Deb et al. indicated that citric acid-stabilised iron oxide nanoparticles (FeNP(C)) (35 nm, tested concentration range was 64, 128, 192, and 256 μM) had antiplatelet properties, which was higher than what citric acid has by itself, as reflected in various molecular events including ATP release of dense granules, the level of tyrosine phosphorylation, and the expression of GpIIb/IIIa and CD62P (P-selectin).125 In another study, IONPs stabilized with citrate (∼57–62 nm) also diminished platelet aggregation.127 In addition, poly(acrylic acid)-coated IONPs presented no effect on platelet activation and aggregation, even up to 62 μg mL−1 of the nanoparticles.124 Polyvinyl alcohol (PVA) coated IONPs showed antiplatelet effects regardless of PVA charge and molecular weight.126 It was demonstrated that PVA-IONPs affected and changed fibrinogen confirmation, which disrupts the bridging between fibrinogen and platelets. Moreover, sodium alginate sulfate (SAS) coated IONPs caused fibrinogen aggregation and solidification as well as reduced PF4 concentration, which was probably due to the excessive presence of sulfonic acid in SAS. These effects led to diminished platelet activation and prolonged clotting time.128
To conclude, IONPs were mostly coated or stabilised with other materials. Coating IONPs with PEG,121 hyaluronic acid,130 chitosan,130 dextran,129 starch,125 PVA,126 and citrate127 attenuated or prevented their effect on the haemostatic balance. PAA-coated IONPs showed stronger effect on platelets, RBCs, and coagulation compared with those with hyaluronic acid (HA) and chitosan, indicating that HA and chitosan were safer coating materials for IONPs.130 In contrast, coating IONPs with SAS128 or citrate125 can lead to anticoagulant effects. Bare,122 silica coated,123 or carbon coated IONPs121 exerted prothrombotic effects.
Silica nanoparticles, as demonstrated by Feng et al., caused hypercoagulation through inducing vascular endothelial cells dysfunction.134 The increased expression of TFs and platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31), as well as the imbalance of the NO/NOS (nitric oxide synthase) system, were detected after the exposure to silica nanoparticles (starting from 1.8 mg kg−1 in rats). Correspondingly, silica nanoparticles (58 nm), especially at high concentrations of 50 and 100 μg mL−1, interrupted the NO balance, leading to HUVECs dysfunction.137 In another study, silica nanoparticles (52.05 ± 8.38 nm, IV dose: 20 mg kg−1) induced platelet activation and aggregation, coagulation hyperactivity, and fibrinolysis resistance, causing prethrombotic and hypercoagulable state in rats.138 Anionic amorphous silica nanoparticles (SiNPs) (10–500 nm) with concentrations varying from 10 to 200 μg mL−1 were reported to induce platelet activation and aggregation, accompanied with GpIIb/IIIa and CD62P upregulation.139 Since the thrombotic activity of SiNPs was hindered by inhibitors of ADP and the matrix metalloproteinase-2 (MMP2) pathway, the author discussed that the nanoparticles interacted with Ca2+ ion channels and resulted in extracellular Ca2+ influx into platelets cytoplasm, leading to the activation of endothelial NOS (eNOS) for NO generation. After the substrate (L-arginine) is used up, eNOS is uncoupled, and superoxide is produced to interact with NO to form ONOO− (peroxynitrite anion). Low ratio of NO/ONOO− is a marker of oxidative stress and diminished NO− availability, which promotes platelet activation. In other studies, silica nanoparticles (around 58 and 245 nm) were reported to enhance the expression of PECAM-1 (starting from 1.8 mg per kg bw of rat), resulting in NO/NOS system imbalance (>1.8 mg per kg bw of rat), and increase in platelet number on endothelial cells (both 10 μg mL−1 and 250 μg mL−1), promoting platelet adhesion and pre-thrombotic state.134,140 Such phenomena is in contrast with another study where PEGylated silica nanoparticles at 20–200 μg mL−1 led to a decrease in adhered platelet number compared with the control and the treatment group at higher concentrations of the nanoparticles (∼1000 μg mL−1).141 The differences between the two studies might be attributed to the PEG coating, porosity, size (50 nm vs. 250 nm), and the fabrication method (Stöber versus mesoporous silica nanoparticles) of the particles.
As explored in a study by Tavano et al., PEGylated organically modified silica nanoparticles (PEG-ORMOSIL) had poor procoagulant activity thanks to a thick superficial PEG coating (accounts for ∼37% w/w of the nanoparticles).142 By contrast, both synthetic amorphous silica nanoparticles (SAS-NPs) and bare ORMOSIL had appreciable procoagulant effect. In another study, not only PEG coated mesoporous silica nanoparticle (MSN), but also phenyl coated MSN, Rhodamine B coated MSN, and bare MSN led to delayed coagulation time in vitro at 1.0 mg mL−1.143
In conclusion, all investigated silica nanoparticles exerted profound prothrombotic effects on the haemostatic balance, except for those with PEG coating141–143 and phenyl surface functionalisation.143
As described in some studies, rectangular zinc oxide nanoparticles (ZnO NPs) caused procoagulant effects by either promoting platelet activation149 or inducing FVIII in the coagulation cascade.75 However, spherical ZnO NPs (bare, citrate, and L-serine coating) suppressed thrombin generation and absorbed coagulation clotting factors that led to prolonged coagulation time in another study.150 Layered double hydroxide nanoparticles (MgAl-Cl-LDH) had no effect on HUVECs at concentrations up to 10 μg mL−1.151 Fibre-like gadolinium oxide (Gd2O3) nanoparticles (diameter: 13.7 ± 6 nm, length: 54.8 ± 29 nm) were both apoptotic and necrotic to HUVECs after 48 h of exposure (IC50 = 304 ± 17 μg mL−1).152 The effects of other types of nanoparticles such as nickel, cerium, tungsten, and hydroxyapatite toward haemostatic balance are presented in Table 2.
3.2 Organic nanoparticles
Physicochemical characteristics of all nanomaterials discussed in this section are detailed in Table 3.| Nanoparticle | Shape | Size | Charge | Coating/stabiliser | Concentration tested | Main finding | Ref. |
|---|---|---|---|---|---|---|---|
| PAMAM dendrimer (G4, G7) | Not specified | 3.4 ± 0.22 nm (G4) (DLS) | Positive | None | Dose >10 mg kg−1 of mice (IV route) | Caused disseminated intravascular coagulation-like manifestation in vivo | 153 |
| 8.1 ± 0.42 nm (G7) (DLS) | |||||||
| PAPAM dendrimer (G3–G6) | Globular | ∼3–8 nm (DLS) | Positive, negative, or neutral | Succinamic acid, amidoethanol, or amine surface functionalization | 1.563–100 μg mL−1 | Induced platelet aggregation by large and cationic PAMAM dendrimer in vitro | 154 |
| PAMAM dendrimer (G7) (amine-modified) | Not specified | 8.1 ± 0.42 nm (DLS) | Positive | None | 100 μg mL−1 | Changed platelet shape, induced platelet activation and aggregation in vitro | 155 |
| Triazine dendrimer (amine-modified) | Not specified | 3.7–13.7 nm | Positive | None | 0.01–1 μM | No appreciable effect on platelet by low generation triazine dendrimer but promoted platelet aggregation with larger generation in vitro | 157 |
| Liposome | Spherical | 109 and 139 nm (DLS) | Negative | PEG | 12.5–400 μg mL−1 | No effect on HUVECs in vitro | 276 |
| Liposome (phosphatidylglycerol, egg phosphatidylcholine, cholesterol) | Spherical | Not specified | Negative | None | 25 mg kg−1 of rat (IV route) | Induced platelet aggregation (reduced platelet number) after the first 5 min of injection. The platelet count was recovered after 60 min post-injection in vivo | 160 |
| Liposome (phosphatidylcholine, phosphatidic acid) | Spherical | Not specified | Negative | None | 0.1–0.4 mL of stock suspension/2 × 105 platelet in vitro | Provoked platelet aggregation in vitro and in vivo | 161 |
| 2 mL kg−1 of Guinea pig per h for 1 h in vivo | |||||||
| Liposome (photopolymerizable phosphatidylcholine derivative) | Spherical | Not specified | Positive, negative, or neutral | None | 100–360 μg per 0.5 mL platelet | Inhibited platelet activation and aggregation in vitro by positive and negatively charged liposomes | 159 |
| Cetyl alcohol/polysorbate | Not specified | Bare NPs: 67.0 ± 17.5 nm (DLS) | Negative | None or PEG | 1–1000 μg mL−1 | Inhibited agonist-induced platelet activation and aggregation | 158 |
| PEGylated NPs: 67.0 ± 11.7 nm (DLS) | Prolonged whole blood clotting time at the concentration >500 μg mL−1 of bare NPs in vitro | ||||||
| Polystyrene latex (pristine, amine- and carboxyl-modified) | Spherical | 50–100 nm (TEM) | Positive, negative, or neutral | None | 15–60 μg mL−1 | Caused platelet aggregation except for the 50 nm amine-modified NPs in vitro | 163 |
| Polystyrene (unmodified, aminated-modified, carboxyl-modified) | Spherical | ∼60–80 nm in buffer containing 0.35% plasma (DLS) | Negative | None | 260 μg mL−1 | Induced platelet aggregation by both carboxyl-modified and aminated-modified polystyrene nanoparticles but not by the unmodified one in vitro | 164 |
| Polystyrene | Not specified | 171.1 ± 3.0 nm (DLS) | Negative | None | NPs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RBCs ratio = 200 RBCs ratio = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1000 1 and 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Reduced RBC deformability and increased PS exposure in vitro | 165 |
| Polystyrene (amine- and carboxyl-modified) | Spherical | Amine-polystyrene: 57.1 and 284 nm (DLS) | Positive or negative | None | 0.5 mg mL−1 | Amine-modified polystyrene NPs bound to FVII, FIX and inhibited thrombin formation | 166 |
| Carboxyl-polystyrene: 27.8 or 223.9 nm (DLS) | Carboxyl-modified polystyrene NPs triggered intrinsic pathway in vitro | ||||||
| Polystyrene | Sphere | Outer diameter: 20 and 200 nm | Not specified | None | 100 μg mL−1 | No effect on platelets in vitro | 72 |
| Lysozyme-dextran | Not specified | 268.2 ± 9.6 nm (DLS) | Negative | None | NPs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RBCs ratio = 1000 RBCs ratio = 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
No effect on RBC in vitro | 165 |
| mPEG-PLA | Not specified | ∼20 nm (DLS) | Not specified | PEG | 12.5–200 μg mL−1 | No effect on HUVECs in vitro | 167 |
| PLA | Spherical | 77.1 ± 4.6 nm to 105.6 ± 3.1 nm (DLS) | Varied | DSPE-PEG (2000) methoxy-terminated, carboxylic acid-terminated, and amino-terminated | 350, 700, and 1400 μg mL−1 | Inhibited P-selectin expression and platelet aggregation in vitro | 277 |
| PLGA | Spherical | ∼420 nm (DLS) | Positive (PEI coating) and negative (BSA coating) | PEI or BSA (bovine serum albumin) | 10–150 μg mL−1 | Impeded on adhesion by NPs with PEI coating but not vWF secretion of endothelial cells in vitro | 278 |
| PLGA | Spherical | 100–500 nm (DLS) | Negative and positive | None | 0.1–500 μg mL−1 | No effect on platelet in vitro | 168 |
| PLGA-macrogol | |||||||
| Chitosan-PLGA | |||||||
| PLGA, Chitosan, and chitosan-PLGA | Spherical | ∼350–800 nm (DLS) | Negative and positive | None | 0.01–100 μg mL−1 | Slightly inhibited platelet aggregation in vitro | 169 |
| PLGA | Not specified | ∼20–100 nm (DLS) | Varied | Bare or functionalized with G2SN dendron or 8D3 antibody | 0.75–3 mg mL−1 | PLGA NPs functionalized with G2SN led to aggregation between fibrinogen and the nanoparticles which led to anticoagulant effect at high concentration (3 mg mL−1) | 170 |
| No effect on coagulation cascade at 1 mg mL−1in vitro | |||||||
| PLGA | Not specified | 113, 321, and 585 nm (DLS) | Not specified | PEG | 0–2.2 mg mL−1 | Reduced platelet aggregation by large nanoparticles (321 and 585 nm) at ≥0.25 mg mL−1in vitro | 279 |
| Chitosan-fucoidan | Spherical | ∼200 nm (DLS) | Positive | None | 0–30 μg mL−1in vitro | Nanoparticles with 1% glutaraldehyde crosslink inhibited highest clot formation in vitro and antithrombotic effect in vivo | 280 |
| 50 mg kg−1 of rat in vivo (oral administration) | |||||||
| Chitosan-fucoidan | Spherical or oval | 198.00 ± 38.84 to 341.70 ± 200.00 nm (DLS) | Positive | None | 0.53–13.3 μg mL−1 | Interfered with the intrinsic pathway and exhibited anticoagulant effect in vitro | 281 |
| Chitosan-acetylsalicylic acid (ASA) | Almost spherical | ∼79.3 ± 24.6 nm (DLS) | Positive | None | 0.25–1 g kg−1 of rat (gastrointestinal perfusion) | Delayed occlusion time in carotid artery thrombosis model in vivo | 282 |
| Polyurethane ionomer | Spherical | 234 nm (DLS) | Slightly negative | None | 0–25 mg mL−1 | Possessed anticoagulant activity in vitro | 171 |
| Polyester | Spherical | 5 and 50 nm (TEM) | Negative | Sulfonic acid | 0.1, 1, 10, 20 mg mL−1 | Inhibited intrinsic and thrombin activity but not extrinsic pathway in vitro | 283 |
| No interference with RBC's morphology in vitro |
In summary, the effect of dendrimer nanoparticles was highly dependent on their surface charge and generation. Large and cationic PAMAM (≥G4) and large generation of triazine dendrimer (G5 and G7) were prothrombotic. Moreover, PAMAM dendrimers exhibited more profound effects on the haemostatic balance than triazine dendrimers.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphatidic acid = 8
phosphatidic acid = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), not cationic and neutral liposomes, provoked platelet aggregation in vitro and in vivo after IV injection in Guinea pigs.161 The effect was probably due to the interaction between anionic liposomes and FXIII/XI. Moreover, Constantinescu et al. suggested that the interaction of liposomes with platelets was independent of opsonisation but dependent on the liposome concentration.162 The discrepancies between studies might be attributed to not only the surface charge but also the composition of the lipid-based nanoparticles.
1), not cationic and neutral liposomes, provoked platelet aggregation in vitro and in vivo after IV injection in Guinea pigs.161 The effect was probably due to the interaction between anionic liposomes and FXIII/XI. Moreover, Constantinescu et al. suggested that the interaction of liposomes with platelets was independent of opsonisation but dependent on the liposome concentration.162 The discrepancies between studies might be attributed to not only the surface charge but also the composition of the lipid-based nanoparticles.
Overall, all lipid-based nanoparticles (without or with PEG coating) had no effect or inhibited thrombotic effect, except for those reported in Reinish et al. and Zbinden et al. studies as discussed above.160,161
Pan et al. demonstrated that the absorption of polystyrene nanoparticles (PSNPs) on murine RBCs significantly reduced RBC deformability as a function of elongation index (EI) value at both sizes (200 nm and 300 nm), as well as both low and high nanoparticle![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RBC ratios (200
RBC ratios (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1000
1 and 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).165 Moreover, PSNPs exerted mechanical, oxidative, and osmotic stresses on murine RBCs.165 As a result, the proportion of RBCs expressing PS increased up to 87% and 92% for low and high nanoparticles
1).165 Moreover, PSNPs exerted mechanical, oxidative, and osmotic stresses on murine RBCs.165 As a result, the proportion of RBCs expressing PS increased up to 87% and 92% for low and high nanoparticles![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RBCs loading ratios, respectively, in comparison with only 0.1% of RBCs and 0.3% of lysozyme-dextran nanogels loading for the control.
RBCs loading ratios, respectively, in comparison with only 0.1% of RBCs and 0.3% of lysozyme-dextran nanogels loading for the control.
Oslakovic et al. reported that amine-modified polystyrene nanoparticles (57.1 and 284 nm in size, 0.5 mg mL−1) bound to FVII and IX inhibited thrombin formation due to the depletion of these coagulation factors in solution.166 Meanwhile, carboxyl-modified polystyrene nanoparticles (27.8 or 223.9 nm in size, 0.5 mg mL−1) act as an active surface to trigger intrinsic pathways.
Inclusively, most examined polystyrene showed procoagulant effects except for the following: 50 nm amine-modified,163 57 and 284 nm amine-modified,166 20 and 200 nm unmodified,72 and ∼73 nm unmodified164 polystyrene nanoparticles (Table 3). There was no physicochemical characteristic that was more important than others in dictating polystyrene nanoparticles’ effect on the haemostatic balance. Their effects could be associated with all parameters along with treatment dose and time.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RBC ratio of 1000
RBC ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1165 and PLGA nanoparticles (bare or functionalized with G2SN dendron or 8D3 antibody) had no effect on coagulation cascade even at 1 mg mL−1in vitro.170
1165 and PLGA nanoparticles (bare or functionalized with G2SN dendron or 8D3 antibody) had no effect on coagulation cascade even at 1 mg mL−1in vitro.170
Besides polymeric nanoparticles having no effect on haemostatic balance and polystyrene nanoparticles that had been specifically discussed above, most of the reported polymeric nanoparticles shift the haemostatic balance to the tendency that reduces procoagulant effects or causes anticoagulant effect (Table 3). For example, PLGA, CS, and PLGA-CS nanoparticles (0.01–100 μg mL−1) had slight inhibitory effects toward platelet aggregation induced by collagen.169 This could be due to the reduced platelet–platelet interaction and/or reduced adsorption of platelets onto collagen fibers. In a study by Mao et al., polyurethane ionomer nanoparticles showed antithrombogenic activity, which could be attributed to its ionomer structure.171 Nevertheless, it is important to note that a wide variety of polymeric nanoparticles have not yet been comprehensively investigated.
4. Effects of nanoparticles on innate immune system in correlation with haemostatic balance
In addition to blood components of the haemostatic networks, nanoparticles in the bloodstream also encounter and affect the immune system, specifically the innate immunity, which could potentially interfere with the haemostatic balance.4.1 Immunothrombosis – effect of innate immunity on haemostatic balance
Recently, influences of innate immunity on the haemostasis balance have been increasingly evidenced.172,173 This relationship was formalised as immunothrombosis, whereby the innate immune system attributes to thrombosis and vice versa, the coagulation activation supports the function of the immunity.174 As far as our knowledge is concerned, the innate immunity regulates haemostasis through the action of leukocytes and complement system.172,173,175Under normal physiological conditions, quiescent leukocytes, namely monocytes, endorse the haemostasis balance by expressing anticoagulant mediators and proteins such as EPCR, TFPI, and TM.172 However, activated leukocytes in apoptotic or proinflammatory conditions (e.g. exposure to foreign agents) will provoke blood coagulation through 3 mechanisms. (1) The first mechanism is expressing or secreting procoagulant factors. For instance, TFs expressed by activated monocytes176,177 and neutrophils178–180 could mediate thrombin generation. MMP, elastase, and cathepsin G secreted by stimulated neutrophils and monocytes trigger the activation of FV, FVIII, and FX181–183 and degrade anticoagulant factors like AT, heparin cofactor II, and TFPI.184–188 Nuclear damage-associated molecular patterns (DAMPs) (i.e. cell-free DNA, high mobility group box 1 (HMGB1), and extracellular histone) secreted by activated/apoptotic leukocytes activate FXI and FXII, mediate thrombin generation, and impair the protein C pathway.189–193 Moreover, neutrophil extracellular traps (NETs) released by neutrophils, monocytes/macrophages, and mast cells can concentrate procoagulant factors (i.e. vWF, TF, fibrinogen, fibronectin, HMGB1, elastase, cathepsin G).179,194,195 (2) The second mechanism is inducing changes in cellular components of coagulation systems (i.e. endothelial cells, platelets, and RBCs). For example, leukocyte-released cytokines such as TNF-α and IL-1β induce EPCR shedding196 and reduce TM expression197 on endothelial cells. Cytokines, histamine, granular enzymes, and DAMPs (i.e. histone and HMGB1) promote endothelium TF activity193,198,199 and exocytosis of Weibel-Palade bodies, thus enhancing the release of P-selectin and/or vWF.200–202 For platelets, it was reported that leukocyte-released cytokines and neutrophil generated oxidants (e.g. HOCl) increased circulating ultra-large vWF multimers,201,203 while NETs facilitated the capture of platelets by vWF.200 Elastase, cathepsin G, extracellular histone, and PAF released by stimulated leukocytes act as platelet activators which induce platelet activation and aggregation.204–208 Extracellular histone also can induce the PS exposure on RBCs.209 (3) The last mechanism is obstructing microcirculation. Activated platelets can interact with leukocytes forming heterotypic leukocyte–platelet aggregates.208,210,211 Platelet activation by extracellular histone provokes the leukocyte–platelet aggregation formation as well.208,211 Besides, platelet-rich microthrombi can be built up by extracellular histone in vivo.207 In addition to regulating coagulation induction, leukocytes also mediate fibrinolysis, thrombus resolution, and coagulation factor clearance.172
A complement system, which is genetically developed from a serine protease reaction cascade evolved from the same ancestor gene as coagulation factors, plays a significant role in haemostasis.173,175 Regardless of activation path, C5b-9 as a membrane attach complex (MAC) or terminal complement complex (TCC) causes platelet activation through the induction of platelet transient membrane depolarisation, PS expression, granule secretion, and thrombin generation.212–215 The combination of MAC and C3 triggers serotonin secretion and thrombin-mediated platelet aggregation.216,217 C3 itself also affects platelet activation.218,219 Anaphylatoxin C3a promotes the activation and aggregation of platelets,220 while anaphylatoxin C5a provokes TF expression on leukocytes221 and promotes procoagulant effects on mast cells.222,223 C5a and MAC prompt neutrophils and endothelial cells for the activation and TF expression, activating the extrinsic pathway of the coagulation cascade.224,225 Activated mannose-binding protein-associated serine protease 2 (MASP-2) is involved in thrombin activation and fibrin generation, whilst MASP-1 boosts fibrin-crosslinking and the cleavage of fibrinogen,226 FXIII, and thrombin activatable fibrinolysis inhibitor (TAFI).227,228 Platelet rolling and platelet activation was caused when C1q interacts with vWF and binds to gC1qR or gC1qR/p33 on platelets, respectively, which could eventually lead to platelet aggregation.229–232 C1 inhibitor inhibits the activity of FXIIa, FXIa, and kallikrein of the coagulation system.233–235 C4b binding protein interferes with protein S of the anticoagulant system.236
4.2 Effect of nanoparticles on innate immunity potentially linked to haemostasis
To the best of our knowledge, the effect of nanoparticles on innate immune systems that directly mediate the haemostatic balance has not been investigated yet. Nevertheless, there were several studies demonstrating the effect of nanoparticles on the immunity, which could potentially interfere with the haemostatic balance, concerning the intrinsic link between the innate immunity and haemostasis. For instance, long needle-like carbon nanotubse (trade name: Mitsui MWCNT-7, outer diameter >50 nm, length ∼13 μm) induced the release of IL-1R and activated the release of IL-1β from bacterial lipopolysaccharide (LPS)-primed human monocyte-derived macrophages at the tested concentration of 100 μg mL−1.237 MWCNTs (diameter: 20–30 nm, length: 10–30 nm) dispersed in serum bovine albumin caused vigorous IL-1β secretion from THP-1 cells in vitro (tested concentrations of 10, 25, 50, and 100 μg mL−1) and in vivo (12.5–100 μg per mice).238 Chowdhury et al. reported that dextran-coated graphene nanoplatelets (60–100 nm) at concentration ≥7 mg mL−1 caused 12–20% increments in complement protein levels.239 However, the level of TNF-α was retained in the normal range and the nanoplatelets did not induce platelet activation. In another study, the secretion of IL-1β and TNF-α by THP-1 cells was induced in a bell-shaped distribution manner after being treated with silica nanoparticles (12.5–200 μg mL−1) of different sizes (10–1000 nm), where the maximum effect was recorded for the 50 nm-nanoparticles and the lesser effect was for those with larger or smaller sizes.240 It was presented that mannan-binding lectin bound to the dextran coating of dextran-coated superparamagnetic iron oxide nanoparticles (Dex-SPIONs) (50 nm, 200 μg per 300 μL mouse plasma).241 Histidine-rich glycoprotein and kininogen tended to bind to the exposed iron oxide part while complement lectin and clotting factors (kininogen, kallikrein, FXI, and FXII) were the secondary binders. Although the bound clotting factors could potentially activate the coagulation intrinsic pathway, the systemic administration of SPIONs was not adequate to promote the clotting in another study examined by the same group.242 Only SPIONs coated with carboxymethyldextran (ferucarbotran, Resosvist®) and dextran (ferumoxtran-10, Sinerem®) significantly activated the complement system, while those with a citric acid (FluidMAG-CT), phosphatidylcholine (FluidMAG-Lipid), starch (FluidMAG-D), and chitosan (FluidMAG-Chitosan) coating did not when incubating nanoparticles with serum at volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.243 Moreover, coating of SPION with human serum albumin or dextran decreased the NETs formation by neutrophils compared with pristine and unstable lauric acid-coated SPIONs (55.8–127.9 nm), both in vitro (200 μg mL−1) and in vivo (500 μg nanoparticles per rabbit).244 Gold nanoparticles (10–100 nm at doses of 2.5–10 μg mL−1) synergised with LPS also induced the release of NETs from human neutrophils.245 For liposomes, those ∼100 nm in size (5–40 mg mL−1) did not interfere with the complement system, while the micro-multilamellar liposomes did.246 Among liposomes with ∼100 nm, negatively charged liposomes containing phosphatidylglycerol, phosphatidic acid, cardiolipin, phosphatidylinositol, or phosphatidylserine activated classical complement pathways whilst positively charged liposomes containing 1,2-bis(oleoyloxy)-3-(trirnethylammonio)propane or stearylamine activated alternative complement pathways, which was in contrast to neutral liposomes.247 C9 and C3b were associated with these liposomes. The C3 opsonization on nanoparticles not only raises the potential risk of haemostatic imbalance but also targets the nanoparticles for reticuloendothelial system (RES) uptake, triggers toxin release, and may likely foul the targeting agents conjugated on nanoparticles.248 Coating the nanoparticles with PEG can reduce C3b-nanoparticle adduct formation. Another approach is infusing complement inhibitors with nanoparticles.242,249
4.243 Moreover, coating of SPION with human serum albumin or dextran decreased the NETs formation by neutrophils compared with pristine and unstable lauric acid-coated SPIONs (55.8–127.9 nm), both in vitro (200 μg mL−1) and in vivo (500 μg nanoparticles per rabbit).244 Gold nanoparticles (10–100 nm at doses of 2.5–10 μg mL−1) synergised with LPS also induced the release of NETs from human neutrophils.245 For liposomes, those ∼100 nm in size (5–40 mg mL−1) did not interfere with the complement system, while the micro-multilamellar liposomes did.246 Among liposomes with ∼100 nm, negatively charged liposomes containing phosphatidylglycerol, phosphatidic acid, cardiolipin, phosphatidylinositol, or phosphatidylserine activated classical complement pathways whilst positively charged liposomes containing 1,2-bis(oleoyloxy)-3-(trirnethylammonio)propane or stearylamine activated alternative complement pathways, which was in contrast to neutral liposomes.247 C9 and C3b were associated with these liposomes. The C3 opsonization on nanoparticles not only raises the potential risk of haemostatic imbalance but also targets the nanoparticles for reticuloendothelial system (RES) uptake, triggers toxin release, and may likely foul the targeting agents conjugated on nanoparticles.248 Coating the nanoparticles with PEG can reduce C3b-nanoparticle adduct formation. Another approach is infusing complement inhibitors with nanoparticles.242,249
5. Physicochemical characteristics affecting interactions between nanoparticles and haemostatic balance
It is important to note that not every nanoparticle is exactly the same. Changes in any nanoparticle's physicochemical characteristics, such as size, shape, surface charge, and coating materials, might correlate with different ways of interaction and lead to alternative effects on haemostatic balance.5.1 Effect of size
The size of a nanoparticle is a critical aspect that significantly affects its biodistribution, circulation and clearance in the body.250 Additionally, size can also impact their interfacial area for blood–nanoparticle and cell–nanoparticle communication.251 Generally, small nanoparticles exhibit larger surface area to volume ratio, hence larger interfacial area to cells and blood components compared with large nanoparticles.251 Intrinsically, smaller sized silica nanoparticles demonstrated a more substantial effect on the haemostatic balance, specifically the coagulation system.48,88,139,252,253 Nanoparticles with the largest size resulted in the release of Weibel-Palade bodies and vWF from endothelial cells among silica nanoparticles with size ranging from 16 to 304 nm after 24 h of incubation, whereas this effect only takes a few hours with smaller sized nanoparticles.252 Previous reported studies have established that silica nanoparticles with a diameter of 10 nm generated greater platelet activation in comparison with nanoparticles larger than 50 nm.139 Correspondingly, due to the increased specific surface area exposed to the coagulation system, smaller sized silica nanoparticles between 30 to 70 nm resulted in enhanced procoagulant activity in comparison with nanoparticles bigger than 100 nm.48,88,253 Nonetheless, another study demonstrated a more significant haemostasis in vivo in larger sized silica nanoparticles (200 nm),153 and interestingly ultrasmall silica nanoparticles (4–7 nm) did not exhibit coagulation activity owing to their higher surface curvatures.133Likewise, AuNPs (≤50 nm) were easily internalised and accumulated in platelets, stimulating platelet activation in comparison with larger size (60 nm) nanoparticles which were inert.111,118 Paradoxically, there were other studies that established that small AuNPs (5–30 nm) had no effect on platelets, while 60 nm-ones blocked platelet aggregation.119 A concentration of 5–40 μM of Au in PRP was employed, which corresponds to 0.94–7.5 μg mL−1 blood in the abovementioned studies. A size-dependent trend was observed with AuNPs within the size range of 12–85 nm, in which 45 and 85 nm-AuNPs induced quicker pro-thrombotic response and 28 nm-ones exhibited the greatest decrement in clot strength.116
The effect of particle size was also verified for silver nanoparticles,87,94 carbon-based nanoparticles,71,254 and iron oxide nanoparticles.130 For instance, longer MWCNTs (both carboxylated and aminated) had a more significant effect on platelet activation than the shorter ones.254 Among those, long carboxylated MWCNTs soften the clot while long aminated MWCNTs increased clot hardness. Interestingly, 30 nm-PVP-AgNPs reduced RBC deformability to a higher degree in comparison with 100 nm-PVP-AgNPs in vitro.94 Small AgNPs (10–15 nm) repressed platelet activation in vitro.87 Size-dependent effects on RBCs were observed with PAA-IONPs where the larger size corresponded to the higher RBCs morphology alteration.130 Furthermore, 5 nm-nanoparticles delayed blood clotting time relative to the 10 and 30 nm-ones.
The discrepancies in the size-dependent effects of nanoparticles on coagulation need to be carefully taken into account because the characterisation of nanoparticle size might not be carried out using similar media and techniques (e.g. water vs. buffer solution or TEM/SEM vs. dynamic light scattering). Additionally, the degree of influence of a particular nanoparticle on the coagulation system may also be associated with other factors, such as the concentration and surface charge of nanoparticles.
5.2 Effect of shape
The shape of a nanoparticle has been presented in previously reported studies as a crucial parameter that has a significant impact on the interaction with haemostatic balance.94,109,117,255,256 Regarding silver nanomaterials coated with PVP, 30 nm spherical AgNPs reduced RBC deformability the most in comparison with 100 nm spherical AgNPs and silver nanowires (40 nm in diameter and 1–2 μm in length).94 Silver nanowires reduced RBC aggregation at 50 μg mL−1 while 30 nm spherical AgNPs did not. Meher et al. reported that heparin coated AgNPs with hexagonal shape displayed the longest delayed coagulation time amongst other shapes in this order: hexagonal > truncated triangular > triangular > spherical.109 Whilst these studies established a correlation of shape to haemostatic balance, there were also conflicting results from studies demonstrating that carbon-based nanoparticles can cause thrombus formation regardless of their shape.256 Also, the shape of AuNPs (spherical, hollow sphere, or rod shape) had no significant effect on endothelial cells.1175.3 Effect of surface charge
A key factor influencing the interaction of nanoparticles with haemostatic balance is their surface charge. Positively charged groups on nanoparticle surfaces can facilitate platelet–platelet interaction and aggregation via neutralizing and forming cross-bridges with negatively charged ionisable sialic acid groups on the platelets’ surface.29,257 Besides, positively charged nanoparticles can induce the changes in the size and number of platelet aggregates by altering platelet morphology156 and disrupting platelet membrane integrity.154 Large and cationic PAMAM (≥G4) and triazine dendrimers (G5 and G7) provoked platelet aggregation, in which the degree of aggregation was proportional to the number of amine groups on the nanoparticles’ surface.154Negatively charged nanoparticle surfaces can initiate the coagulation cascade which eventually disrupts haemostatic balance.62–65 For instance, the upregulation of activation markers (P-selectin or PAC-1) of platelets was triggered by anionic polystyrene (carboxyl-modification) whilst the interruption of the platelet membrane was initiated by cationic polystyrene (amine-modification).164 Positively and negatively charged polystyrene nanoparticles164 can eventually lead to thrombotic events, except for the 50 nm amine-modified nanoparticles reported by Smyth et al.163 and the 57 and 284 nm amine-modified ones reported by Oslakovic et al.166 This is in contradiction with liposomes where both anionic and cationic nanoparticles inhibited platelet activation and aggregation.159 Nevertheless, there are still contradictory studies that reported evoked platelet aggregation effects of anionic liposomes,160,161 or the independence of the surface charge of polystyrene nanoparticles towards platelet activation.163
In conclusion, it is apparent that the charge-dependent effect of nanoparticles on the haemostatic balance is unpredictable. The influence of nanoparticle charge is even more difficult to clarify in physiological conditions due to the absorption of plasma proteins on the surface of nanoparticles.
5.4 Effect of surface functionality
Reactivity of a nanoparticle to the haemostatic balance can be altered by a layer of coating material on its surface. Among all, polyethylene glycol (PEG) is the most commonly used polymeric material. As reported in previous studies, the presence of PEG on the nanoparticles surface reduced their interference with endothelial cells and platelets, probably due to the capability to prevent protein binding.90,142,158,258–260 Therefore, unattended haemostasis is reduced and the compatibility of nanoparticles is improved. However, PEGylation of nanoparticles is not successful for all nanoparticles.74 In addition to PEG, other polymers, namely dextran,261 albumin,84 and starch,125 did not cause any effect on endothelial cells and platelets, or reduced platelet aggregation. Citrate coating either had no effect92,96,111,112,119 or was one factor contributing to antiplatelet activity of the nanoparticles.87,125,127,150 Nevertheless, citrate coating/stabilising also synergises with other factors such as size and dose, indicating the influences of nanoparticles on the haemostatic balance, which is the reason for the procoagulant effects of some citrate coated nanoparticles.85,111,116,120 It was reported that PAA conjugated on the surface of gold nanoparticles binds to fibrinogen and promotes changes in its conformation (unfolding).115 However, gold nanoparticles coated with PEI and PVP induced platelet aggregation.118 Interestingly, superparamagnetic iron oxide nanoparticles at the same size of 5–6 nm functionalized with either hyaluronic acid or chitosan showed less effect on platelets, RBCs, and coagulation than those with PAA coating (tested concentration of 4–1000 μg mL−1).130 All the findings above have demonstrated that specific coating material is worth investigating for these commonly used nanoparticles during their interactions with the haemostatic balance.5.5 Effect of other characteristics
The concentration of nanoparticle metal cores (such as gold) had an impact on haemostasis. Hsu et al. revealed that a lower gold concentration (43.5 ppm) incorporated with polyurethane (PU) nanocomposites resulted in less platelet adhesion and activation compared with a higher amount of gold incorporated (174 ppm).262Because of the binding of plasma proteins or simply pH value, the surface charges of the nanoparticle can be altered in the physiological fluids, which could come along with the change in nanoparticle's hydrophobicity.24,31 A study clarifying the influence of latex polystyrene nanoparticles’ hydrophobicity on the blood haemostatic balance was carried out by Miyamoto et al.263 The results revealed that hydrophobic latex nanoparticles provoked platelet aggregation to a higher extent than the hydrophilic ones. This could be due to their ability to interact more closely with the cell membrane and activate the platelets.264 In another study, TiO2 nanotubes with a superhydrophobic surface had a tendency to prevent platelet adhesion.265 Not only were very few adhered platelets detected on their surface, but also these adhered platelets were not activated, which was opposite to bare TiO2 nanotubes and superhydrophilic TiO2 nanotubes. However, further investigation relating to the relationship between hydrophobicity of nanoparticles and the haemostatic balance is rarely found.
6. Challenges and future directions
According to ISO 10993-4 guidelines, thrombosis, haematology (hemolysis and leukocyte count), and complement activation are blood incompatibilities that should be considered for in vivo study, while coagulation, platelet activation, platelet aggregation, haematology, and complement activation are blood incompatibilities considered for in vitro study, acquired prior to clinical translation of blood-contacting biomaterials.266,267 As systematically reviewed by Urbán et al. in 2019, thrombosis, haematology, and complement activation are the main blood toxicities triggered by inorganic, lipid-based, and polymeric nanoparticles.267 Thrombosis, as a consequence of the haemostatic imbalance, accounts for 61% of the total reported cases of in vivo blood toxicities in nanomedicine. It is therefore the most common blood incompatibility caused by nanomedicine. Anticoagulant and antifibrinolytic effects, as other consequences of haemostatic imbalance, also imply hidden risk of haemorrhage triggered by nanoparticles.There are several things that should be taken into consideration when it comes to the nanoparticle-haemostatic balance:
(1) There is still room for more studies in the future as not all commonly examined nanoparticles are comprehensively investigated and fully understood regarding the underlying mechanisms. Establishing an approach for the systemic investigation of nanoparticle effects on the haemostatic balance, especially with the focus on clinically and preclinically used nanoparticles, is tremendously encouraged.
(2) Further research investigating the effects of nanoparticles on RBCs and specific plasma factors of the haemostatic balance will be of high interest as most of the current studies focus more on their interactions with platelets and endothelial cells. Regarding their interactions with RBCs, a haemolysis assay is usually utilised to demonstrate hemocompatibility but cannot be used independently to evaluate the influences of nanoparticles on haemostatic balance. RBC aggregation, deformability, and PS exposure should also be taken into account.
(3) Alterable interferences with the haemostatic balance in correlation to changes in nanoparticle physiochemical parameters have been examined in many studies but not in a systematic way. The discrepancies in results need to be treated with caution since the characterisation techniques adopted might not be comparable in terms of methods, setting, and media. More importantly, the effects of a specific nanoparticle on haemostasis could be associated with a myriad of synergistic physicochemical characteristics.
(4) As discussed, coating material is an important factor that can alter nanoparticle reactivity to the haemostatic balance. Nevertheless, there is a limited variety of investigated polymeric materials. The effect of metal coating of core–shell nanoparticles, regarding types of metal, thickness of metal coating, and coating method, on the haemostasis balance has not been explored yet. Therefore, more studies are needed to elucidate the effects of coating materials on haemostatic balance. It is worth examining specific coating materials for commonly used nanoparticles.
(5) The interface between the haemostatic balance and other characteristics of nanoparticles, such as hydrophobicity, porosity, lipid composition of lipid-based nanoparticles, and surface topography, may attract much interest in the future.
(6) Apparently, in vivo studies are encouraged since the behaviour of nanoparticles is not always predictable in physiological conditions due to the absorption of plasma proteins on their surface. Moreover, the discrepancies in administration routes (i.e. inhalation, instillation, or IV) must be taken into account when interpreting in vivo results.
(7) Along with the physicochemical characteristics of nanoparticles that were discussed, the dose multiplied by the duration of exposure is one of the most crucial factors in deciding nanoparticle effects on the haemostatic balance.
(8) Different types of nanoparticles will affect the haemostatic balance in different ways. Slight changes in one or more parameters of a specific type of nanoparticle, even the well-established ones, could significantly alter their behaviour to an extent that we can no longer predict.
(9) There are many degradable nanoparticles intentionally designed to stay at the target site for a long time for therapeutic treatment. Hence, we also need to take extreme care to evaluate the influence of their degradation products on the haemostatic balance over time.
(10) Nanoparticles can encounter and affect the innate immune system (i.e. leukocytes and complement system), which could potentially interfere with the haemostatic balance concerning the intrinsic link between the innate immunity and haemostasis. There were several studies demonstrating the effect of nanoparticles on immunity. However, the effect of nanoparticles on innate immune systems that directly mediate the haemostatic balance has not been examined yet to the best of our knowledge, encouraging more studies in the future.
7. Conclusion
Nanoparticles in the bloodstream always interfere with the haemostatic balance through interactions with one or more components of the blood coagulation, anticoagulation, and fibrinolytic system. Our review presents a thorough outline of the haemostatic balance and possible interference of various inorganic and organic nanoparticles by focusing on the underlying mechanisms and factors that could have some effects. The effect of nanoparticles on the innate immune system that could potentially link to haemostasis is discussed as well. This collated information is valuable for the establishment of nanoparticles that can either avoid unintended interferences with the haemostatic balance or purposely downregulate/upregulate their components under a controlled manner, thus speeding up their successful translation to the clinic and market.Abbreviations
| ADP | Adenosine diphosphate |
| AFM | Atomic force microscopy |
| AgNPs | Silver nanoparticles |
| APC | Activated protein C |
| AT | Antithrombin III |
| AuNPs | Gold nanoparticles |
| DLS | Dynamic light scattering |
| DAMPs | Nuclear damage-associated molecular patterns |
| EPCR | Endothelial protein C receptor |
| GpIIb/IIIa | Glycoprotein IIb/IIIa |
| GSH | Reduced glutathione |
| HMWK | High-molecular-weight kininogen |
| HUVECs | Human umbilical vein endothelial cells |
| HMGB1 | High mobility group box 1 |
| IONPs | Iron oxide nanoparticles |
| IV | Intravenous injection |
| LPS | Bacterial lipopolysaccharide |
| MMP | Matrix metalloproteinase |
| MWCNT | Multiple wall carbon nanotubes |
| MAC | Membrane attach complex |
| MASP | Mannose-binding protein-associated serine protease |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NETs | Neutrophil extracellular traps |
| PAA | Poly(acrylic acid) |
| PAF | Platelet-activating factor |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAMAM | Poly(amidoamine) |
| PAR | Protease-activated receptor |
| PECAM-1 | Platelet endothelial cell adhesion molecule-1 |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PGI2 | Prostacyclin |
| PK | Prekallikrein |
| PLA | Poly(D,L-lactide) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PRP | Platelet rich plasma |
| PS | Phosphatidylserine |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| PZ | Protein Z |
| RBCs | Red blood cells |
| SAED | Selected area (electron) diffraction |
| SOCE | Store-operated Ca2+ entry |
| SWCNT | Single wall carbon nanotubes |
| TEM | Transmission electron microscopy |
| TF | Tissue factor |
| TFPI | Tissue factor pathway inhibitor |
| TiO2 | Rutile titanium |
| TM | Thrombomodulin |
| t-PA | Tissue-type plasminogen activator |
| TXA2 | Thromboxane A2 |
| TCC | Terminal complement complex |
| TAFI | Thrombin activatable fibrinolysis inhibitor |
| u-PA | Urokinase-type plasminogen activator |
| vWF | Von Willebrand factor |
| XRD | X-ray powder diffraction |
| ZnO NPs | Zinc oxide nanoparticles |
| ZPI | Z-Dependent protease inhibitor |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by National Health and Medical Research Council (HTT: APP1037310, APP1182347, APP2002827) and Heart Foundation (HTT: 102761).References
- J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne and M. K. Danquah, Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations, Beilstein J. Nanotechnol., 2018, 9, 1050–1074 CrossRef CAS.
- K. M. de la Harpe, P. P. Kondiah, Y. E. Choonara, T. Marimuthu, L. C. du Toit and V. Pillay, The hemocompatibility of nanoparticles: a review of cell–nanoparticle interactions and hemostasis, Cells, 2019, 8, 1209 CrossRef CAS.
- M. F. Matus, C. Vilos, B. A. Cisterna, E. Fuentes and I. Palomo, Nanotechnology and primary hemostasis: Differential effects of nanoparticles on platelet responses, Vasc. Pharmacol., 2018, 101, 1–8 CrossRef CAS.
- T. T. H. Thi, D. H. T. Nguyen, D. T. D. Nguyen, D. H. Nguyen and M.-D. Truong, Decellularized Porcine Epiphyseal Plate-Derived Extracellular Matrix Powder: Synthesis and Characterization, Cells Tissues Organs, 2020, 209, 1–9 CrossRef.
- H. D. Tran, K. D. Park, Y. C. Ching, C. Huynh and D. H. Nguyen, A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering, J. Ind. Eng. Chem., 2020, 89, 58–82 CrossRef CAS.
- E. Reimhult, Nanoparticle interactions with blood proteins and what it means: a tutorial review, 2019 Search PubMed.
- N. Arndt, H. D. Tran, R. Zhang, Z. P. Xu and H. T. Ta, Different approaches to develop nanosensors for diagnosis of diseases, Adv. Sci., 2020, 7, 2001476 CrossRef CAS PubMed.
- H. Adelnia, H. D. Tran, P. J. Little, I. Blakey and H. T. Ta, Poly (aspartic acid) in Biomedical Applications: From Polymerization, Modification, Properties, Degradation, and Biocompatibility to Applications, ACS Biomater. Sci. Eng., 2021, 7, 2083–2105 CrossRef CAS.
- T. N. T. Nguyen, D.-H. Nguyen-Tran, L. G. Bach, T. H. Du Truong, N. T. T. Le and D. H. Nguyen, Surface PEGylation of hollow mesoporous silica nanoparticles via aminated intermediate, Prog. Nat. Sci.: Mater. Int., 2019, 29, 612–616 CrossRef.
- D. H. Nguyen, L. G. Bach, D.-H. Nguyen Tran, V. D. Cao, T. N. Q. Nguyen, T. T. H. Le, T. T. Tran and T. T. H. Thi, Partial surface modification of low generation polyamidoamine dendrimers: Gaining insight into their potential for improved carboplatin delivery, Biomolecules, 2019, 9, 214 CrossRef CAS.
- D. H. N. Tran, T. H. Nguyen, T. N. N. Vo, L. P. T. Pham, D. M. H. Vo, C. K. Nguyen, L. G. Bach and D. H. Nguyen, Self–assembled poly (ethylene glycol) methyl ether–grafted gelatin nanogels for efficient delivery of curcumin in cancer treatment, J. Appl. Polym. Sci., 2019, 136, 47544 CrossRef.
- T. T. Hoang Thi, D.-H. Nguyen Tran, L. G. Bach, H. Vu-Quang, D. C. Nguyen, K. D. Park and D. H. Nguyen, Functional magnetic core-shell system-based iron oxide nanoparticle coated with biocompatible copolymer for anticancer drug delivery, Pharmaceutics, 2019, 11, 120 CrossRef.
- V. M. H. Do, L. G. Bach, D.-H. N. Tran, T. N. Q. Nguyen, D. T. Hoang, D. H. Nguyen and T. T. H. Thi, Effective Elimination of Charge-associated Toxicity of Low Generation Polyamidoamine Dendrimer Eases Drug Delivery of Oxaliplatin, Biotechnol. Bioprocess Eng., 2020, 1–11 Search PubMed.
- B. Q. Bao, N. H. Le, D. H. T. Nguyen, T. V. Tran, L. P. T. Pham, L. G. Bach, H. M. Ho, T. H. Nguyen and D. H. Nguyen, Evolution and present scenario of multifunctionalized mesoporous nanosilica platform: A mini review, Mater. Sci. Eng., C, 2018, 91, 912–928 CrossRef CAS.
- Y. Wu, R. Zhang, H. D. Tran, N. D. Kurniawan, S. S. Moonshi, A. K. Whittaker and H. T. Ta, Chitosan Nanococktails Containing Both Ceria and Superparamagnetic Iron Oxide Nanoparticles for Reactive Oxygen Species-Related Theranostics, ACS Appl. Nano Mater., 2021, 4, 3604–3618 CrossRef CAS.
- A. U. Rehman, Y. Wu, H. D. Tran, K. Vazquez-Prada, Y. Liu, H. Adelnia, N. D. Kurniawan, M. N. Anjum, S. S. Moonshi and H. T. Ta, Silver/Iron Oxide Nano-Popcorns for Imaging and Therapy, ACS Appl. Nano Mater., 2021, 4(10), 10136–10147 CrossRef CAS.
- N. N. M. Yusof, A. McCann, P. J. Little and H. T. Ta, Non-invasive imaging techniques for the differentiation of acute and chronic thrombosis, Thromb. Res., 2019, 177, 161–171 CrossRef CAS.
- A. Zia, Y. Wu, T. Nguyen, X. Wang, K. Peter and H. T. Ta, The choice of targets and ligands for site-specific delivery of nanomedicine to atherosclerosis, Cardiovasc. Res., 2020, 116, 2055–2068 CrossRef CAS PubMed.
- Y. Liu, Y. Wu, R. Zhang, J. Lam, J. C. Ng, Z. P. Xu, L. Li and H. T. Ta, Investigating the use of layered double hydroxide nanoparticles as carriers of metal oxides for theranostics of ROS-related diseases, ACS Appl. Bio Mater., 2019, 2, 5930–5940 CrossRef CAS.
- K. X. Vazquez-Prada, J. Lam, D. Kamato, Z. P. Xu, P. J. Little and H. T. Ta, Targeted Molecular Imaging of Cardiovascular Diseases by Iron Oxide Nanoparticles, Arterioscler., Thromb., Vasc. Biol., 2021, 41, 601–613 CrossRef CAS.
- H. T. Ta, Z. Li, C. Hagemeyer, Y. Wu, H. J. Lim, W. Wang, J. Wei, G. Cowin, A. Whittaker and K. Peter, Novel bionanotechnological solutions based on metal oxide and metal to preserve and assess organs for transplantation, Cryobiology, 2018, 81, 233 CrossRef.
- Y. Wu, K. X. Vazquez-Prada, Y. Liu, A. K. Whittaker, R. Zhang and H. T. Ta, Recent Advances in the Development of Theranostic Nanoparticles for Cardiovascular Diseases, Nanotheranostics, 2021, 5, 499 CrossRef.
- Y. Wu and H. T. Ta, Different approaches to synthesise cerium oxide nanoparticles and their corresponding physical characteristics, ROS scavenging and anti-inflammatory capabilities, J. Mater. Chem. B, 2021, 9, 7291–7301 RSC.
- M. I. Setyawati, C. Y. Tay, D. Docter, R. H. Stauber and D. T. Leong, Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood, Chem. Soc. Rev., 2015, 44, 8174–8199 RSC.
- H. D. Tran, F. Akther, Z. P. Xu and H. T. Ta, Effects of nanoparticles on the blood coagulation system (nanoparticle interface with the blood coagulation system), in Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood, Elsevier, 2022, pp. 113–140 Search PubMed.
- G. Blanco and A. Blanco, Medical biochemistry, Academic Press, 2017 Search PubMed.
- T. Astrup, The Haemostatic Balance, Thromb. Haemostasis, 1958, 2, 347–357 CrossRef CAS.
- P. J. Gaffney, T. A. Edgell and C. M. Whitton, The haemostatic balance–Astrup revisited, Pathophysiol. Haemostasis Thromb., 1999, 29, 58–71 CrossRef CAS PubMed.
- N. K. Hante, C. Medina and M. J. Santos-Martinez, Effect on Platelet Function of Metal-Based Nanoparticles Developed for Medical Applications, Front. Cardiovasc. Med., 2019, 6, 139 CrossRef CAS.
- H. Rasche, Haemostasis and thrombosis: an overview, Eur. Heart J. Suppl., 2001, 3, Q3–Q7 CrossRef CAS.
- E. Fröhlich, Action of nanoparticles on platelet activation and plasmatic coagulation, Curr. Med. Chem., 2016, 23, 408–430 CrossRef.
- D. Sobot, S. Mura, P. Couvreur, S. Kobayashi and K. Müllen, Nanoparticles: blood components interactions, in Encyclopedia of polymeric nanomaterials, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014, pp. 1–10 Search PubMed.
- A. N. Ilinskaya and M. A. Dobrovolskaia, Nanoparticles and the blood coagulation system, in Handbook of immunological properties of engineered nanomaterials: Volume 2: Haematocompatibility of Engineered Nanomaterials, World Scientific, 2016, pp. 261–302 Search PubMed.
- M. Franchini and P. M. Mannucci, The hemostatic balance revisited through the lessons of mankind evolution, Intern. Emerg. Med., 2008, 3, 3–8 CrossRef PubMed.
- K. Martin, A. D. Ma and N. S. Key, Molecular basis of hemostatic and thrombotic diseases, in Molecular Pathology, Elsevier, 2018, pp. 277–297 Search PubMed.
- A. N. Ilinskaya and M. A. Dobrovolskaia, Nanoparticles and the blood coagulation system. Part I: benefits of nanotechnology, Nanomedicine, 2013, 8, 773–784 CrossRef CAS.
- R. E. Rumbaut and P. Thiagarajan, Platelet-vessel wall interactions in hemostasis and thrombosis, in Synthesis Lectures on Integrated Systems Physiology: From Molecule to Function, 2010, vol. 2, pp. 1–75 Search PubMed.
- E. Gaston, J. F. Fraser, Z. P. Xu and H. T. Ta, Nano-and micro-materials in the treatment of internal bleeding and uncontrolled hemorrhage, Nanomedicine, 2018, 14, 507–519 CrossRef CAS PubMed.
- J. A. Ezihe-Ejiofor and N. Hutchinson, Anticlotting mechanisms 1: physiology and pathology, Anaesth. Crit. Care Pain Med., 2013, 13, 87–92 CrossRef.
- S. Reitsma, D. W. Slaaf, H. Vink, M. A. Van Zandvoort and M. G. oude Egbrink, The endothelial glycocalyx: composition, functions, and visualization, Pflügers Arch., 2007, 454, 345–359 CrossRef CAS.
- P. Hangge, J. Stone, H. Albadawi, Y. S. Zhang, A. Khademhosseini and R. Oklu, Hemostasis and nanotechnology, Cardiovasc. Diagn. Ther., 2017, 7, S267 CrossRef PubMed.
- K. N. Ekdahl, K. Fromell, C. Mohlin, Y. Teramura and B. Nilsson, A human whole-blood model to study the activation of innate immunity system triggered by nanoparticles as a demonstrator for toxicity, Sci. Technol. Adv. Mater., 2019, 20, 688–698 CrossRef CAS.
- J. W. Yau, H. Teoh and S. Verma, Endothelial cell control of thrombosis, BMC Cardiovasc. Disord., 2015, 15, 1–11 CrossRef.
- S. Palta, R. Saroa and A. Palta, Overview of the coagulation system, Indian J. Anaesth., 2014, 58, 515 CrossRef CAS PubMed.
- K. Broos, H. B. Feys, S. F. De Meyer, K. Vanhoorelbeke and H. Deckmyn, Platelets at work in primary hemostasis, Blood Rev., 2011, 25, 155–167 CrossRef CAS.
- E. Demir, D. Burgucu, F. Turna, S. Aksakal and B. Kaya, Determination of TiO2, ZrO2, and Al2O3 nanoparticles on genotoxic responses in human peripheral blood lymphocytes and cultured embyronic kidney cells, J. Toxicol. Environ. Health, Part A, 2013, 76, 990–1002 CrossRef CAS PubMed.
- G. G. De La Cruz, P. Rodríguez-Fragoso, J. Reyes-Esparza, A. Rodríguez-López, R. Gómez-Cansino and L. Rodriguez-Fragoso, Interaction of nanoparticles with blood components and associated pathophysiological effects, in Unraveling the Safety Profile of Nanoscale Particles and Materials-From Biomedical to Environmental Applications, 2018 Search PubMed.
- H. Nabeshi, T. Yoshikawa, K. Matsuyama, Y. Nakazato, A. Arimori, M. Isobe, S. Tochigi, S. Kondoh, T. Hirai and T. Akase, Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure, Nanotechnology, 2012, 23, 045101 CrossRef PubMed.
- P. J. Sims, M. Ginsberg, E. Plow and S. Shattil, Effect of platelet activation on the conformation of the plasma membrane glycoprotein IIb-IIIa complex, J. Biol. Chem., 1991, 266, 7345–7352 CrossRef CAS.
- A. Matzdorff and R. Voss, Upregulation of GP IIb/IIIa receptors during platelet activation: Influence on efficacy of receptor blockade, Thromb. Res., 2006, 117, 307–314 CrossRef CAS.
- N. S. Kleiman, A. E. Raizner, R. Jordan, A. L. Wang, D. Norton, K. F. Mace, A. Joshi, B. S. Coller and H. F. Weisman, Differential inhibition of platelet aggregation induced by adenosine diphosphate or a thrombin receptor-activating peptide in patients treated with bolus chimeric 7E3 Fab: implications for inhibition of the internal pool of GPIIb/IIIa receptors, J. Am. Coll. Cardiol., 1995, 26, 1665–1671 CrossRef CAS PubMed.
- J. Weisel and R. Litvinov, Red blood cells: the forgotten player in hemostasis and thrombosis, J. Thromb. Haemostasis, 2019, 17, 271–282 CrossRef CAS.
- V. X. Du, D. Huskens, C. Maas, R. Al Dieri, P. G. de Groot and B. de Laat, New insights into the role of erythrocytes in thrombus formation, in Seminars in thrombosis and hemostasis, Thieme Medical Publishers, 2014, pp. 072–080 Search PubMed.
- R. Mehri, C. Mavriplis and M. Fenech, Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system, PLoS One, 2018, 13, e0199911 CrossRef PubMed.
- K. Sriram, M. Intaglietta and D. M. Tartakovsky, Non–Newtonian flow of blood in arterioles: consequences for wall shear stress measurements, Microcirculation, 2014, 21, 628–639 CrossRef PubMed.
- B. L. Walton, M. Lehmann, T. Skorczewski, L. A. Holle, J. D. Beckman, J. A. Cribb, M. J. Mooberry, A. R. Wufsus, B. C. Cooley and J. W. Homeister, Elevated hematocrit enhances platelet accumulation following vascular injury, Blood, 2017, 129, 2537–2546 CrossRef CAS.
- Y.-F. Wu, P.-S. Hsu, C.-S. Tsai, P.-C. Pan and Y.-L. Chen, Significantly increased low shear rate viscosity, blood elastic modulus, and RBC aggregation in adults following cardiac surgery, Sci. Rep., 2018, 8, 1–10 Search PubMed.
- S. Yedgar, A. Koshkaryev and G. Barshtein, The red blood cell in vascular occlusion, Pathophysiol. Haemostasis Thromb., 2002, 32, 263–268 CrossRef CAS.
- H. C. Kwaan and M. Samama, Clinical thrombosis, CRC Press, 2019 Search PubMed.
- L. Guo, D. Tong, M. Yu, Y. Zhang, T. Li, C. Wang, P. Zhou, J. Jin, B. Li and Y. Liu, Phosphatidylserine-exposing cells contribute to the hypercoagulable state in patients with multiple myeloma, Int. J. Oncol., 2018, 52, 1981–1990 CAS.
- S. A. Smith, R. J. Travers and J. H. Morrissey, How it all starts: Initiation of the clotting cascade, Crit. Rev. Biochem. Mol. Biol., 2015, 50, 326–336 CrossRef CAS PubMed.
- J. Simak and S. De Paoli, The effects of nanomaterials on blood coagulation in hemostasis and thrombosis, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9, e1448 Search PubMed.
- R. C. Wiggins and C. C. Cochrane, The autoactivation of rabbit Hageman factor, J. Exp. Med., 1979, 150, 1122–1133 CrossRef CAS PubMed.
- F. van der Graaf, F. Keus, R. Vlooswijk and B. N. Bouma, The contact activation mechanism in human plasma: activation induced by dextran sulfate, 1982 Search PubMed.
- D. L. Tankersley, B. M. Alving and J. Finlayson, Activation of factor XII by dextran sulfate: the basis for an assay of factor XII, 1983 Search PubMed.
- P. K. Bendapudi, K. Deceunynck, S. Koseoglu, R. H. Bekendam, S. D. Mason, J. Kenniston and R. C. Flaumenhaft, Stimulated platelets but not endothelium generate thrombin via a factor XIIa-dependent mechanism requiring phosphatidylserine exposure, American Society of Hematology Washington, DC, 2016 Search PubMed.
- Y. Buyue, T. M. Misenheimer and J. P. Sheehan, Low molecular weight heparin inhibits plasma thrombin generation via direct targeting of factor IXa: contribution of the serpin–independent mechanism, J. Thromb. Haemostasis, 2012, 10, 2086–2098 CrossRef CAS.
- J. C. Chapin and K. A. Hajjar, Fibrinolysis and the control of blood coagulation, Blood Rev., 2015, 29, 17–24 CrossRef CAS PubMed.
- H. Sun, L. Lv, Y. Bai, H. Yang, H. Zhou, C. Li and L. Yang, Nanotechnology-enabled materials for hemostatic and anti-infection treatments in orthopedic surgery, Int. J. Nanomed., 2018, 13, 8325 CrossRef CAS PubMed.
- K. D. Patel, R. K. Singh and H.-W. Kim, Carbon-based nanomaterials as an emerging platform for theranostics, Mater. Horiz., 2019, 6, 434–469 RSC.
- A. Radomski, P. Jurasz, D. Alonso-Escolano, M. Drews, M. Morandi, T. Malinski and M. W. Radomski, Nanoparticle–induced platelet aggregation and vascular thrombosis, Br. J. Pharmacol., 2005, 146, 882–893 CrossRef CAS.
- J. Semberova, S. H. De Paoli Lacerda, O. Simakova, K. Holada, M. P. Gelderman and J. Simak, Carbon nanotubes activate blood platelets by inducing extracellular Ca2+ influx sensitive to calcium entry inhibitors, Nano Lett., 2009, 9, 3312–3317 CrossRef CAS PubMed.
- S. H. De Paoli Lacerda, J. Semberova, K. Holada, O. Simakova, S. D. Hudson and J. Simak, Carbon nanotubes activate store-operated calcium entry in human blood platelets, ACS Nano, 2011, 5, 5808–5813 CrossRef CAS.
- A. R. Burke, R. N. Singh, D. L. Carroll, J. D. Owen, N. D. Kock, R. D'Agostino Jr., F. M. Torti and S. V. Torti, Determinants of the thrombogenic potential of multiwalled carbon nanotubes, Biomaterials, 2011, 32, 5970–5978 CrossRef CAS PubMed.
- K. Luyts, S. Smulders, D. Napierska, K. Poels, H. Scheers, B. Hemmeryckx, B. Nemery, M. F. Hoylaerts and P. H. Hoet, Pulmonary and hemostatic toxicity of multi-walled carbon nanotubes and zinc oxide nanoparticles after pulmonary exposure in Bmal1 knockout mice, Part. Fibre Toxicol., 2014, 11, 1–15 CrossRef.
- J. Luo, M. Zhang, J. Cheng, S. Wu, W. Xiong, H. Kong, Y. Zhao and H. Qu, Hemostatic effect of novel carbon dots derived from Cirsium setosum Carbonisata, RSC Adv., 2018, 8, 37707–37714 RSC.
- T.-Y. Lee, T. Jayakumar, P. Thanasekaran, K.-C. Lin, H.-M. Chen, P. Veerakumar and J.-R. Sheu, Carbon dot nanoparticles exert inhibitory effects on human platelets and reduce mortality in mice with acute pulmonary thromboembolism, Nanomaterials, 2020, 10, 1254 CrossRef CAS.
- S. Kumari, M. K. Singh, S. K. Singh, J. J. Grácio and D. Dash, Nanodiamonds activate blood platelets and induce thromboembolism, Nanomedicine, 2014, 9, 427–440 CrossRef CAS.
- T. Avsievich, A. Popov, A. Bykov and I. Meglinski, Mutual interaction of red blood cells influenced by nanoparticles, Sci. Rep., 2019, 9, 1–6 Search PubMed.
- M. P. Gelderman, O. Simakova, J. D. Clogston, A. K. Patri, S. F. Siddiqui, A. C. Vostal and J. Simak, Adverse effects of fullerenes on endothelial cells: Fullerenol C60 (OH) 24 induced tissue factor and ICAM-1 membrane expression and apoptosis in vitro, Int. J. Nanomed., 2008, 3, 59 CAS.
- S. Xia, J. Li, M. Zu, J. Li, J. Liu, X. Bai, Y. Chang, K. Chen, W. Gu and L. Zeng, Small size fullerenol nanoparticles inhibit thrombosis and blood coagulation through inhibiting activities of thrombin and FXa, Nanomedicine, 2018, 14, 929–939 CrossRef CAS.
- S. K. Singh, M. K. Singh, M. K. Nayak, S. Kumari, S. Shrivastava, J. J. Grácio and D. Dash, Thrombus inducing property of atomically thin graphene oxide sheets, ACS Nano, 2011, 5, 4987–4996 CrossRef CAS PubMed.
- S. K. Singh, M. K. Singh, P. P. Kulkarni, V. K. Sonkar, J. J. Grácio and D. Dash, Amine-modified graphene: thrombo-protective safer alternative to graphene oxide for biomedical applications, ACS Nano, 2012, 6, 2731–2740 CrossRef CAS.
- T. V. Vakhrusheva, A. A. Gusev, S. A. Gusev and I. I. Vlasova, Albumin reduces thrombogenic potential of single-walled carbon nanotubes, Toxicol. Lett., 2013, 221, 137–145 CrossRef CAS PubMed.
- X. Sun, J. Shi, X. Zou, C. Wang, Y. Yang and H. Zhang, Silver nanoparticles interact with the cell membrane and increase endothelial permeability by promoting VE-cadherin internalization, J. Hazard. Mater., 2016, 317, 570–578 CrossRef CAS.
- P. H. Danielsen, Y. Cao, M. Roursgaard, P. Møller and S. Loft, Endothelial cell activation, oxidative stress and inflammation induced by a panel of metal-based nanomaterials, Nanotoxicology, 2015, 9, 813–824 CrossRef.
- S. Shrivastava, T. Bera, S. K. Singh, G. Singh, P. Ramachandrarao and D. Dash, Characterization of antiplatelet properties of silver nanoparticles, ACS Nano, 2009, 3, 1357–1364 CrossRef CAS.
- E.-A. Jun, K.-M. Lim, K. Kim, O.-N. Bae, J.-Y. Noh, K.-H. Chung and J.-H. Chung, Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity, Nanotoxicology, 2011, 5, 157–167 CrossRef CAS.
- S. Krajewski, R. Prucek, A. Panacek, M. Avci-Adali, A. Nolte, A. Straub, R. Zboril, H. P. Wendel and L. Kvitek, Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood, Acta Biomater., 2013, 9, 7460–7468 CrossRef CAS PubMed.
- V. Ragaseema, S. Unnikrishnan, V. K. Krishnan and L. K. Krishnan, The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces, Biomaterials, 2012, 33, 3083–3092 CrossRef CAS.
- J. Laloy, V. Minet, L. Alpan, F. Mullier, S. Beken, O. Toussaint, S. Lucas and J.-M. Dogné, Impact of silver nanoparticles on haemolysis, platelet function and coagulation, Nanobiomedicine, 2014, 1, 4 CrossRef PubMed.
- H. Huang, W. Lai, M. Cui, L. Liang, Y. Lin, Q. Fang, Y. Liu and L. Xie, An evaluation of blood compatibility of silver nanoparticles, Sci. Rep., 2016, 6, 1–15 CrossRef CAS.
- M. Marulasiddeshwara, S. Dakshayani, M. S. Kumar, R. Chethana, P. R. Kumar and S. Devaraja, Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: A promising therapeutic agent, Mater. Sci. Eng., C, 2017, 81, 182–190 CrossRef CAS.
- M. J. Kim and S. Shin, Toxic effects of silver nanoparticles and nanowires on erythrocyte rheology, Food Chem. Toxicol., 2014, 67, 80–86 CrossRef CAS.
- D. Bandyopadhyay, H. Baruah, B. Gupta and S. Sharma, Silver nano particles prevent platelet adhesion on immobilized fibrinogen, Indian J. Clin. Biochem., 2012, 27, 164–170 CrossRef CAS.
- J. H. Lee, M. Gulumian, E. M. Faustman, T. Workman, K. Jeon and I. J. Yu, Blood biochemical and hematological study after subacute intravenous injection of gold and silver nanoparticles and coadministered gold and silver nanoparticles of similar sizes, BioMed Res. Int., 2018, 2018, 8460910 Search PubMed.
- J. Hajtuch, N. Hante, E. Tomczyk, M. Wojcik, M. W. Radomski, M. J. Santos-Martinez and I. Inkielewicz-Stepniak, Effects of functionalized silver nanoparticles on aggregation of human blood platelets, Int. J. Nanomed., 2019, 14, 7399 CrossRef CAS.
- V. Trivedi, A. Boire, B. Tchernychev, N. C. Kaneider, A. J. Leger, K. O'Callaghan, L. Covic and A. Kuliopulos, Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site, Cell, 2009, 137, 332–343 CrossRef CAS PubMed.
- S. Shrivastava, S. K. Singh, A. Mukhopadhyay, A. S. Sinha, R. K. Mandal and D. Dash, Negative regulation of fibrin polymerization and clot formation by nanoparticles of silver, Colloids Surf., B, 2011, 82, 241–246 CrossRef CAS PubMed.
- K. Heise, M. Hobisch, L. Sacarescu, U. Maver, J. Hobisch, T. Reichelt, M. Sega, S. Fischer and S. Spirk, Low-molecular-weight sulfonated chitosan as template for anticoagulant nanoparticles, Int. J. Nanomed., 2018, 13, 4881 CrossRef CAS PubMed.
- R. N. Krishnaraj and S. Berchmans, In vitro antiplatelet activity of silver nanoparticles synthesized using the microorganism Gluconobacter roseus: an AFM-based study, RSC Adv., 2013, 3, 8953–8959 RSC.
- M. Jeyaraj, S. Varadan, K. J. P. Anthony, M. Murugan, A. Raja and S. Gurunathan, Antimicrobial and anticoagulation activity of silver nanoparticles synthesized from the culture supernatant of Pseudomonas aeruginosa, J. Ind. Eng. Chem., 2013, 19, 1299–1303 CrossRef CAS.
- K. Roy, A. K. Srivastwa and C. K. Ghosh, Anticoagulant, thrombolytic and antibacterial activities of Euphorbia acruensis latex-mediated bioengineered silver nanoparticles, Green Process. Synth., 2019, 8, 590–599 CAS.
- A. Lateef, B. I. Folarin, S. M. Oladejo, P. O. Akinola, L. S. Beukes and E. B. Gueguim-Kana, Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract, Prep. Biochem. Biotechnol., 2018, 48, 646–652 CrossRef CAS PubMed.
- A. Lateef, S. A. Ojo, J. A. Elegbede, M. A. Azeez, T. A. Yekeen and A. Akinboro, Evaluation of some biosynthesized silver nanoparticles for biomedical applications: hydrogen peroxide scavenging, anticoagulant and thrombolytic activities, J. Cluster Sci., 2017, 28, 1379–1392 CrossRef CAS.
- A. Lateef, M. A. Akande, S. A. Ojo, B. I. Folarin, E. B. Gueguim-Kana and L. S. Beukes, Paper wasp nest-mediated biosynthesis of silver nanoparticles for antimicrobial, catalytic, anticoagulant, and thrombolytic applications, 3 Biotech, 2016, 6, 1–10 CrossRef.
- A. Lateef, M. A. Akande, M. A. Azeez, S. A. Ojo, B. I. Folarin, E. B. Gueguim-Kana and L. S. Beukes, Phytosynthesis of silver nanoparticles (AgNPs) using miracle fruit plant (Synsepalum dulcificum) for antimicrobial, catalytic, anticoagulant, and thrombolytic applications, Nanotechnol. Rev., 2016, 5, 507–520 CAS.
- B. Harish, K. B. Uppuluri and V. Anbazhagan, Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent, Carbohydr. Polym., 2015, 132, 104–110 CrossRef CAS PubMed.
- M. K. Meher and K. M. Poluri, Anticoagulation and antibacterial properties of heparinized nanosilver with different morphologies, Carbohydr. Polym., 2021, 266, 118124 CrossRef CAS PubMed.
- J. Berry, B. Arnoux, G. Stanislas, P. Galle and J. Chretien, A microanalytic study of particles transport across the alveoli: role of blood platelets, Biomedicine, 1977, 27, 354–357 CAS.
- S. Deb, H. K. Patra, P. Lahiri, A. K. Dasgupta, K. Chakrabarti and U. Chaudhuri, Multistability in platelets and their response to gold nanoparticles, Nanomedicine, 2011, 7, 376–384 CrossRef CAS.
- S. A. Love, J. W. Thompson and C. L. Haynes, Development of screening assays for nanoparticle toxicity assessment in human blood: preliminary studies with charged Au nanoparticles, Nanomedicine, 2012, 7, 1355–1364 CrossRef CAS PubMed.
- G. Chen, N. Ni, J. Zhou, Y.-J. Chuang, B. Wang, Z. Pan and B. Xu, Fibrinogen clot induced by gold-nanoparticle in vitro, J. Nanosci. Nanotechnol., 2011, 11, 74–81 CrossRef CAS.
- M. A. Dobrovolskaia, A. K. Patri, J. Zheng, J. D. Clogston, N. Ayub, P. Aggarwal, B. W. Neun, J. B. Hall and S. E. McNeil, Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles, Nanomedicine, 2009, 5, 106–117 CrossRef CAS PubMed.
- Z. J. Deng, M. Liang, M. Monteiro, I. Toth and R. F. Minchin, Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation, Nat. Nanotechnol., 2011, 6, 39–44 CrossRef CAS PubMed.
- N. Ajdari, C. Vyas, S. L. Bogan, B. A. Lwaleed and B. G. Cousins, Gold nanoparticle interactions in human blood: a model evaluation, Nanomedicine, 2017, 13, 1531–1542 CrossRef CAS.
- D. Bartczak, O. L. Muskens, S. Nitti, T. Sanchez-Elsner, T. M. Millar and A. G. Kanaras, Interactions of human endothelial cells with gold nanoparticles of different morphologies, Small, 2012, 8, 122–130 CrossRef CAS PubMed.
- M. Hecold, R. Buczkowska, A. Mucha, J. Grzesiak, O. Rac-Rumijowska, H. Teterycz and K. Marycz, The effect of PEI and PVP-stabilized gold nanoparticles on equine platelets activation: potential application in equine regenerative medicine, J. Nanomater., 2017, 2017, 8706921 Search PubMed.
- A. Aseychev, O. Azizova, E. Beckman, L. Dudnik and V. Sergienko, Effect of gold nanoparticles coated with plasma components on ADP-induced platelet aggregation, Bull. Exp. Biol. Med., 2013, 155, 685–688 CrossRef CAS PubMed.
- M. J. Santos-Martinez, I. Inkielewicz-Stepniak, C. Medina, K. Rahme, D. M. D'Arcy, D. Fox, J. D. Holmes, H. Zhang and M. W. Radomski, The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation, Int. J. Nanomed., 2012, 7, 243 CrossRef CAS PubMed.
- L. Bircher, O. M. Theusinger, S. Locher, P. Eugster, B. Roth-Z'graggen, C. M. Schumacher, J.-D. Studt, W. J. Stark, B. Beck-Schimmer and I. K. Herrmann, Characterization of carbon-coated magnetic nanoparticles using clinical blood coagulation assays: effect of PEG-functionalization and comparison to silica nanoparticles, J. Mater. Chem. B, 2014, 2, 3753–3758 RSC.
- Q. Ran, Y. Xiang, Y. Liu, L. Xiang, F. Li, X. Deng, Y. Xiao, L. Chen, L. Chen and Z. Li, Eryptosis indices as a novel predictive parameter for biocompatibility of Fe 3 O 4 magnetic nanoparticles on erythrocytes, Sci. Rep., 2015, 5, 16209 CrossRef CAS.
- C. Achilli, S. Grandi, G. Guidetti, A. Ciana, C. Tomasi, D. Capsoni and G. Minetti, Fe 3 O 4@ SiO 2 core–shell nanoparticles for biomedical purposes: adverse effects on blood cells, Biomater. Sci., 2016, 4, 1417–1421 RSC.
- M. G. Villegas, M. T. Ceballos, J. Urquijo, E. Y. Torres, B. L. Ortiz-Reyes, O. L. Arnache-Olmos and M. R. López, Poly (acrylic acid)-Coated Iron Oxide Nanoparticles interact with mononuclear phagocytes and decrease platelet aggregation, Cell. Immunol., 2019, 338, 51–62 CrossRef CAS.
- S. Deb, S. Raja, A. K. Dasgupta, R. Sarkar, A. P. Chattopadhyay, U. Chaudhuri, P. Guha and P. Sardar, Surface tunability of nanoparticles in modulating platelet functions, Blood Cells, Mol., Dis., 2012, 48, 36–44 CrossRef CAS.
- R. K. Kottana, L. Maurizi, B. Schnoor, K. Morris, J. A. Webb, M. A. Massiah, N. Millot and A. l. Papa, Anti–Platelet Effect Induced by Iron Oxide Nanoparticles: Correlation with Conformational Change in Fibrinogen, Small, 2021, 17, 2004945 CrossRef CAS PubMed.
- D. Cabrera, K. Walker, S. Moise, N. D. Telling and A. G. Harper, Controlling human platelet activation with calcium-binding nanoparticles, Nano Res., 2020, 13, 2697–2705 CrossRef CAS PubMed.
- C. Li, M. Zhang, X. Liu, W. Zhao and C. Zhao, Immobilization of heparin-mimetic biomacromolecules on Fe3O4 nanoparticles as magnetic anticoagulant via mussel-inspired coating, Mater. Sci. Eng., C, 2020, 109, 110516 CrossRef CAS PubMed.
- S. L. Easo and P. Mohanan, In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles, Colloids Surf., B, 2015, 134, 122–130 CrossRef CAS PubMed.
- T. Liu, R. Bai, H. Zhou, R. Wang, J. Liu, Y. Zhao and C. Chen, The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility, RSC Adv., 2020, 10, 7559–7569 RSC.
- S. E. Baker, A. M. Sawvel, J. Fan, Q. Shi, N. Strandwitz and G. D. Stucky, Blood clot initiation by mesocellular foams: dependence on nanopore size and enzyme immobilization, Langmuir, 2008, 24, 14254–14260 CrossRef CAS PubMed.
- T. Yoshida, Y. Yoshioka, S. Tochigi, T. Hirai, M. Uji, K.-I. Ichihashi, K. Nagano, Y. Abe, H. Kamada and S.-I. Tsunoda, Intranasal exposure to amorphous nanosilica particles could activate intrinsic coagulation cascade and platelets in mice, Part. Fibre Toxicol., 2013, 10, 1–12 CrossRef PubMed.
- T. Kushida, K. Saha, C. Subramani, V. Nandwana and V. M. Rotello, Effect of nano-scale curvature on the intrinsic blood coagulation system, Nanoscale, 2014, 6, 14484–14487 RSC.
- L. Feng, X. Yang, S. Liang, Q. Xu, M. R. Miller, J. Duan and Z. Sun, Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway, Part. Fibre Toxicol., 2019, 16, 1–13 CrossRef PubMed.
- D. Kudela, S. A. Smith, A. May-Masnou, G. B. Braun, A. Pallaoro, C. K. Nguyen, T. T. Chuong, S. Nownes, R. Allen and N. R. Parker, Clotting activity of polyphosphate–functionalized silica nanoparticles, Angew. Chem., 2015, 127, 4090–4094 CrossRef.
- X. Liu and J. Sun, Time-course effects of intravenously administrated silica nanoparticles on blood coagulation and endothelial function in rats, J. Nanosci. Nanotechnol., 2013, 13, 222–228 CrossRef CAS PubMed.
- C. Guo, Y. Xia, P. Niu, L. Jiang, J. Duan, Y. Yu, X. Zhou, Y. Li and Z. Sun, Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling, Int. J. Nanomed., 2015, 10, 1463 CrossRef CAS.
- L. Jiang, Y. Li, Y. Li, C. Guo, Y. Yu, Y. Zou, Y. Yang, Y. Yu, J. Duan and W. Geng, Silica nanoparticles induced the pre-thrombotic state in rats via activation of coagulation factor XII and the JNK-NF-κB/AP-1 pathway, Toxicol. Res., 2015, 4, 1453–1464 CrossRef CAS.
- J. J. Corbalan, C. Medina, A. Jacoby, T. Malinski and M. W. Radomski, Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis, Int. J. Nanomed., 2012, 7, 631 CrossRef CAS.
- J. Saikia, R. Mohammadpour, M. Yazdimamaghani, H. Northrup, V. Hlady and H. Ghandehari, Silica Nanoparticle–Endothelial Interaction: Uptake and Effect on Platelet Adhesion under Flow Conditions, ACS Appl. Bio Mater., 2018, 1, 1620–1627 CrossRef CAS.
- D. Kim, S. Finkenstaedt-Quinn, K. R. Hurley, J. T. Buchman and C. L. Haynes, On-chip evaluation of platelet adhesion and aggregation upon exposure to mesoporous silica nanoparticles, Analyst, 2014, 139, 906–913 RSC.
- R. Tavano, D. Segat, E. Reddi, J. Kos, M. Rojnik, P. Kocbek, S. Iratni, D. Scheglmann, M. Colucci and I. M. R. Echevarria, Procoagulant properties of bare and highly PEGylated vinyl-modified silica nanoparticles, Nanomedicine, 2010, 5, 881–896 CrossRef CAS PubMed.
- A. Yildirim, E. Ozgur and M. Bayindir, Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption, J. Mater. Chem. B, 2013, 1, 1909–1920 RSC.
- A. Nemmar, K. Melghit and B. H. Ali, The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats, Exp. Biol. Med., 2008, 233, 610–619 CrossRef CAS PubMed.
- N. Haberl, S. Hirn, M. Holzer, G. Zuchtriegel, M. Rehberg and F. Krombach, Effects of acute systemic administration of TiO2, ZnO, SiO2, and Ag nanoparticles on hemodynamics, hemostasis and leukocyte recruitment, Nanotoxicology, 2015, 9, 963–971 CrossRef PubMed.
- P. Bihari, M. Holzer, M. Praetner, J. Fent, M. Lerchenberger, C. A. Reichel, M. Rehberg, S. Lakatos and F. Krombach, Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation, Toxicology, 2010, 269, 148–154 CrossRef CAS PubMed.
- P. Akinola, A. Lateef, T. Asafa, L. Beukes, A. Hakeem and H. Irshad, Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: antimicrobial, dye degradation, antioxidant and anticoagulant activities, Heliyon, 2020, 6, e04610 CrossRef CAS PubMed.
- K. Lingaraju, R. Basavaraj, K. Jayanna, S. Bhavana, S. Devaraja, H. K. Swamy, G. Nagaraju, H. Nagabhushana and H. R. Naika, Biocompatible fabrication of TiO2 nanoparticles: Antimicrobial, anticoagulant, antiplatelet, direct hemolytic and cytotoxicity properties, Inorg. Chem. Commun., 2021, 127, 108505 CrossRef CAS.
- M. Šimundić, B. Drašler, V. Šuštar, J. Zupanc, R. Štukelj, D. Makovec, D. Erdogmus, H. Hägerstrand, D. Drobne and V. Kralj-Iglič, Effect of engineered TiO 2 and ZnO nanoparticles on erythrocytes, platelet-rich plasma and giant unilamelar phospholipid vesicles, BMC Vet. Res., 2013, 9, 7 CrossRef.
- J.-Y. Yang, J. Bae, A. Jung, S. Park, S. Chung, J. Seok, H. Roh, Y. Han, J.-M. Oh and S. Sohn, Surface functionalization-specific binding of coagulation factors by zinc oxide nanoparticles delays coagulation time and reduces thrombin generation potential in vitro, PLoS One, 2017, 12, e0181634 CrossRef PubMed.
- Z. Gu, S. Yan, S. Cheong, Z. Cao, H. Zuo, A. C. Thomas, B. E. Rolfe and Z. P. Xu, Layered double hydroxide nanoparticles: Impact on vascular cells, blood cells and the complement system, J. Colloid Interface Sci., 2018, 512, 404–410 CrossRef CAS PubMed.
- M. J. Akhtar, M. Ahamed and H. Alhadlaq, Gadolinium oxide nanoparticles induce toxicity in human endothelial HUVECs via lipid peroxidation, mitochondrial dysfunction and autophagy modulation, Nanomaterials, 2020, 10, 1675 CrossRef CAS PubMed.
- K. Greish, G. Thiagarajan, H. Herd, R. Price, H. Bauer, D. Hubbard, A. Burckle, S. Sadekar, T. Yu and A. Anwar, Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles, Nanotoxicology, 2012, 6, 713–723 CrossRef CAS PubMed.
- M. A. Dobrovolskaia, A. K. Patri, J. Simak, J. B. Hall, J. Semberova, S. H. De Paoli Lacerda and S. E. McNeil, Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro, Mol. Pharm., 2012, 9, 382–393 CrossRef CAS.
- S. J. Marrink, A. H. De Vries and A. E. Mark, Coarse grained model for semiquantitative lipid simulations, J. Phys. Chem. B, 2004, 108, 750–760 CrossRef CAS.
- C. F. Jones, R. A. Campbell, Z. Franks, C. C. Gibson, G. Thiagarajan, A. Vieira-de-Abreu, S. Sukavaneshvar, S. F. Mohammad, D. Y. Li and H. Ghandehari, Cationic PAMAM dendrimers disrupt key platelet functions, Mol. Pharm., 2012, 9, 1599–1611 CrossRef CAS.
- A. E. Enciso, B. Neun, J. Rodriguez, A. P. Ranjan, M. A. Dobrovolskaia and E. E. Simanek, Nanoparticle effects on human platelets in vitro: A comparison between PAMAM and triazine dendrimers, Molecules, 2016, 21, 428 CrossRef PubMed.
- J. Koziara, J. Oh, W. Akers, S. Ferraris and R. Mumper, Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles, Pharm. Res., 2005, 22, 1821–1828 CrossRef CAS PubMed.
- R. Juliano, M. Hsu, D. Peterson, S. Regen and A. Singh, Interactions of conventional or photopolymerized liposomes with platelets in vitro, Exp. Cell Res., 1983, 146, 422–427 CrossRef CAS PubMed.
- L. Reinish, M. Bally, H. Loughrey and P. Cullis, Interactions of liposomes and platelets, Thromb. Haemostasis, 1988, 59, 518–523 Search PubMed.
- G. Zbinden, H. Wunderli-Allenspach and L. Grimm, Assessment of thrombogenic potential of liposomes, Toxicology, 1989, 54, 273–280 CrossRef CAS PubMed.
- I. Constantinescu, E. Levin and M. Gyongyossy-Issa, Liposomes and blood cells: a flow cytometric study, Artif. Cells, Blood Substitutes, Biotechnol., 2003, 31, 395–424 CrossRef CAS.
- E. Smyth, A. Solomon, A. Vydyanath, P. K. Luther, S. Pitchford, T. D. Tetley and M. Emerson, Induction and enhancement of platelet aggregation in vitro and in vivo by model polystyrene nanoparticles, Nanotoxicology, 2015, 9, 356–364 CrossRef CAS.
- C. McGuinnes, R. Duffin, S. Brown, N. L. Mills, I. L. Megson, W. MacNee, S. Johnston, S. L. Lu, L. Tran and R. Li, Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro, Toxicol. Sci., 2011, 119, 359–368 CrossRef CAS PubMed.
- D. C. Pan, J. W. Myerson, J. S. Brenner, P. N. Patel, A. C. Anselmo, S. Mitragotri and V. Muzykantov, Nanoparticle properties modulate their attachment and effect on carrier red blood cells, Sci. Rep., 2018, 8, 1–12 Search PubMed.
- C. Oslakovic, T. Cedervall, S. Linse and B. Dahlbäck, Polystyrene nanoparticles affecting blood coagulation, Nanomedicine, 2012, 8, 981–986 CrossRef CAS PubMed.
- F. Liu, H. Huang, Y. Gong, J. Li, X. Zhang and Y. Cao, Evaluation of in vitro toxicity of polymeric micelles to human endothelial cells under different conditions, Chem.-Biol. Interact., 2017, 263, 46–54 CrossRef CAS PubMed.
- Z. Ramtoola, P. Lyons, K. Keohane, S. W. Kerrigan, B. P. Kirby and J. G. Kelly, Investigation of the interaction of biodegradable micro–and nanoparticulate drug delivery systems with platelets, J. Pharm. Pharmacol., 2011, 63, 26–32 CrossRef CAS.
- X. Li, A. Radomski, O. I. Corrigan, L. Tajber, F. De Sousa Menezes, S. Endter, C. Medina and M. W. Radomski, Platelet compatibility of PLGA, chitosan and PLGA–chitosan nanoparticles, Nanomedicine, 2009, 4, 735–746 CrossRef CAS.
- C. Fornaguera, G. Calderó, M. Mitjans, M. P. Vinardell, C. Solans and C. Vauthier, Interactions of PLGA nanoparticles with blood components: protein adsorption, coagulation, activation of the complement system and hemolysis studies, Nanoscale, 2015, 7, 6045–6058 RSC.
- C. Mao, L.-C. Jiang, W.-P. Luo, H.-K. Liu and J.-C. Bao, Novel blood-compatible polyurethane ionomer nanoparticles, Macromolecules, 2009, 42, 9366–9368 CrossRef CAS.
- L. L. Swystun and P. C. Liaw, The role of leukocytes in thrombosis, Blood, 2016, 128, 753–762 CrossRef CAS PubMed.
- S. Luo, D. Hu, M. Wang, P. F. Zipfel and Y. Hu, Complement in hemolysis-and thrombosis-related diseases, Front. Immunol., 2020, 11, 1212 CrossRef CAS PubMed.
- B. Engelmann and S. Massberg, Thrombosis as an intravascular effector of innate immunity, Nat. Rev. Immunol., 2013, 13, 34–45 CrossRef CAS PubMed.
- V. Afshar-Kharghan, Complement and clot, Blood, 2017, 129, 2214–2215 CrossRef CAS PubMed.
- M. M. Aleman, C. Gardiner, P. Harrison and A. S. Wolberg, Differential contributions of monocyte–and platelet–derived microparticles towards thrombin generation and fibrin formation and stability, J. Thromb. Haemostasis, 2011, 9, 2251–2261 CrossRef CAS.
- A. Angelillo-Scherrer, Leukocyte-derived microparticles in vascular homeostasis, Circ. Res., 2012, 110, 356–369 CrossRef CAS.
- N. Maugeri, M. Brambilla, M. Camera, A. Carbone, E. Tremoli, M. Donati, G. De Gaetano and C. Cerletti, Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation 1, J. Thromb. Haemostasis, 2006, 4, 1323–1330 CrossRef CAS.
- M.-L. von Brühl, K. Stark, A. Steinhart, S. Chandraratne, I. Konrad, M. Lorenz, A. Khandoga, A. Tirniceriu, R. Coletti and M. Köllnberger, Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo, J. Exp. Med., 2012, 209, 819–835 CrossRef PubMed.
- R. Darbousset, G. M. Thomas, S. Mezouar, C. Frere, R. Bonier, N. Mackman, T. Renné, F. Dignat-George, C. Dubois and L. Panicot-Dubois, Tissue factor–positive neutrophils bind to injured endothelial wall and initiate thrombus formation, Blood, 2012, 120, 2133–2143 CrossRef CAS PubMed.
- D. H. Allen and P. B. Tracy, Human Coagulation Factor V Is Activated to the Functional Cofactor by Elastase and Cathepsin G Expressed at the Monocyte Surface (*), J. Biol. Chem., 1995, 270, 1408–1415 CrossRef CAS.
- A. J. Gale and D. Rozenshteyn, Cathepsin G, a leukocyte protease, activates coagulation factor VIII, Thromb. Haemostasis, 2008, 99, 44–51 CrossRef CAS PubMed.
- J. Plescia and D. C. Altieri, Activation of Mac-1 (CD11b/CD18)-bound factor X by released cathepsin G defines an alternative pathway of leucocyte initiation of coagulation, Biochem. J., 1996, 319, 873–879 CrossRef CAS.
- M. Jochum, S. Lander, N. Heimburger and H. Fritz, Effect of human granulocytic elastase on isolated human antithrombin III, 1981 Search PubMed.
- C. W. Pratt, R. B. Tobin and F. C. Church, Interaction of heparin cofactor II with neutrophil elastase and cathepsin G, J. Biol. Chem., 1990, 265, 6092–6097 CrossRef CAS.
- S. Massberg, L. Grahl, M.-L. von Bruehl, D. Manukyan, S. Pfeiler, C. Goosmann, V. Brinkmann, M. Lorenz, K. Bidzhekov and A. B. Khandagale, Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases, Nat. Med., 2010, 16, 887–896 CrossRef CAS PubMed.
- A. azzaq Belaaouaj, A. Li, T.-C. Wun, H. G. Welgus and S. D. Shapiro, Matrix metalloproteinases cleave tissue factor pathway inhibitor: effects on coagulation, J. Biol. Chem., 2000, 275, 27123–27128 CrossRef.
- D. A. Higuchi, T.-C. Wun, K. M. Likert and G. J. Broze, The effect of leukocyte elastase on tissue factor pathway inhibitor, 1992 Search PubMed.
- L. L. Swystun, S. Mukherjee and P. C. Liaw, Breast cancer chemotherapy induces the release of cell–free DNA, a novel procoagulant stimulus, J. Thromb. Haemostasis, 2011, 9, 2313–2321 CrossRef CAS PubMed.
- T. J. Gould, T. T. Vu, L. L. Swystun, D. J. Dwivedi, S. H. Mai, J. I. Weitz and P. C. Liaw, Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms, Arterioscler., Thromb., Vasc. Biol., 2014, 34, 1977–1984 CrossRef CAS PubMed.
- S. Barranco-Medina, N. Pozzi, A. D. Vogt and E. Di Cera, Histone H4 promotes prothrombin autoactivation, J. Biol. Chem., 2013, 288, 35749–35757 CrossRef CAS PubMed.
- C. T. Ammollo, F. Semeraro, J. Xu, N. Esmon and C. Esmon, Extracellular histones increase plasma thrombin generation by impairing thrombomodulin–dependent protein C activation, J. Thromb. Haemostasis, 2011, 9, 1795–1803 CrossRef CAS PubMed.
- T. Ito, K. Kawahara, T. Nakamura, S. Yamada, T. Nakamura, K. Abeyama, T. Hashiguchi and I. Maruyama, High–mobility group box 1 protein promotes development of microvascular thrombosis in rats, J. Thromb. Haemostasis, 2007, 5, 109–116 CrossRef CAS PubMed.
- T. A. Fuchs, A. Brill and D. D. Wagner, Neutrophil extracellular trap (NET) impact on deep vein thrombosis, Arterioscler., Thromb., Vasc. Biol., 2012, 32, 1777–1783 CrossRef CAS PubMed.
- I. Mitroulis, K. Kambas, A. Chrysanthopoulou, P. Skendros, E. Apostolidou, I. Kourtzelis, G. I. Drosos, D. T. Boumpas and K. Ritis, Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout, PLoS One, 2011, 6, e29318 CrossRef CAS PubMed.
- M. Menschikowski, A. Hagelgans, G. Eisenhofer and G. Siegert, Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways, Exp. Cell Res., 2009, 315, 2673–2682 CrossRef CAS PubMed.
- B. Nan, P. Lin, A. B. Lumsden, Q. Yao and C. Chen, Effects of TNF-α and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells, Thromb. Res., 2005, 115, 417–426 CrossRef CAS PubMed.
- X. Yang, L. Li, J. Liu, B. Lv and F. Chen, Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1, Thromb. Res., 2016, 137, 211–218 CrossRef CAS PubMed.
- J. Herbert, P. Savi, M. Laplace and A. Lale, IL-4 inhibits LPS-, IL-1β- and TNFα-induced expression of tissue factor in endothelial cells and monocytes, FEBS Lett., 1992, 310, 31–33 CrossRef CAS PubMed.
- A. Michels, L. L. Swystun, S. Albánez, J. Mewburn, K. Sponagle and D. Lillicrap, Histones induce endothelial von Willebrand factor release and subsequent platelet capture in in vitro and in vivo models, American Society of Hematology Washington, DC, 2014 Search PubMed.
- A. Bernardo, C. Ball, L. Nolasco, J. F. Moake and J.-F. Dong, Effects of inflammatory cytokines on the release and cleavage of the endothelial cell–derived ultralarge von Willebrand factor multimers under flow, Blood, 2004, 104, 100–106 CrossRef CAS PubMed.
- K. K. Hamilton and P. J. Sims, Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1, J. Clin. Invest., 1987, 79, 600–608 CrossRef CAS PubMed.
- J. Chen, X. Fu, Y. Wang, M. Ling, B. McMullen, J. Kulman, D. W. Chung and J. A. López, Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13, Blood, 2010, 115, 706–712 CrossRef CAS PubMed.
- P. Renesto and M. Chignard, Enhancement of cathepsin G-induced platelet activation by leukocyte elastase: consequence for the neutrophil-mediated platelet activation, 1993 Search PubMed.
- C. A. LaRosa, M. J. Rohrer, S. E. Benoit, L. J. Rodino, M. R. Barnard and A. D. Michelson, Human neutrophil cathepsin G is a potent platelet activator, J. Vasc. Surg., 1994, 19, 306–320 CrossRef CAS.
- P. Seth, R. Kumari, M. Dikshit and R. Srimal, Effect of platelet activating factor antagonists in different models of thrombosis, Thromb. Res., 1994, 76, 503–512 CrossRef CAS.
- T. A. Fuchs, A. A. Bhandari and D. D. Wagner, Histones induce rapid and profound thrombocytopenia in mice, Blood, 2011, 118, 3708–3714 CrossRef CAS PubMed.
- F. Semeraro, C. T. Ammollo, J. H. Morrissey, G. L. Dale, P. Friese, N. L. Esmon and C. T. Esmon, Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4, Blood, 2011, 118, 1952–1961 CrossRef CAS.
- F. Semeraro, C. Ammollo, N. Esmon and C. Esmon, Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells, J. Thromb. Haemostasis, 2014, 12, 1697–1702 CrossRef CAS PubMed.
- S. R. Clark, A. C. Ma, S. A. Tavener, B. McDonald, Z. Goodarzi, M. M. Kelly, K. D. Patel, S. Chakrabarti, E. McAvoy and G. D. Sinclair, Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood, Nat. Med., 2007, 13, 463–469 CrossRef CAS.
- A. Carestia, L. Rivadeneyra, M. A. Romaniuk, C. Fondevila, S. Negrotto and M. Schattner, Functional responses and molecular mechanisms involved in histone-mediated platelet activation, Thromb. Haemostasis, 2013, 110, 1035–1045 CrossRef CAS PubMed.
- P. J. Sims and T. Wiedmer, The response of human platelets to activated components of the complement system, Immunol. Today, 1991, 12, 338–342 CrossRef CAS.
- B. Ando, T. Wiedmer, K. Hamilton and P. Sims, Complement proteins C5b-9 initiate secretion of platelet storage granules without increased binding of fibrinogen or von Willebrand factor to newly expressed cell surface GPIIb-IIIa, J. Biol. Chem., 1988, 263, 11907–11914 CrossRef CAS.
- M. J. Polley and R. L. Nachman, Human complement in thrombin-mediated platelet function: uptake of the C5b-9 complex, J. Exp. Med., 1979, 150, 633–645 CrossRef CAS.
- P. J. Sims, E. Faioni, T. Wiedmer and S. Shattil, Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity, J. Biol. Chem., 1988, 263, 18205–18212 CrossRef CAS.
- T. Wiedmer, C. T. Esmon and P. J. Sims, Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase, 1986 Search PubMed.
- M. J. Polley and R. Nachman, The human complement system in thrombin-mediated platelet function, J. Exp. Med., 1978, 147, 1713–1726 CrossRef CAS PubMed.
- S. Subramaniam, K. Jurk, L. Hobohm, S. Jäckel, M. Saffarzadeh, K. Schwierczek, P. Wenzel, F. Langer, C. Reinhardt and W. Ruf, Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development, Blood, 2017, 129, 2291–2302 CrossRef CAS PubMed.
- E. Peerschke and B. Ghebrehiwet, C1q augments platelet activation in response to aggregated Ig, J. Immunol., 1997, 159, 5594–5598 CAS.
- T. Wiedmer, S. Hall, T. Ortel, W. Kane, W. Rosse and P. Sims, Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria, 1993 Search PubMed.
- P. F. Zipfel and C. Skerka, Complement regulators and inhibitory proteins, Nat. Rev. Immunol., 2009, 9, 729–740 CrossRef CAS PubMed.
- J. Wojta, C. Kaun, G. Zorn, M. Ghannadan, A. W. Hauswirth, W. R. Sperr, G. Fritsch, D. Printz, B. R. Binder and G. Schatzl, C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils, Blood, 2002, 100, 517–523 CrossRef CAS PubMed.
- J. Wojta, K. Huber and P. Valent, New aspects in thrombotic research: complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype, Pathophysiol. Haemostasis Thromb., 2003, 33, 438–441 CrossRef PubMed.
- K. Ikeda, K. Nagasawa, T. Horiuchi, T. Tsuru, H. Nishizaka and Y. Niho, C5a induces tissue factor activity on endothelial cells, Thromb. Haemostasis, 1997, 77, 394–398 CrossRef CAS.
- K. Ritis, M. Doumas, D. Mastellos, A. Micheli, S. Giaglis, P. Magotti, S. Rafail, G. Kartalis, P. Sideras and J. D. Lambris, A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways, J. Immunol., 2006, 177, 4794–4802 CrossRef CAS PubMed.
- K. C. Gulla, K. Gupta, A. Krarup, P. Gal, W. J. Schwaeble, R. B. Sim, C. D. O'Connor and K. Hajela, Activation of mannan–binding lectin–associated serine proteases leads to generation of a fibrin clot, Immunology, 2010, 129, 482–495 CrossRef CAS PubMed.
- K. Hess, R. Ajjan, F. Phoenix, J. Dobó, P. Gál and V. Schroeder, Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation, PLoS One, 2012, 7, e35690 CrossRef CAS PubMed.
- H. Kozarcanin, C. Lood, L. Munthe-Fog, K. Sandholm, O. A. Hamad, A. A. Bengtsson, M. O. Skjoedt, M. Huber-Lang, P. Garred and K. N. Ekdahl, The lectin complement pathway serine proteases (MASPs) represent a possible crossroad between the coagulation and complement systems in thromboinflammation, J. Thromb. Haemostasis, 2016, 14, 531–545 CrossRef CAS PubMed.
- R. Kölm, M. Schaller, L. T. Roumenina, I. Niemiec, J. A. K. Hovinga, E. Khanicheh, B. A. Kaufmann, H. Hopfer and M. Trendelenburg, Von Willebrand factor interacts with surface-bound C1q and induces platelet rolling, J. Immunol., 2016, 197, 3669–3679 CrossRef PubMed.
- E. I. Peerschke, T. K. Murphy and B. Ghebrehiwet, Activation-dependent surface expression of gC1qR/p33 on human blood platelets, Thromb. Haemostasis, 2003, 89, 331–339 CrossRef CAS.
- K. N. Ekdahl and B. Nilsson, Alterations in C3 activation and binding caused by phosphorylation by a casein kinase released from activated human platelets, J. Immunol., 1999, 162, 7426–7433 CAS.
- P. J. Sims and T. Wiedmer, Repolarization of the membrane potential of blood platelets after complement damage: evidence for a Ca++-dependent exocytotic elimination of C5b-9 pores, 1986 Search PubMed.
- A. E. Davis III, P. Mejia and F. Lu, Biological activities of C1 inhibitor, Mol. Immunol., 2008, 45, 4057–4063 CrossRef.
- E. Conway, Reincarnation of ancient links between coagulation and complement, J. Thromb. Haemostasis, 2015, 13, S121–S132 CrossRef CAS PubMed.
- B. Ghebrehiwet, A. P. Kaplan, K. Joseph and E. I. Peerschke, The complement and contact activation systems: partnership in pathogenesis beyond angioedema, Immunol. Rev., 2016, 274, 281–289 CrossRef CAS PubMed.
- D. Ermert and A. M. Blom, C4b-binding protein: the good, the bad and the deadly. Novel functions of an old friend, Immunol. Lett., 2016, 169, 82–92 CrossRef CAS PubMed.
- J. Palomaki, E. Valimaki, J. Sund, M. Vippola, P. A. Clausen, K. A. Jensen, K. Savolainen, S. Matikainen and H. Alenius, Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism, ACS Nano, 2011, 5, 6861–6870 CrossRef CAS PubMed.
- X. Wang, T. Xia, S. Addo Ntim, Z. Ji, S. Lin, H. Meng, C.-H. Chung, S. George, H. Zhang and M. Wang, Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung, ACS Nano, 2011, 5, 9772–9787 CrossRef CAS PubMed.
- S. M. Chowdhury, S. Kanakia, J. D. Toussaint, M. D. Frame, A. M. Dewar, K. R. Shroyer, W. Moore and B. Sitharaman, In vitro hematological and in vivo vasoactivity assessment of dextran functionalized graphene, Sci. Rep., 2013, 3, 1–10 Search PubMed.
- N. Nishijima, T. Hirai, K. Misato, M. Aoyama, E. Kuroda, K. J. Ishii, K. Higashisaka, Y. Yoshioka and Y. Tsutsumi, Human scavenger receptor A1-mediated inflammatory response to silica particle exposure is size specific, Front. Immunol., 2017, 8, 379 Search PubMed.
- D. Simberg, J.-H. Park, P. P. Karmali, W.-M. Zhang, S. Merkulov, K. McCrae, S. N. Bhatia, M. Sailor and E. Ruoslahti, Differential proteomics analysis of the surface heterogeneity of dextran iron oxide nanoparticles and the implications for their in vivo clearance, Biomaterials, 2009, 30, 3926–3933 CrossRef CAS PubMed.
- H. Gaikwad, Y. Li, G. Gifford, E. Groman, N. K. Banda, L. Saba, R. Scheinman, G. Wang and D. Simberg, Complement Inhibitors Block Complement C3 Opsonization and Improve Targeting Selectivity of Nanoparticles in Blood, Bioconjugate Chem., 2020, 31, 1844–1856 CrossRef CAS PubMed.
- T. Fülöp, R. Nemes, T. Mészáros, R. Urbanics, R. J. Kok, J. A. Jackman, N.-J. Cho, G. Storm and J. Szebeni, Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: Correlation with physicochemical features and clinical information, J. Controlled Release, 2018, 270, 268–274 CrossRef PubMed.
- R. Bilyy, H. Unterweger, B. Weigel, T. Dumych, S. Paryzhak, V. Vovk, Z. Liao, C. Alexiou, M. Herrmann and C. Janko, Inert coats of magnetic nanoparticles prevent formation of occlusive intravascular co-aggregates with neutrophil extracellular traps, Front. Immunol., 2018, 9, 2266 CrossRef PubMed.
- Y. Yang, N. Wang, Y. Zhu, Y. Lu, Q. Chen, S. Fan, Q. Huang, X. Chen, L. Xia and Y. Wei, Gold nanoparticles synergize with bacterial lipopolysaccharide to enhance class A scavenger receptor dependent particle uptake in neutrophils and augment neutrophil extracellular traps formation, Ecotoxicol. Environ. Saf., 2021, 211, 111900 CrossRef CAS PubMed.
- J. Szebeni, L. Baranyi, S. Savay, M. Bodo, D. S. Morse, M. Basta, G. L. Stahl, R. Bünger and C. R. Alving, Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction, Am. J. Physiol.: Heart Circ. Physiol., 2000, 279, H1319–H1328 CrossRef CAS PubMed.
- A. Chonn, P. Cullis and D. Devine, The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes, J. Immunol., 1991, 146, 4234–4241 CAS.
- Z. Wang and J. S. Brenner, The Nano-War Against Complement Proteins, AAPS J., 2021, 23, 1–8 CrossRef PubMed.
- G. Gifford, V. P. Vu, N. K. Banda, V. M. Holers, G. Wang, E. V. Groman, D. Backos, R. Scheinman, S. M. Moghimi and D. Simberg, Complement therapeutics meets nanomedicine: overcoming human complement activation and leukocyte uptake of nanomedicines with soluble domains of CD55, J. Controlled Release, 2019, 302, 181–189 CrossRef CAS PubMed.
- F. Alexis, E. Pridgen, L. K. Molnar and O. C. Farokhzad, Factors affecting the clearance and biodistribution of polymeric nanoparticles, Mol. Pharm., 2008, 5, 505–515 CrossRef CAS PubMed.
- M. A. Ashraf, W. Peng, Y. Zare and K. Y. Rhee, Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites, Nanoscale Res. Lett., 2018, 13, 1–7 CrossRef CAS PubMed.
- A. T. Bauer, E. A. Strozyk, C. Gorzelanny, C. Westerhausen, A. Desch, M. F. Schneider and S. W. Schneider, Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells, Biomaterials, 2011, 32, 8385–8393 CrossRef CAS PubMed.
- A. Nemmar, S. Albarwani, S. Beegam, P. Yuvaraju, J. Yasin, S. Attoub and B. H. Ali, Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation, Int. J. Nanomed., 2014, 9, 2779 CrossRef PubMed.
- J. Meng, X. Cheng, J. Liu, W. Zhang, X. Li, H. Kong and H. Xu, Effects of long and short carboxylated or aminated multiwalled carbon nanotubes on blood coagulation, PLoS One, 2012, 7, e38995 CrossRef PubMed.
- Y. He, J. Xu, X. Sun, X. Ren, A. Maharjan, P. York, Y. Su, H. Li and J. Zhang, Cuboidal tethered cyclodextrin frameworks tailored for hemostasis and injured vessel targeting, Theranostics, 2019, 9, 2489 CrossRef CAS.
- M. Holzer, P. Bihari, M. Praetner, B. Uhl, C. Reichel, J. Fent, M. Vippola, S. Lakatos and F. Krombach, Carbon–based nanomaterials accelerate arteriolar thrombus formation in the murine microcirculation independently of their shape, J. Appl. Toxicol., 2014, 34, 1167–1176 CrossRef CAS PubMed.
- O. L. Gobbo, K. Sjaastad, M. W. Radomski, Y. Volkov and A. Prina-Mello, Magnetic nanoparticles in cancer theranostics, Theranostics, 2015, 5, 1249 CrossRef CAS PubMed.
- Y. Su, L. Zhao, F. Meng, Q. Wang, Y. Yao and J. Luo, Silver nanoparticles decorated lipase-sensitive polyurethane micelles for on-demand release of silver nanoparticles, Colloids Surf., B, 2017, 152, 238–244 CrossRef CAS PubMed.
- M. Yu, S. Huang, K. J. Yu and A. M. Clyne, Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture, Int. J. Mol. Sci., 2012, 13, 5554–5570 CrossRef CAS PubMed.
- M. J. Santos-Martinez, K. Rahme, J. J. Corbalan, C. Faulkner, J. D. Holmes, L. Tajber, C. Medina and M. W. Radomski, Pegylation increases platelet biocompatibility of gold nanoparticles, J. Biomed. Nanotechnol., 2014, 10, 1004–1015 CrossRef CAS PubMed.
- S. M. Chowdhury, S. Kanakia, J. D. Toussaint, M. D. Frame, A. M. Dewar, K. R. Shroyer, W. Moore and B. Sitharaman, In vitro hematological and in vivo vasoactivity assessment of dextran functionalized graphene, Sci. Rep., 2013, 3, 2584 CrossRef PubMed.
- S. H. Hsu, C. M. Tang and H. J. Tseng, Biocompatibility of poly (ether) urethane–gold nanocomposites, J. Biomed. Mater. Res., Part A, 2006, 79, 759–770 CrossRef PubMed.
- M. Miyamoto, S. Sasakawa, T. Ozawa, H. Kawaguchi and Y. Ohtsuka, Mechanisms of blood coagulation induced by latex particles and the roles of blood cells, Biomaterials, 1990, 11, 385–388 CrossRef CAS PubMed.
- L. Kou, J. Sun, Y. Zhai and Z. He, The endocytosis and intracellular fate of nanomedicines: Implication for rational design, Asian J. Pharm. Sci., 2013, 8, 1–10 CrossRef CAS.
- Y. Yang, Y. Lai, Q. Zhang, K. Wu, L. Zhang, C. Lin and P. Tang, A novel electrochemical strategy for improving blood compatibility of titanium-based biomaterials, Colloids Surf., B, 2010, 79, 309–313 CrossRef CAS PubMed.
- I. Standard, ISO 10993–4: Biological Evaluation of Medical Devices Part 4—Selection of Tests for Interactions with Blood, International Organization for Standardization, Geneva, Switzerland, 2017 Search PubMed.
- P. Urbán, N. J. Liptrott and S. Bremer, Overview of the blood compatibility of nanomedicines: A trend analysis of in vitro and in vivo studies, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1546 Search PubMed.
- H. K. Kim, M.-J. Choi, S.-H. Cha, Y. K. Koo, S. H. Jun, S. Cho and Y. Park, Earthworm extracts utilized in the green synthesis of gold nanoparticles capable of reinforcing the anticoagulant activities of heparin, Nanoscale Res. Lett., 2013, 8, 1–7 CrossRef.
- V. Gryshchuk and N. Galagan, Silica nanoparticles effects on blood coagulation proteins and platelets, Biochem. Res. Int., 2016, 2016, 2959414 Search PubMed.
- A. Guildford, T. Poletti, L. Osbourne, A. Di Cerbo, A. Gatti and M. Santin, Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response, J. R. Soc., Interface, 2009, 6, 1213–1221 CrossRef CAS PubMed.
- C. Santos, S. Turiel, P. S. Gomes, E. Costa, A. Santos-Silva, P. Quadros, J. Duarte, S. Battistuzzo and M. H. Fernandes, Vascular biosafety of commercial hydroxyapatite particles: discrepancy between blood compatibility assays and endothelial cell behavior, J. Nanobiotechnol., 2018, 16, 1–15 CrossRef PubMed.
- A. K. Sharma, A. K. Swami, M. Saran and M. Mathur, Synthesis of tungsten nanoparticles for their biomedical application, Int. J. Pharm. Sci. Res., 2020, 11, 4070–4077 CAS.
- H. Derakhshankhah, M. J. Hajipour, E. Barzegari, A. Lotfabadi, M. Ferdousi, A. A. Saboury, E. P. Ng, M. Raoufi, H. Awala and S. Mintova, Zeolite nanoparticles inhibit Aβ–fibrinogen interaction and formation of a consequent abnormal structural clot, ACS Appl. Mater. Interfaces, 2016, 8, 30768–30779 CrossRef CAS PubMed.
- H. Li, X. Zhang, X. Lin, S. Zhuang, Y. Wu, Z. Liu, J. Rong and J. Zhao, CaCO 3 nanoparticles pH-sensitively induce blood coagulation as a potential strategy for starving tumor therapy, J. Mater. Chem. B, 2020, 8, 1223–1234 RSC.
- S. Del Turco, G. Ciofani, V. Cappello, P. Parlanti, M. Gemmi, C. Caselli, R. Ragusa, A. Papa, D. Battaglia and L. Sabatino, Effects of cerium oxide nanoparticles on hemostasis: Coagulation, platelets, and vascular endothelial cells, J. Biomed. Mater. Res., Part A, 2019, 107, 1551–1562 CrossRef CAS PubMed.
- J. Matuszak, J. Baumgartner, J. Zaloga, M. Juenet, A. E. Da Silva, D. Franke, G. Almer, I. Texier, D. Faivre and J. M. Metselaar, Nanoparticles for intravascular applications: physicochemical characterization and cytotoxicity testing, Nanomedicine, 2016, 11, 597–616 CrossRef CAS PubMed.
- E. Fuentes, B. Yameen, S.-J. Bong, C. Salvador-Morales, I. Palomo and C. Vilos, Antiplatelet effect of differentially charged PEGylated lipid-polymer nanoparticles, Nanomedicine, 2017, 13, 1089–1094 CrossRef CAS PubMed.
- D. Yu, Y. Zhang, X. Zhou, Z. Mao and C. Gao, Influence of surface coating of PLGA particles on the internalization and functions of human endothelial cells, Biomacromolecules, 2012, 13, 3272–3282 CrossRef CAS PubMed.
- R. Bakhaidar, S. O'Neill and Z. Ramtoola, PLGA-PEG Nanoparticles Show Minimal Risks of Interference with Platelet Function of Human Platelet-Rich Plasma, Int. J. Mol. Sci., 2020, 21, 9716 CrossRef CAS PubMed.
- L. C. R. P. da Silva, V. Todaro, F. A. do Carmo, F. S. Frattani, V. P. de Sousa, C. R. Rodrigues, P. C. Sathler and L. M. Cabral, A promising oral fucoidan-based antithrombotic nanosystem: development, activity and safety, Nanotechnology, 2018, 29, 165102 CrossRef PubMed.
- L. C. da Silva, T. Garcia, M. Mori, G. Sandri, M. C. Bonferoni, P. V. Finotelli, L. P. Cinelli, C. Caramella and L. M. Cabral, Preparation and characterization of polysaccharide-based nanoparticles with anticoagulant activity, Int. J. Nanomed., 2012, 7, 2975 CrossRef CAS PubMed.
- S. Luo, H. Man, X. Jia, Y. Li, A. Pan, X. Zhang and Y. Song, Preparation and characterization of acetylsalicylic acid/chitosan nanoparticles and its antithrombotic effects, Des. Monomers Polym., 2018, 21, 172–181 CrossRef CAS PubMed.
- Q. Han, X. Chen, Y. Niu, B. Zhao, B. Wang, C. Mao, L. Chen and J. Shen, Preparation of water-soluble hyperbranched polyester nanoparticles with sulfonic acid functional groups and their micelles behavior, anticoagulant effect and cytotoxicity, Langmuir, 2013, 29, 8402–8409 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |