Recent advances in in situ oxygen-generating and oxygen-replenishing strategies for hypoxic-enhanced photodynamic therapy
Shuheng
Qin
,
Yue
Xu
,
Hua
Li
,
Haiyan
Chen
 * and
Zhenwei
Yuan
* and
Zhenwei
Yuan
 *
*
Department of Biomedical Engineering, School of Engineering, China Pharmaceutical University, 639 Longmian Road, Jiangning District, Nanjing 210009, China. E-mail: yuanzhenwei@cpu.edu.cn; chenhaiyan@cpu.edu.cn; Fax: +86-25-83271046; Tel: +86-25-83271080
First published on 3rd April 2021
Abstract
Cancer is a leading cause of death worldwide, accounting for an estimated 10 million deaths by 2020. Over the decades, various strategies for tumor therapy have been developed and evaluated. Photodynamic therapy (PDT) has attracted increasing attention due to its unique characteristics, including low systemic toxicity and minimally invasive nature. Despite the excellent clinical promise of PDT, hypoxia is still the Achilles’ heel associated with its oxygen-dependent nature related to increased tumor proliferation, angiogenesis, and distant metastases. Moreover, PDT-mediated oxygen consumption further exacerbates the hypoxia condition, which will eventually lead to the poor effect of drug treatment and resistance and irreversible tumor metastasis, even limiting its effective application in the treatment of hypoxic tumors. Hypoxia, with increased oxygen consumption, may occur in acute and chronic hypoxia conditions in developing tumors. Tumor cells farther away from the capillaries have much lower oxygen levels than cells in adjacent areas. However, it is difficult to change the tumor's deep hypoxia state through different ways to reduce the tumor tissue's oxygen consumption. Therefore, it will become more difficult to cure malignant tumors completely. In recent years, numerous investigations have focused on improving PDT therapy's efficacy by providing molecular oxygen directly or indirectly to tumor tissues. In this review, different molecular oxygen supplementation methods are summarized to alleviate tumor hypoxia from the innovative perspective of using supplemental oxygen. Besides, the existing problems, future prospects and potential challenges of this strategy are also discussed.
1. Introduction
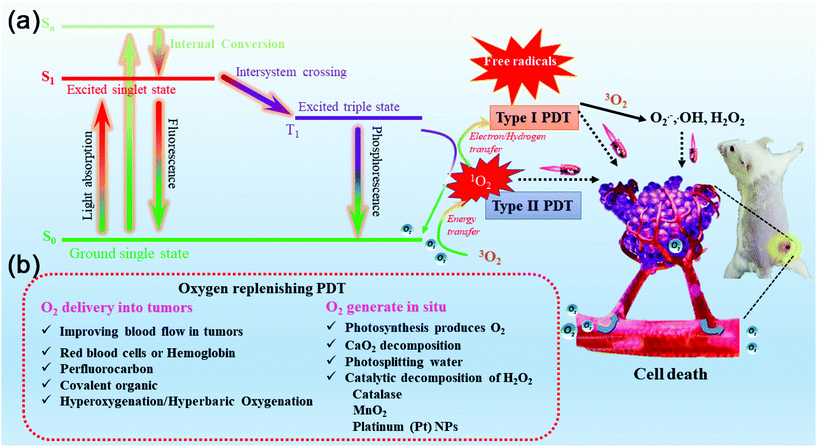
PDT is a promising modality for cancer treatment.1 It uses a photosensitizer in the presence of oxygen to ablate cancer cells by generating reactive oxygen (ROS) at a specific wavelength of irradiation.2 ROS species include singlet oxygen 1O2 (Type II PDT) and superoxide anion radical (O2˙−), hydroxyl radical (HO˙), and hydrogen peroxide (H2O2) (Type I PDT).3,4 The two photochemical pathways for the photo-inducement of photosensitizers are classified as type I and type II. In most cases, the effect is achieved through a type II mechanism involving the conversion of triplet ground molecular oxygen (3O2) to its highly reactive singlet oxygen (1O2) analog.3,5 However, the treatment method of type I PDT has also been reported in recent years.6–9 This is the direct transfer of electrons or hydrogen atoms between photosensitizer (PS) and substrate molecules, usually resulting in the production of free radicals.10,11 This process does not involve a large amount of O2 consumption, and the fluorophore is a phenothiazine derivative.12 Peng's research group has conducted a study on photodynamic therapy to improve hypoxia by using this mechanism.11,12 The difference between the two different types of photodynamic therapy lies in whether the therapy process is oxygen-dependent, and the mechanism of the two treatment modes is shown in Fig. 1a. Most of the existing PDT systems (Type II) are highly dependent on O2 and consume a large amount of O2 during treatment.13 The critical problem affecting the result of PDT is that the tumor's hypoxia and oxygen consumption during treatment will aggravate the degree of hypoxia,14 thus making the treatment effect worse.15,16 In solid tumors, O2 concentration varies with location, and oxygen levels in some internal regions are very low (partial pressure of O2 < 2.5 mmHg, severe tumor hypoxia),17,18 contributing to the limited effectiveness of drug (doxorubicin) treatment,19 simultaneously due to the disruption of vascular growth factor balance, tumor invasiveness and resistance. To make matters worse, hypoxia in tumor tissue increases genetic instability, tumor progression, and metastasis-related problems.20,21 while inhibiting the tumor response to cytotoxicity and targeted therapy; hypoxia is present in most cancer types,22,23 common ones include pancreatic, breast, lung and prostate cancer. In recent years, many efforts have been made to defeat the photodynamic disturbance caused by hypoxia; among them, the method of increasing the O2 supply of cancer tissues to treat cancer has also been proposed.17,24,25 The core factor why anticancer drugs can hardly reach the tumor site is the separation of hypoxic tumor cells from blood.26 In this case, only a few given drugs can finally get to hypoxic tumors due to the hypoxia of the microenvironment, the decrease in drug reactivity and the decrease in affinity with DNA molecules, which are the Achilles’ heel of the treatment process.27,28 The hyperbaric oxygen (HBO) treatment method has been proposed and related studies have been carried out.29 Pure oxygen is provided to patients in a pressurized chamber, breathing hyperbaric oxygen increases the pressure of oxygen, and when breathing, the oxygen content increases from 20% (50 Torr) to 100% (150–350 Torr) at 1 atm, which can advantageously reduce the hypoxia in tumor tissue and increase the sensitivity of tumor cells to drugs, increasing the curative effect in the treatment of tumor. The direct transport of oxygen molecules has been achieved using perfluorocarbon (PFC) materials approved by the FDA, such as C5F12. However, the problems of oxygen leakage and a frequent dose of tumor oxygenation strategy based on PFC have not been solved until now, which worsens the results of photodynamic therapy. Great achievements have been made in the study of anti-hypoxia-mediated tumor PDT; commonly used strategies of O2 supplementation include using organic or inorganic catalysts to catalyze endogenous H2O2,30–32 water splitting,33,34 oxygen transportation,35 PFC, and respiratory inhibitors,36,37 and the primary purpose is to improve the therapeutic effect of PDT by relieving hypoxia.38–41 So far, the primary methods are divided into two categories, namely: (1) improve the tumor microenvironment and relieve hypoxia; and (2) improve tumor hypoxia through various oxygen delivery carriers, for example, nanoparticles designed according to different mechanisms for tumor oxygenation and enhanced cancer therapy, including fluorocarbons, hemoglobin, and oxygen-carrying nano-platforms.2. Improve the tumor microenvironment and relieve hypoxia
2.1 Photosynthesis produces O2
In the emergence and evolution of life on Earth, the primitive photosynthesis of microorganisms such as cyanobacteria and Archaea has been essential for the generation of molecular oxygen.42 Photosynthetic cyanobacteria are a kind of prokaryote that can grow with solar energy and CO2 as the only energy and carbon source.43 Microalgae are naturally occurring single-celled microorganisms capable of photosynthesis that are used for biofuel and nutrition applications and animal feed, and as myocardial oxygenation factors for tissue-healing myocardial repair.44 It has been reported that engineered living microalgae are transported to anoxic tumor areas to increase local oxygen levels and re-sensitize drug-resistant cancer cells to radiation and light, and heat. Through the study, we found that the oxygen produced in situ through photosynthesis mediated by microalgae significantly improves the anoxic environment in the tumor, resulting in a significant effect of radiotherapy, and many literature studies have reported that Chlorella contains a variety of immunostimulatory substances, which can be used for the immunotherapy of tumors at the same time.45–47 Oxygen production mainly depends on the active oxygen produced by the chlorophyll of microalgae during laser irradiation, which further enhances photosensitivity and tumor cell apoptosis. It is an innovative treatment strategy for photosynthesis to produce oxygen to alleviate tumor hypoxia.The tumor's hypoxic environment limits the cytotoxicity of many chemotherapeutic agents by blocking the ROS that are produced within the cell and leads to the inevitable recurrence and metastasis of cancer. Therefore, hypoxic tumors are rarely cured, but hypoxia in tumor tissues has been explored. Microbiology has provided some important reference value for researchers in agriculture, industry and public health research fields. As early as the late 19th century, W. Busch and W. Coley began trying to treat tumors with microorganisms.48 They treated sarcoma patients independently with Streptococcus pyogenes and Serratia marcescens and observed significant improvements in malignant tumors after treatment. This application is more promising because bacteria have excellent tumor targeting ability and are easy to obtain.49 In recent years, masses of studies have used these characteristics of bacteria to target tumor tissue, activate the nano-system in different ways in situ, and finally achieve drug release or combination therapy and accurate cancer treatment44,50
Against this background, Shi and collaborators have reported a chlorin e6 (Ce6)-integrated photosensitive cell.51 This was established by the modification of cyanobacteria (producing O2 by photosynthesis) with the photosensitizer Ce6. With the irradiation of a 660 nm laser, photosynthesis produces O2, which is directly used by the PDT process and increases the PDT effect (Fig. 2a). In addition to the application of Ce6 photosensitizer, the study was also extended to another negatively charged chlorin-based photosensitizer, protoporphyrin (Ppix), to construct Ppix hybrid cyanobacteria cells (Ppix-Cyan). With free Ce6, with different laser power densities (10, 20, and 30 mW cm−2), the change of dissolved oxygen (DO) in solution fluctuates over the range of 10 mmol−1, indicating the dynamic equilibrium of oxygen dissolution–evaporation. Hybrid bacteria ceCyan can increase the DO to 24.7 μmol L−1 after 10 minutes of 20 mW cm−2 excitation power, and on increasing this to 30 mW cm−2, DO significantly increased by 2.34 times (compared with the lower power of 20 mW cm−2). This indicated that cyanobacteria had high photosynthetic and oxygenation performance (Fig. 2b). When using the free radical trapping agent 2,2,6,6-tetramethylpiperidine (TEMP) to verify the free radicals’ production, cyanobacteria were added, and the free radicals increased gradually with the increase of concentration, which further indicated that O2 supplementation would increase the production of ROS in the process of PDT. The promoting effect of oxygen mitigation on ROS was also explored, and a Singlet Oxygen Sensor Green (SOSG) probe was used to observe the production of 1O2 in cells. After cyanobacteria, free Ce6 photosensitizer and ceCyan cells were co-incubated with the same intensity and time of light exposure and were observed by confocal fluorescence microscope; bright fluorescence of SOSG could only be observed in ceCyan-PDT treated cells, which indicated that significant 1O2 was produced by cascade photosynthesis and photodynamics. The hypoxia indicator [Ru(dpp)3]Cl2 was also used to further verify that the hybrid bacteria irradiated by laser changed the state of hypoxia (Fig. 2c). The oxygenation effect on tumor in vivo was studied by photoacoustic (PA) imaging accompanied by B-mode ultrasound (US) imaging using oxygen saturation mode (SO2), showing the continuous effect of oxygen production in vivo (Fig. 2d).
 | ||
| Fig. 2 (a) Schematic diagram of the photodynamic process of photosynthesis enhancement. (b) After 660 nm laser irradiation for 10 minutes, the DO level changes (with or without cyanobacteria). (c) A hypoxia-sensitive probe and ROS indicator probe indicate the degree of hypoxia and ROS production of 4T1 cells after different treatments. (d) US and PA imaging and their combined images were performed on the tumor sections after intracranial injection of cyanobacteria and subsequent laser irradiation for tumor oxidation in vivo. Reproduced from ref. 51 with permission from Wiley, copyright 2019. | ||
Gold nanorods (AuNRs) showed excellent performance in expanding the tumor vasculature; after continuously irradiating with an NIR laser, the local temperature would increase to 42 °C,52 and thus the results of this can be utilized for promoting the release of oxygen-dependent drugs in hypoxic tumors.53 Similarly, a Chlorella AuNRs BSA-Gel was developed by Yu Seok Youn's research group;54 this is a BSA-PEG-based hydrogel library system containing Chlorella and gold nanorods. AuNRs are an attractive material, as high temperature can cause irreversible damage to tumor cells.55,56 Chlorella can produce oxygen in local tumor tissue through photosynthesis under 808 nm excitation, without increasing whole-body oxygen content. The tumor will be ablated, together with using the drug doxorubicin after relieving tumor hypoxia. CT imaging of the tumor tissue with Chlorella AuNRsBSA-Gel was undertaken.
In contrast to the PBS control group, the imaging ability of this system was good. The photosynthetic oxygen-production capacity of AuNRs BSA-Gel and the control group with or without 660 nm laser irradiation was evaluated. The data show its superior performance in oxygen production. The intracellular ROS production level of each group of 4T1 cells was observed by CLSM under normoxic and anoxic conditions. It was also proved that drug treatment with Dox was needed to produce ROS under the condition of oxygen to realize the ablation of tumor cells. The study on the anti-tumor effect of Chlorella AuNRs BSA-Gel on mice transplanted with 4T1 cells showed that the production of oxygen in the presence of Chlorella could promote tumor ablation.
Through Chlorella photosynthesis, the oxygen generation strategy can be combined into different nano-systems, effectively providing oxygen to hypoxic tumor cells.57,58 Both chemotherapy drugs and photodynamic therapy have high application expectations in treating hypoxic tumors.59 Jiang's research group designed an autotrophic light-triggered green oxygen supply machine (ALGAE),60 which was constituted of Chlorella pyrenoidosa and alginate by calcium cross-linking. The photodynamic oxygen generation ability induced by the algal system is different. After verification, they found that the oxygen production and repetitive oxygen production of Chlorella under irradiation conditions were better than in the dark. When Ce6 is irradiated by a 635 nm laser, the energy is transferred from Ce6 to the supplementary oxygen produced by the algae system, creating more singlet oxygen. Oxygen produced by photosynthesis can alleviate hypoxia during PDT therapy, down-regulate HIF-1α and VEGF expression, and up-regulate the expression of E-cadherin. This is consistent with the previously reported hypoxia-inducible HIF-1α inducing up-regulation of VEGF expression and E-cadherin loss, even with tumor progression and metastasis.61,62 The wound healing experiment showed the comparison group's cell fusion rate, and those of the routine PDT treatment group, and algae PDT treatment group after 24 hours, and indicated that PDT with algae treatment can better inhibit cell metastasis. Through H&E staining and TUNEL, and in vivo anticancer therapy, we can conclude that oxygen production by photosynthesis, which relied on algae implanted near the tumor tissue, can increase the proportion of apoptosis induced by PDT. The anti-cancer treatment in vivo was studied by using the model of the 4T1 tumor-bearing mouse, and the therapeutic potential of algae was proved again. The active photosynthetic bacteria Syne was also used for PDT.50 The photosensitized encapsulated nanoparticles (HSA/ICG) were assembled by intermolecular disulfide cross-linking and linked to the surface of the Syne. Then takes shape a biomimetic system (S/HSA/ICG) with photosynthesis and PDT effects (Fig. 3a). Syne is used as the targeted photosensitizer promoting the delivery of ICG to the tumor (FL images of the mice up to 72 h) and photocatalytic oxygen production in situ. It is verified by PA imaging that Syne can achieve an anti-tumor immune response with 660 nm laser irradiation through mediation of O2 self-sufficient PDT and immunogenic cell death (ICD). And, the temperature of S/HSA/ICG irradiated by 808 nm laser is as high as 77.8 °C. The synergistic effect of PDT/PTT can induce 84% cell death, achieve good bactericidal ability, and affect the viability and growth of bacteria to a great extent. After intravenous injection of S/HSA/ICG into mice, it accumulates effectively under laser irradiation through photosynthesis, continuously produces oxygen, significantly relieves tumor hypoxia, and enhances active oxygen production. Thus it achieved complete ablation of triple-negative breast cancer without primary tumor metastasis, and there was no tumor recurrence after 24 days of continuous treatment.
 | ||
| Fig. 3 (a) Schematic diagram of the photosensitizer (HSA/ICG) conjugated with Syne as an in situ photocatalytic oxygen generation system for PDT. Syne and HAS have strong tumor-targeting ability. Syne accumulates in the tumor tissue and produces oxygen under laser irradiation, enhancing the efficacy of PDT therapy and reversing the tumor immunosuppressive microenvironment. In the 4T1 MTNBC mouse model, Syne (S/HSA/ICG NP coated) can synergistically inhibit local and metastatic tumors. Reproduced from ref. 50 with permission from Wiley, copyright 2020. (b) During tumor hypoxia, laser irradiation produces oxygen through photosynthesis, and oxygen collection is used for sustainable PDT. Reproduced from ref. 63 with permission from Elsevier, copyright 2020. | ||
Similarly, one of the advantages of PDT therapy is that it promotes an anti-tumor immune response and sustained oxygen release. A self-enriched oxygen photodynamic therapy (Oxy-PDT) was established through loading a photosensitizer into PFC nanodroplets (Fig. 3b).63 This is a novel, long-lasting PDT that is controlled by light and enables photosynthesis to produce oxygen. With the high oxygen-carrying capacity of PFC, a high oxygen content can be sustained. Therefore, although the tumor oxygen content is still limited in the PDT process, sufficient O2 could be enriched in perfluorinated carbon nanodroplets for consumption by loaded photosensitizers. Thus, by improving the photodynamic efficiency, persistent PDT also promoted the activation of DCs and enhanced the anti-tumor immunity. After stopping the light, Chlorella further served as an adjuvant to promote (DC) activation in dendritic cells and promoted the anti-tumor immune response. Moreover, 1O2 exists much longer in PFC than in the cellular environment or water. Therefore, Oxy-PDT can help photosensitizers reach their full therapeutic potential. In vivo studies have revealed that tumor growth in Oxy-PDT-treated mice was inhibited after Oxy-PDT was injected into the tumor. Besides, when Oxy-PDT was injected intravenously into tumor-bearing mice, the treatment results showed that it could significantly inhibit tumor growth. At the same time, normal PDT didn't get such impressive results.
Due to the use of chlorella, the tumor tissues of mice need to be dissected before the chlorella can be implanted into the tumor tissues, and after the implantation, the chlorella needs to be sutured. Therefore, Zhang's team developed a new strategy, which relies on an injectable hydrogel (ALG-MI-S2973) to achieve the same photosynthetic function to produce oxygen, and the oxygen pump based on S.2973 is compounded with silica nanoparticles loaded with ICG.64 After evaluating the effect of PDT, they found that PDT is feasible. This novel strategy of photo-oxygen-dynamic therapy (PODT) combined a light-driven biological oxygen pump and PDT for the rapid growth of Synechococcus elongatus UTEX 2973. This method reduces the damage to the body. Still, the biggest advantage is that the treatment process becomes fast and convenient and is expected to be used in clinic by its hypoxia relieving properties. Besides, most of the previous studies used the same excitation wavelength for photosynthesis and the PDT trigger. Zhang's team creatively separated the growth of S.2973 and the stimulus of PDT through disparate wavelengths of laser, avoiding the damaging effect of ROS on S.2973 induced by PDT in the early stages of treatment. Hence, a result of maximum oxygen production can be ensured. The excellent oxygen production of ALG-MI-S2973 can significantly reduce HIF-1α under the action of an oxygen pump, and the inhibition rate of 4T1 tumor cells is almost 100%.
Cyanobacteria are prokaryotes with a highly differentiated membrane system capable of photosynthesis. They have thylakoid membranes that perform fully functional photosynthetic and respiratory electron transport chain functions. The existence of different membrane systems gives a unique complexity to the cells of these bacteria. The efficient O2 production of chloroplasts is attributed to their delicate Z-type structure, which is composed of two different light systems (PS-I and PS-II) that located on the chloroplast thylakoid membrane (TM).65 Yan et al. constructed UCTM NPs by combining thylakoid membrane with upconversion nanoparticles, which was formed by modifying chloroplast thylakoid membrane with upconversion nanoparticles to release oxygen to promote the production of ROS.66 Under the irradiation by a 980 nm laser, UCNPs can emit red light to activate PS-I and PS-II of the TM to promote the production of oxygen in water. In vivo experiments have proved that UCTM NPs have excellent biocompatibility, and 980 nm laser irradiation in PDT can effectively remove anoxic tumors in mice. Also, the publications about photosynthesis to produce oxygen were summaried in Table 1.
| Application | O2 source | Excitation requirements (excitation wavelength, power density, time) | ROS species | PSs | Cyanobacterial cells | In vitro (cells) | In vivo (tumor-bearing mice) | DO | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: chlorin e6 (Ce6); Synechococcus elongatus PCC 7942 strain (S. elongatus PCC 7942); Synechococcus elongatus UTEX 2973 (S. elongatus UTEX 2973); hypoxia alleviation assessment (HAA), photosystem-I (PS-I) and photosystem-II (PS-II), thylakoid membranes (TM). | |||||||||
| PDT | Photosynthesis | In vitro: 660 nm, 20 mW cm−2, 15 min | 1O2 | Ce6 | S. elongatus PCC 7942 | 4T1 | 4T1 | 57.9 μmol L−1 (30 mW cm−2) | 51 |
| In vivo: 660 nm, 50 mW cm−2 | |||||||||
| PDT | Photosynthesis | 635 nm, 50 mW cm−2 | 1O2 | Ce6 | Chlorella | 4T1 | 4T1 | ∼10.0 mg L−1 (50 mW cm−2) | 60 |
| PDT | Photosynthesis | In vitro: 660 nm, 0.1 W cm−2, 5 min | 1O2 | ICG | S. elongatus PCC 7942 | 4T1 | 4T1 mTNBC | ∼6.0 mg L−1 | 50 |
| 808 nm, 0.8 W cm−2, 5 min | |||||||||
| In vivo: 660/808 (5 min), sequential radiation | |||||||||
| PDT | Photosynthesis | In vitro: 660 nm, 1 W cm−2 | 1O2 | Ce6 | Chlorella | CT26 | CT26 | ∼90 μM (170 mW cm−2) | 63 |
| In vivo: 660 nm, 170 mW cm−2, 5 min (HAA); 660 nm, 1 W cm−2, 30 s (PDT) | |||||||||
| PDT | Photosynthesis | In vitro: 808 nm, 2 W cm−2 | 1O2 | ICG | Cyanobacteria | 4T1 | 4T1 | 8 mg L−1 (0.25 W cm−2) | 64 |
| In vivo: 640 nm, 0.25 W cm−2, 5 min | |||||||||
| 808 nm, 0.25 W cm−2, 5 min | |||||||||
| PDT | Photosynthesis | In vitro: 980 nm, 0.5 W cm−2, 10 min | 1O2 | PS-I, PS-II | TM | 3T3, 4T1 | 4T1 | 200 µg mL−1 | 65 |
| In vivo: 980 nm, 0.5 W cm−2, 5 min | |||||||||
2.2 Improving tumor hypoxia by generating O2in situ
For example, the polymer vesicle named HC@P1-Vesicle was designed,68 and H2O2 and polyamide dendrimer (CC-PAMAM) was assembled with ROS responsive triblock copolymers. When different excitation light was used as a trigger for oxygen production and the release of CC-PAMAM, it showed different therapeutic effects. Under irradiation (805 nm), the photothermal treatment effect was triggered, resulting in the thermal decomposition of H2O2. Under excitation light irradiation of 660 nm, photoactive CC-PAMAM is allowed to be released from the vesicles to achieve complete ablation of hypoxic, low-osmotic pancreatic tumors, and the therapeutic effect of this method is the best (Fig. 4a). Aiming at tumor therapy, Lei's group used nanoparticles for PDT and PTT. They designed BQ-MIL@cat-MIL nanoparticles based on the metal–organic skeleton (MOF).69 By accurately wrapping black phosphorus quantum dots (BQ) and catalase in the inner and outer layers of the MOF, H2O2 was converted into O2 in the stable outer layer of catalase in the MOF. Then O2 was directly injected into MOF-sensitized BQ (Fig. 5a). A high singlet oxygen quantum yield was obtained, and photodynamic-thermal synergistic therapy was achieved in vitro and in vivo.
 | ||
| Fig. 4 (a) The treatment results of BxPC-3 tumor-bearing BALB/c nude mice with different therapies. Reproduced from ref. 68 with permission from Wiley, copyright 2017. (b) The therapeutic process and mechanism demonstration of multifunctional CMGCC nanoclusters. Reproduced from ref. 72 with permission from the American Chemical Society, copyright 2020. | ||
 | ||
| Fig. 5 (a) A schematic diagram of the stepwise assembly process of BQ and catalase in homologous MOF aims to enhance therapy against hypoxic tumor cells. Reproduced from ref. 69 with permission from Wiley, copyright 2018. (b) Schematic illustration for the preparation of O2@UiO-66@ICG@RBC and the NIR-triggered O2 releasing and enhanced PDT mechanism. Reproduced from ref. 70 with permission from Elsevier B.V., copyright 2018. (c) The nanoparticles of PLGA-FA/IR780-H2O2, and illustrations of their application for PTT/PDT. Reproduced from ref. 71 with permission from the Royal Society of Chemistry, copyright 2018. (d) A schematic diagram of a nano-catalase system (Cat@PDS) that enhances chemotherapy–photodynamic therapy by decomposing and releasing oxygen through the phospholipid membrane coating. Reproduced from ref. 74 with permission from Elsevier B.V., copyright 2020. | ||
Progress has been made in photodynamic-thermal synergistic therapy both in vivo and in vitro, and it further promotes apoptosis and activates caspase-3. Combining all the merits, it is considered a promising therapeutic platform for the anti-hypoxic tumor microenvironment. With the metal–organic skeleton (MOF), it makes use of its gas adsorption ability to prepare a bionic oxygen evolution PDT nano-platform with long cycle characteristics.70 Simultaneously, the photothermal effect of a near-infrared thermo-sensitizer was used to catalyze oxygen production from hydrogen peroxide. UiO-66@ICG@RBC nanoparticles were designed using zirconium-based MOF (UIO-66) as an oxygen storage carrier (Fig. 5b). Afterwards this was coupled with ICG (coordination reaction) and this then continued by coating with the erythrocyte membrane. The photothermal properties of ICG can promote the sudden release of O2 in UIO-66. Afterward, the production of O2 was able to improve the therapeutic effect of PDT on hypoxic tumors. The same strategy used triggered photo-thermotherapy to thermally decompose H2O2.71 The nanoparticles (PLGA–FA/IR780–H2O2NPs) were constructed from core–shell poly(lactic-co-glycolic acid) nanoparticles (PLGA) and a H2O2/poly(vinylpyrrolidone) complex (O2 source) covalently bound to the targeted unit of folic acid and embedded in a shell IR780 diode (Fig. 5c). In in vitro experiments, the cell survival rate was analyzed by irradiation for one minute (808 nm, 0.5 mW cm−2), and the cell viability was reduced by about 80%, indicating the photothermal effect and ROS production ability of this nano-system. The in vivo experiment confirmed that the photothermal effect promoted the thermal decomposition of H2O2 and increased the local O2 concentration of the tumor during photothermal therapy. Simultaneously, a therapeutic experiment in HepG2 tumor-bearing mice further demonstrated the good tumor-therapeutic effect of this nano-system.
The core–shell nano-system has some special advantages; the nanoparticle RC@RFC was constructed that can combat tumor resistance to PDT, change the hypoxia state of the tumor and inhibit the expression of HIF-1α at the same time.73 This nano-system, through self-assembly of hydrophobic rapamycin (RAP) and photosensitizer Ce6, forms the carrier-free double drug and then coats the surface with a layer of the metal–organic skeleton (MOF) to support catalase. The total drug loading reaches 60%, and this nano-system can be passively accumulated in the tumor tissue. For evaluation of the RC@RFC nanoparticles’ effect on tumor growth by activating the nanoparticles’ activity with the help of light irradiation, the therapeutic effect is reflected directly according to the change of tumor volume. Hyper-hypoxic tumors showed a strong effect of PDT. The in situ O2 production of RC@TFC nanoparticles and the dissolved oxygen level under laser irradiation were analyzed, which showed that RC@TFC showed a concentration dependence and adequate continuous oxygen production capacity. Besides, the nanoparticles of Cat@PDS could target tumor tissue by wrapping catalase nanoparticles in a polyethylene glycol phospholipid membrane (Fig. 5d).74 After the process of PDT, the tumor O2 content was increased, and the expression of HIF-1α decreased, which finally had a remarkable inhibitory effect on tumor growth and led to 97.2% inhibition of lung metastasis. Using the catalase-catalyzed hydrogen peroxide oxygen production strategy, a tumor-targeted bioreactor LIP-IR-CAT was designed by co-loading liposomes with IR780 and catalase.75 Based on the high fluorescence quantum yield (up to 0.127) of the IR780 photosensitizer and the specific targeting of mitochondria, the physical and chemical problems of IR780 as a single photosensitizer were solved by using this method of a NIR fluorescence probe loaded on liposomes. Intra-tumor oxygen saturation was monitored by PA imaging. The results show that the oxygen produced by LIP-IR-CAT in the tumor can supplement the O2 consumption during PDT and provide additional O2 to relieve tumor hypoxia. At the same time, after the application of this nano-reactor, the PDT effect in tumor-bearing mice was noteworthily stronger than that in the other control groups, and the level of ATP was significantly lower (about 68.8%). The content of glutathione (GSH) in tumor cells is at least 4 times higher than that in normal cells.76,77 It is worth noting that the GSH in intracellular fluid is also 10–1000 times higher than in extracellular fluid.78 Therefore, the efficiency of PDT can be improved by adding MnO2 to nano-systems capable of targeting tumor tissue by reacting with glutathione. Based on this strategy of consuming intracellular GSH to enhance the therapeutic effect of PDT, a multi-functional nano-platform CMGCC was developed,72 which consists of catalase (CAT) and MnO2 nanoparticles integrated with Ce6-modified ethylene glycol chitosan polymer micelles, in which CAT catalyzes the decomposition of hydrogen peroxide and MnO2 can consume intracellular GSH, and produce Mn2+ for T1-weighted magnetic resonance imaging (as a contrast agent). In addition, the pH-sensitive surface charge can be switched from neutral to positively charged GC polymer, and this shift can improve the accumulation of PS in the tumor area. The process of collaborative treatment is shown in Fig. 4b. After proving the good PDT performance in vitro, they continued to study the potential of the CMGCC nanoclusters as a T1-weighted MR imaging contrast agent, and the final in vivo PDT shows that this multi-functional nano-therapy is effective at the level of the therapeutic effect on the tumor. Also, the publications about the decomposition of H2O2 by catalase or heat were summaried in Table 2.
| Application | Catalyst | O2 source | PSs | Excitation requirements (excitation wavelength, power density, time) | TROS species | In vitro (cell line) | In vivo (tumor-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: chlorin e6 (Ce6); cyanine 3 (Cy3); NIR dye (IR780 iodide); phospholipid membrane coated nanocatalase system (Cat@PDS). | ||||||||
| PDT | Heat | H2O2 | Ce6 | 805/660 nm, 3/5 min, 1 W cm−2/0.1 W cm−2 | 1O2 | BxPC-3 | BxPC-3 | 68 |
| PDT/PTT | Catalase | H2O2 | Cy3 | PTT: 808 nm, 1 W cm−2, 10 min | 1O2 | HeLa | HeLa | 69 |
| PDT: 660 nm, 150 mW cm−2, 3 min | ||||||||
| PDT/PTT | None | UiO-66 | ICG | In vitro: 808 nm, 0.06 W cm−2, 5 min | 1O2 | RAW264.7 and MCF-7 | MCF-7 | 70 |
| In vivo: 808 nm, 0.06 W cm−2, 1 min | ||||||||
| PDT/PTT | Heat | H2O2 | IR780 | In vitro: 808 nm, 0.5 W cm−2, 30 s | 1O2 | HepG2 and EC | HepG2 | 71 |
| In vivo: 808 nm, 0.5 W cm−2, 3 min | ||||||||
| PDT | Catalase | H2O2 | Ce6 | In vitro: 635 nm, 0.75 W cm−2, 2 min | 1O2 | MDA-MB-231 | MDA-MB-231 | 73 |
| In vivo: 635 nm, 0.75 W cm−2, 5 min | ||||||||
| PDT | Catalase | H2O2 | Cat@PDS | In vitro: 655 nm, 1 W cm−2, 2 min | 1O2 | 4T1 | 4T1 | 74 |
| In vivo: 655 nm, 1 W cm−2, 5 min | ||||||||
| PDT | Catalase | H2O2 | IR780 | In vitro: 808 nm, 1 W cm−2, 20 s | 1O2 | MDA-MB-231 | MDA-MB-231 | 75 |
| In vivo: 808 nm, 1.5 W cm−2, 20 s | ||||||||
| PDT | Catalase | H2O2 | Ce6 | In vitro: 660 nm, 100 mW cm−2, 5 min | 1O2 | HEK 293 and HeLa | HeLa | 72 |
| In vivo: 660 nm, 100 mW cm−2, 10 min | ||||||||
To improve the abnormal oxidative state of tumor cells and reduce oxidative damage, tumor cells take measures by activating several pathways to clear ROS, including GSH, catalase (CAT), superoxide dismutase (SOD), and vitamins.81–83 Among them, GSH is the highest antioxidant in cells,84 which seriously hinders the efficacy of PDT. However, GSH-mediated drug resistance produces extensive challenges for various treatments,85,86 so the consumption of GSH content helps improve the therapeutic effect.87–89 In the tumor microenvironment, when the H2O2 met the MnO2 nanosheets, the MnO2 was reduced to Mn2+ together with the generation of O2. The process is shown in the following equations:90
pH = 5.5
| MnO2 + 2H+ → Mn2+ + H2O + 1/2O2 | (1) |
| MnO2 + H2O2 + 2H+ → Mn2+ + H2O + O2 | (2) |
pH = 7.4
 | (3) |
Combined with the combination of MnO2 and glucose depletion reaction, a tumor-targeting MnO2 motor nano-system (MG/HA) was designed,91 which can effectively consume glucose to inhibit tumor progression and relieve hypoxia, inhibit metabolism by reducing the expression of Glut1, and further inhibit tumor invasion metastasis. In the nanocomposite, after the cancer cells with overexpression of CD44 specifically ingest MG/HA, GOD catalyzes the oxidation of glucose to gluconic acid and H2O2. When consuming O2, it generates at the same time, by the reaction, H2O2 and acid, which seems to be a circular process (Fig. 6a). The abnormal expression of Glut1 is related to tumor proliferation, metastasis, and poor prognosis.92In vitro experiments showed that the expression of Glut1 in normal COS-7 cells was lower than that in CT-26 and HeLa cells. There was a highly positive correlation between the expression level of GLT1 and the concentration of glucose. The decrease of sugar content caused by effective glucose consumption in the MG/HA group could significantly inhibit the high expression of Glut1, and the starvation toxicity of MG/HA was considerably higher than that of G/HA. In vivo observation showed that the MG/HA group showed a potent anti-tumor effect during the two-week treatment, showing an apparent O2 cycle starvation effect. The slight weight gain in all groups indicated that each sample had good biocompatibility. In general, compared with the traditional nano-system, the overexpression of Glut1 in tumor cells can be directly suppressed by combining the MnO2 motor with the designed MG/HA nano-system, thereby reducing tumor invasion and metastasis, thus further promoting treatment and being used to overcome the obstacle of starvation treatment. Lin's team also developed a nano-system containing MnO2 to couple photosensitizers (Au25 nanoclusters) with platinum(IV) (Pt(IV)) prodrugs using MnO2 nanotablets as carriers to achieve synergistic PDT and chemotherapy.93 GSH can bring the content of MnO2 nano-tablets down. Pt(IV) prodrugs in tumor tissues cooperate with MnO2 to catalyze H2O2 to produce oxygen, increasing the therapeutic effect of PDT. Mn2+ can be used as a contrast agent in MRI to realize the integration of diagnosis and treatment.
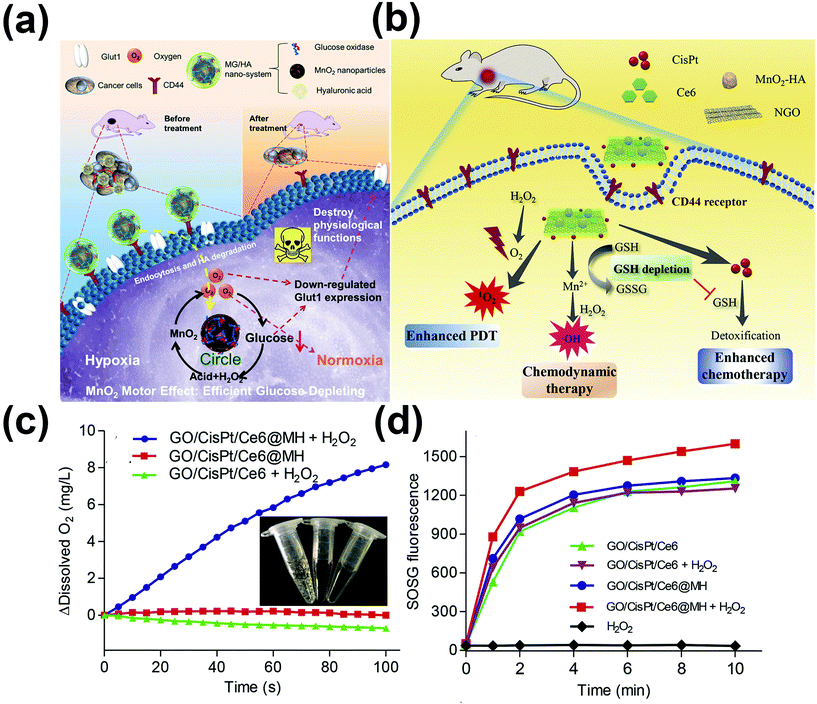 | ||
| Fig. 6 (a) The MG/HA nano-system interferes with the up-regulation of Glut1 and explains the process of ablation of tumor cells. Reproduced with permission. Reproduced from ref. 91 with permission from the American Chemical Society, copyright 2018. (b) A schematic diagram of the process of tumor treated with GO/CisPt/Ce6@MH nanoparticle CDT/PDT. (c) The kinetics of O2 generation catalyzed by GO/CisPt/Ce6 and GO/CisPt/Ce6@MH in presence or absence of 1 mmol/L H2O2. Inset: photographs of each reaction tube after reaction. (d) The kinetics of 1O2 generation for GO/CisPt/Ce6 or GO/CisPt/Ce6@MH in the presence or absence of 1 mmol/L H2O2 under laser irradiation. Reproduced from ref. 97 with permission from Elsevier B.V., copyright 2020. | ||
In recent years, two-dimensional graphene oxide (GO) has been extensively used as a drug carrier for nano-drugs because of its large surface, effective adsorption of aromatic drugs, and wealth of functional groups for drug coupling.94–96 Therefore, Zhou's team made use of the better carrier function of GO,97 and also used the strategy of MnO2 catalysis to alleviate tumor hypoxia and reduce intracellular glutathione levels (Fig. 6b), to improve the sensitivity of chemo-photodynamic therapy.94,98,99 A nanometer platform GO/CisPt/Ce6@MH was developed, CisPt was covalently bonded to the edge of the GO by ester bonds, and MnO2 nanoparticles were further modified on a 2D surface. In in vitro experiments, dissolved oxygen was measured. This nano-platform significantly produces O2 in the presence of hydrogen peroxide (Fig. 6c and d). The ability of this self-oxygen supply system to enhance PDT is explored. With the SOSG probe for monitoring the 1O2, together with H2O2, GO/CisPt/Ce6@MH shows a swift rate of 1O2 formation, which confirms the advantage of MnO2 in enhancing PDT therapy. In in vivo tests, BALB/c mice were treated with Ce6 and CisPt (0.5 mg kg−1, 1.25 mg kg−1), respectively. The results showed that synergistic treatment with GO/CisPt/Ce6@MH was very useful.
A new Intelligent Nano platform that was composed of mesoporous organosilicon dioxide nanoparticles (MONS) coated with glucose oxidase (Gox) and MnO2 nanoparticles-Ce6 was designed and named MONsGOx@MnO2-Ce6.100 Gox oxidizes glucose to gluconic acid and produces H2O2 at the same time. The released MnO2 nanowires can regenerate O2 in the presence of H2O2 and react with GSH (as an oxidant) to improve the photodynamic treatment effect. MONS-Gox@MnO2-Ce6 can realize the imaging of cancer cells and reduce intracellular glucose uptake and Glut1 expression and inhibit the metabolism of cancer cells. The new Nano platform continued to be designed for relieving tumor hypoxia; through assembling a Mn ferromanganese (Mn) nanoparticle, a hollow ferromanganese (Mn) nanoparticle was designed.101 When exposed to a tumor hypoxic microenvironment, the vesicles disintegrate and release drugs and ferromanganese oxide nanoparticles quickly. The ferromanganese oxide can catalyze the excess H2O2 to produce oxygen. Combined with immunotherapy, the nanovesicles showed good efficacy in combination with chemotherapy and immunotherapy. Also, the publications about the decomposition of H2O2 by MnO2 were summaried in Table 3.
| Application | Catalyst | O2 source | PS | Excitation requirements (excitation wavelength, power density, time) | ROS species | In vitro (cell line) | In vivo (tumor-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: starving therapy (ST); glucose (GLU); chemotherapy (CT). | ||||||||
| ST | MnO2 | H2O2, GLU | NO | NO | H2O2 | CT-26, COS-7 and HeLa | CT-26 | 91 |
| PDT/CT | MnO2 | H2O2 | Au25 | In vitro: 650 nm, 5 min | 1O2 | HeLa | U14 | 93 |
| In vivo: 650 nm, 15 min | ||||||||
| PDT | MnO2 | H2O2 | Ce6 | In vitro: 635 nm, 0.1 W cm−2, 1 min | 1O2 | MDA-MB-231 | MDA-MB-231 | 97 |
| In vivo: 635 nm, 0.1 W cm−2, 5 min | ||||||||
| PDT | MnO2 | H2O2 | Ce6 | 660 nm, 7.2 mW cm−2, 30 min | 1O2 | HeLa | NO | 100 |
There are fatal shortcomings in the application of metal–organic framework (MOF) integrated photosensitizers in the process of PDT, but Pt-modified MOFs can be used in other treatments (chemotherapy, radiotherapy, sonodynamic therapy), although the poor efficiency of the treatment is also limited by hypoxia.104,105 The PDT efficiency of MOFs is seriously limited by tumor hypoxia. The PCN-224-Pt nanoparticles were designed, therefore, to solve the above problems by decorating platinum nano-enzymes on the photosensitizer-integrated MOF as a new strategy.106 In an in vivo experiment, in a subcutaneous tumor model, PCN-224-PT was given by intratumor injection for photodynamic therapy. Under 638 nm excitation light irradiation (1 W cm−2) for 10 min, the PCN-224-PT + light group's tumor ablation ability was the most significant compared with the control group after intravenous injection.
A new strategy of in situ growth was applied that decorated black phosphorus (BP) nanosheets with Pt nanoparticles (NPs), forming Pt@BP nanoparticles.107 As an artificial catalase, Pt@NPs can effectively decompose the accumulated H2O2 and relieve tumor hypoxia in the tumor, and then improve the PDT effect of BP nanoparticles. When exposed to tumor hypoxia, BP nanosheets will not cause the decomposition of H2O2, while the Pt@BP nano-hydride will cause the deterioration of H2O2. The hypoxia environment was simulated in a cell experiment. As a hypoxia indicator probe,108,109 the fluorescence intensity was decreased significantly when using Pt@BP nanocomposites, which indicated that Pt@BP nanohybrids had high efficiency in regulating intracellular hypoxia. In addition, after Pt@BP nanohybrid treatment, immunohistochemical staining confirmed that HIF-1α was significantly down-regulated, indicating that Pt@BP nanohybrids can also effectively reverse the hypoxia state of the tumor, and inhibit the hypoxia-related signal pathway. Zhang's research group designed and synthesized Pda-Pt@PCN-FA,110 which is composed of a polydopamine (Pda) nucleus, PTNP mesosphere, Zr-TCPP(PCN) porphyrin shell, and Zr6 coordination with a folic acid carboxyl group (Fig. 7a). The platinum nanoparticle intermediate layer can catalyze endogenous H2O2 to O2, which plays a dual role in inhibiting the tumor cells’ growth and promoting death. Under irradiation, oxygen is converted through the PCN shell to lethal ROS. In an in vitro experiment, O2 was measured by a dissolved oxygen meter, the liquid surface was sealed with liquid paraffin, and the O2 production reached the maximum (about 50 mg L−1) within 25 minutes after adding H2O2. The disodium salt of 9,10-anthracene-dipropionic acid (ADPA) was used as the probe for detecting 1O2,111 and the hypoxia microenvironment in the tumor was simulated by blowing nitrogen for half an hour. The fluorescence intensity of ADPA decreased rapidly after treatment with Pda-Pt@PCN. Cell experiments were carried out again to check the oxygen-production capacity and ROS-production capacity of Pda-Pt@PCN-FA.
 | ||
| Fig. 7 (a) The simple preparation process of PCN-224-Pt and the schematic diagram of reactive oxygen species produced by PCN-224-Pt to kill tumor cells. Reproduced from ref. 110 with permission from John Wiley & Sons, copyright 2018. (b) Preparation of Pt@BP nano-hybrids and the scheme of tumor cell death caused by a series of reactions under NIR illumination. Reproduced from ref. 112 with permission from the American Chemical Society, copyright 2019. | ||
They confirmed that metastasis's driving force could be caused by an anoxic microenvironment, detected cell movement by a wound healing test, and found that Pda-Pt@PCN-FA could inhibit tumor metastasis by reducing the degree of hypoxia. In in vivo experiments, the imaging and distribution of Pda-Pt@PCN-FA were studied by the small animal imaging system. Fluorescence imaging was executed accompanied by intravenous injection of the nanomaterial, and the fluorescence intensity was most substantial at 28 hours after injection, indicating a good imaging ability. Simultaneously, measurements in animals showed no significant metastatic sites in the lungs of mice injected with Pda-Pt@PCN-FA, regardless of whether radiation was or was not used. In the end, they confirmed that the inhibition of tumor metastasis is caused by the improvement of hypoxia in tumor tissue and explained the correlation between them. For overcoming the poor results of PDT, caused by premature leakage of photosensitizer and hypoxia, FA/PtBSA@MB-MSNS nanocomposites were designed and synthesized.112 Platinum nanoclusters (Pt-BSA) were coated with bovine serum albumin (BSA) with oxygen and combined with mesoporous silica nanospheres to develop intelligent nano-aggregates to achieve a better therapeutic effect on anoxic tumors. Disulfide bond bridged mesoporous silica nanospheres (MSNS) contain methylene blue (MB), with BSA-encapsulated Pt nanoparticles (Pt-BSA) as a biological coating (Fig. 7b). In in vitro experiments, they evaluated the photodynamic conversion efficiency of biological tissue after nano-drug release under light irradiation. Chicken skin has a similar biological barrier effect to human tissue, so they used chicken skin with a thickness of 1 mm to stimulate the production of 1O2, which decreased to 72.15% after being irradiated by 635 nm laser (6 mW cm−2), with DPBF in FA/PtBSA@MB-MSNS aqueous solution for 120 s. This result confirmed that FA/PtBSA@MB-MSNS could produce 1O2 under the skin, highlighting its potential to kill cancer cells in tumor therapy. In an in vivo experiment, the ablation ability of PDT towards tumor cells in vivo was studied by using the cancer cell (HeLa) tumor model. Different treatments were used to track tumor volume change, and the therapeutic effect was by PDT treatment. Both the MB and MB-MSNS treatment groups slightly hindered tumor growth, while the PtBSA@MB-MSNS treatment group had the most significant ablation ability.
For making up for the instability of most photosensitizers at present, a new nano-platform HCS@Pt-Ce6 NPs was designed,113 which has been developed to load and deliver photosensitizers. There are reported two different Pt/carbon (Pt/C) nano-enzymes that were loaded as Ce6 nano-carriers. Pt NPs as a nano-enzyme had excellent peroxidase-like and oxidase-like activity. It was verified that Pt showed higher enzyme imitation and anti-tumor activity when loaded outside the carbon shell. In an in vivo experiment, 4 hours after injection of 7.5 mg kg−1 nano-particles into the tail vein, with 660 nm laser for 10 minutes, a comparison of treatment results between the experimental group and the control group showed that the tumor volumes of the HCS@Pt nanoparticle group and HCS@PtCe6 nanoparticle group were inhibited, indicating that catalytic therapy based on HCS@Pt nanoparticles has a specific effect in vivo. It also shows that HCS@Pt-Ce6 nanoparticles as photodynamic and catalytic synergistic therapy are feasible. In particular, a nano-sheet, which was constructed from a two-dimensional (2D) metal–organic skeleton Sm-tetrakis(4-carboxyphenyl) porphyrin (TCPP),114 was assembled with transition metal ions (Sm3+) and PSs (TCPP) and grew platinum enzymes mimicking catalase (CAT) on them in situ. As a new type of 2D porous nanomaterial, the 2D metal–organic framework has excellent properties for gas adsorption,115–117 photocatalysis,118,119 drug delivery,120,121etc. In this nanomaterial system, the 2D MOF has a simplified one-dimensionality, which can remove the diffusion restriction on the generation of ROS, thus showing higher PDT efficiency than three-dimensional (3D) MOF. Due to the improvement of the physical and chemical properties of bulky Sm nodes and the enhancement of intersystem crossing, 1O2 generation capacity was enhanced. More importantly, the CAT-mimicking Pt nanoenzyme on Sm-TCPP nanoparticles can alleviate the hypoxia of the tumor microenvironment, mainly by using the decomposition of overexpressed H2O2 to O2. The most important organelles that produce H2O2 and are very sensitive to ROS are the mitochondria. The triphenylphosphine (TPP) molecule that was introduced to Sm-TCPP-Pt achieved the function of good mitochondrion-targeting, and became an O2 self-supply PDT system. The prodrug nanoparticles of CPT-TK-HPPH/Pt NP that were created were loaded with platinum nanoparticles (Pt NP) in order to solve the problems of targeting defects and poor efficacy of PDT in hypoxia-related solid tumors.122 The excellent therapeutic effects of camptothecin (CPT) and the photosensitizer – 2-(1-hexoxy ethyl)-2-decadienyl pyropheophorbide pheophorbide (HPPH) were used, respectively.123–125 The prodrug consists of a thioketal bond connecting the CPT and HPPH, and the loaded Pt NPs are used to catalyze the release of O2 from H2O2 (Fig. 8). In current studies, many photosensitizers can produce ROS and effectively decompose ROS-responsive covalent bonds by using this positive feedback regulation, such as thioketal, aryl boron ester, thioether, diselenide ether, and peroxyacetate.126–128 By introducing drugs and photosensitizers linked with a thioketal bond as prodrugs, the ROS produced by excitation light breaks down the covalent bonds and releases the drugs. The in vitro ROS-production ability of the nano-system was verified. In order to explore the ability for intracellular ROS production, a commercial probe for detecting ROS was used. Different groups were irradiated with or without excitation, and the results showed that the CPT-TK-HPPH NP and CPT-TK-HPPH/Pt NP groups produced ROS, and the ROS contents under excitation light were 1.5 and 2.3 times higher than those without the laser, respectively. In in vivo imaging experiments, they studied whether the drug would accumulate in the tumor, directly affecting the anti-tumor effect in vivo. They evaluated the biological distribution and tumor-targeting ability of CPT-TK-HPPH/Pt NPs by NIR imaging and PA imaging. Its accumulation ability in tumor tissue was strong, and the fluorescence intensity was enhanced within 24 hours. The ablation ability of the tumor in vivo was also verified. The inhibition ability in the CPT-TK-HPPH/Pt NP + laser group was the strongest, which was due to Pt NPs in CPT-TK-HPPH/PtNP catalyzing the decomposition of hydrogen peroxide in tumor tissue to produce oxygen and improving tumor hypoxia.
 | ||
| Fig. 8 Schematic diagram of combined treatment of tumor cells with CPT-TK-HPPH/Pt NP under 660 nm laser irradiation. Reproduced from ref. 122 with permission from Wiley, copyright 2020. | ||
In a recent investigation, for enhancing oxygen delivery, nanoparticle-stabilized oxygen microcapsules were prepared by interfacial polymerization.129 The novel vehicle of polydopamine–nanoparticle-stabilized oxygen microcapsules applied for oxygen delivery is effective. In aqueous solution, it shows good biocompatibility; moreover, oxygen placed in the microcapsule can diffuse well from the core to the outside of the capsule, and thus can increase the oxygen content in the microenvironment. The feasibility of this strategy has been well proved by in vivo and in vitro experiments. In in vivo experiments, when it was injected subcutaneously, the hypoxia could be improved, and in the end suppressed the growth of the tumor. This oxygen supplementation strategy can be used in a wider range of applications, such as photodynamic therapy or other treatments with more aerobic therapy. Also, the publications about H2O2 decomposition by Pt NPs were summaried in Table 4
| Application | Catalyst | O2 source | Excitation requirements (excitation wavelength, power density, time) | PSs | ROS species | In vitro (cell line) | In vivo (tumor-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: tetrakis (4-carboxyphenyl) porphyrin (TCPP); black phosphorus nanosheets (BP); chlorin e6 (Ce6); Pt/carbon (Pt/C) nanozymes; catalase (CAT)-mimicking platinum nanozymes. | ||||||||
| PDT | Pt NP | H2O2 | In vitro: 638 nm, 1 W cm−2, 10 min | TCPP | 1O2 | RAW264.7, 4T1 and HeLa | H22 | 106 |
| In vivo: 638 nm, 1 W cm−2, 8 min | ||||||||
| PDT | Pt NP | H2O2 | In vitro: 660 nm, 1 W cm−2, 5 min | BP | 1O2 | 4T1 | 4T1 | 107 |
| In vivo: 660 nm, 1 W cm−2, 10 min | ||||||||
| PDT | Pt NP | H2O2 | In vitro: 660 nm, 30 mW cm−2, 2 min | H2TCPP | 1O2 | CT26 and COS7 | CT26 | 110 |
| In vivo: 660 nm, 220 mW cm−2, 5 min | ||||||||
| PDT | Pt BSA | H2O2 | In vitro: 635 nm, 45 mW cm−2, 30 min | MB | 1O2 | HeLa and A549 | HeLa | 112 |
| In vivo: 635 nm, 100 mW cm−2, 15 min | ||||||||
| PDT | Pt/C | H2O2 | In vitro (vivo): 660 nm, 0.346 W cm−2, 10 min | Ce6 | 1O2 | 4T1 and A549 | CT26 | 113 |
| PDT | Pt NP | H2O2 | In vitro (vivo): 660 nm, 100 mW cm−2, 15 min | TCPP | 1O2 | MCF-7 | MCF-7 | 114 |
| PDT | Pt NP | H2O2 | In vitro (vivo): 660 nm, 200 mW cm−2, 5 min | CPT-TK-HPPH | 1O2 | CT26 | CT26 | 122 |
2.3 CaO2 decomposition
The ability of calcium peroxide to produce O2 is widely used in tissue engineering, agriculture, aquaculture and relieving hypoxia in tumor tissue.130–132 As a supplementary source of oxygen, it can release oxygen for a long time to improve the viability of organisms when they are in constant contact with water.133 Calcium peroxide (CaO2) is a promising material for regulating tumor hypoxia and improving PDT efficiency because of its good biocompatibility and high efficiency and long-lasting O2 production. PDT uses light-activated photosensitizers to convert O2 to ROS, while CDT uses in situ Fenton or Fenton-like reactions between H2O2 and the catalyst to produce ˙OH. However, a large amount of GSH in the tumor microenvironment can deplete ROS. CaO2 is a safe solid inorganic peroxide that decomposes when it meets with water and releases O2 and H2O2 at the same time. Therefore, the strategy of introducing CaO2 into therapies involving ROS to enhance ROS production is widely used. The CaO2 component in the nano-system is reactive to water, causing O2 to be released in the water for a long time (eqn (4) and (5))134–136| 2CaO2 + 2H2O → 2Ca(OH)2 + O2 | (4) |
| 2CaO2 + 4H2O → 2Ca(OH)2 + 2H2O2 → 2Ca(OH)2 + 2H2O + O2 | (5) |
A NIR rechargeable “optical battery” for avoiding irradiating PDT was designed, which was formed by embedding three different components into biocompatible polydimethylsiloxane (namely, upconversion materials, persistent luminescence materials, photosensitizer) (Fig. 9a).137 They used NIR irradiation (980 nm, 2 W cm−2) for a short charging process of 5 s, and the luminescence could be sustained for a long time (about 30 min). According to previous studies, the battery was tested using different thicknesses of pork tissue to simulate human tissue (the penetration depth is up to 1 cm). After using this way to test it, they found that when the NIR irradiation was shone at pork tissue, 1O2 could be produced at a depth of 4 mm. What is interesting is that the NIR rechargeable irradiation-free PDT implants can easily be made in any size and shape. In addition, the cell has good biocompatibility in the human colon adenocarcinoma cell line HT29 without light and will produce strong cytotoxicity with laser irradiation. When they encapsulated the CaO2 in the battery, they found a higher molecular oxygen concentration. Hence, they further used oxygen production to alleviate tumor hypoxia; they implanted this device into an HT29 solid tumor, used NIR radiation for treatment (660 nm, 30 mW cm−2), and finally successfully solved the hypoxia condition of the tumor tissue, and a good tumor ablation effect was achieved (Fig. 9c), so it also promoted the therapeutic effect of PDT.
 | ||
| Fig. 9 (a) Fast charging with NIR light can achieve a long-lasting luminescence activation of a PDT “optical battery” implanted into the tumor to produce reactive oxygen species. Reproduced with permission from ref. 137. Copyright 2020, Royal Society of Chemistry (b) CaO2/B1/NH4HCO3 liposome can generate O2 for PDT under the NIR light irradiation; the bottom of the picture shows the mechanism for killing cancer cells. Reproduced from ref. 139 with permission from the American Chemical Society, copyright 2014. (c) and (d) Curve of tumor volume changes in mice during different treatments. Reproduced from ref. 137 with permission from the Royal Society of Chemistry, copyright 2020 (c). Reproduced from ref. 139 with permission from the Royal Society of Chemistry, copyright 2019 (d). | ||
Encouraged by previous research methods, Song's research group developed a nanomaterial.138 Through the implanted oxygen production bank, the local oxygen therapy enhanced the ablation of malignant tumors by Dox in a highly specific manner without increasing oxygen levels in tissues and organs in the whole body. The O2 production pool's design method puts CaO2 (contained in alginate solution) and catalase into a calcium chloride (CaCl2) bath to form calcium cross-linked microcapsule particles. When the oxygen-producing reservoir is implanted subcutaneously near the tumor, the exposed interstitial medium will react with it, and then the O2 is produced in situ, thus effectively reducing the anoxic area in the tumor tissue. In vitro experiments showed that the ROS level and apoptosis rate of Hep3B cells treated with Dox increased under normoxic conditions, but the ability of Dox to induce ROS was significantly decreased under hypoxic pressure, which enhanced the resistance of anoxic cells to Dox. Simultaneously, it is suggested that the oxygen-producing nanoparticles significantly enhance the cytotoxicity of Dox on ROS-induced cells. In vivo, the improvement of cytotoxicity of Dox on the tumor induced by the oxygen transport pool was further evaluated by positron emission tomography (PET). The maximum uptake of 18F-fludeoxyglucose (18F-FDG) in pellets + Dox (1.68 ± 0.06 %ID g−1) was significantly lower than that in other groups, indicating the therapeutic effect of this nano-system and slowing down the metabolism and proliferation of tumor cells.
Similarly, an O2-self-sufficient nano-platform (LipoMB/CaO2) for PDT of anoxic tumors was designed.140 In this system, a photosensitizer (MB) and CaO2 nanoparticles are wrapped within a hydrophilic cavity. The nano-platform can amplify the production of O2 under dual-stage light-driven PDT, regulate intracellular hypoxia and enhance the production of 1O2 in the process of PDT. After the first-stage laser irradiation, the release of O2 originating from the reaction of CaO2 and H2O alleviated tumor hypoxia. In vivo experiments also proved that LipoMB/CaO2 has good biocompatibility and can reduce tumor hypoxia and have an anti-tumor metastasis effect. In addition, a liposome was produced that achieved the function of being oxygen self-sufficient, named CaO2/B1/NH4HCO3 liposome.139 The liposome consists of hydrophobic aza-BODIPY dye (B1), oxygen-producing nanoparticles and hydrophilic thermally active ammonium bicarbonate (NH4HCO3) (Fig. 9b). The results of in vitro experiments showed that CaO2/B1/NH4HCO3 had an excellent ability to produce ROS after the tumor tissue was irradiated by 730 nm and 100 mW cm−2 for 5 minutes. At the same time, the in vivo experiments showed that this nano-platform had an excellent tumor therapeutic effects (Fig. 9d). The heat generated promotes the thermal decomposition of NH4HCO3 to produce oxygen, which increases the therapeutic effect of PDT and confirming the feasibility of this strategy.
In tumor therapy, the lack of substrates needed in treatment, such as hypoxia in PDT and deficiency of hydrogen peroxide in CDT, will limit the therapeutic effect of ROS. Professor Dong's team jointly designed an H2O2/O2 self-sufficient nano agent (MSNs@CaO2-ICG)@LA.141 This nano-system consists of manganese silicate (MSN) loaded calcium peroxide (CaO2) and the photosensitizer indocyanine green (ICG), with further shaping of the surface, that was modified with phase-change material (LA), as an open-source and cost-saving ROS generation strategy for CDT/PDT synergistic therapy. In this nano-system, the strategy of laser irradiation to generate heat is also adopted to expose the CaO2 in the nano-system. CaO2 is protected by outer LA to avoid direct contact with water. Under NIR irradiation, which induces the photothermal action of photosensitizer ICG, the outer LA can melt, and the exposed CaO2 reacts with water, resulting in the rapid formation of H2O2 and O2, and further exposure of the internal MSN. The oxygen released can alleviate the hypoxia in the cells, thereby enhancing ICG-mediated PDT. The interaction between MSNs and GSH leads to the release of the Fenton-like agent Mn2+, which is used in H2O2-supplemented CDT and magnetic resonance imaging (MRI)-guided therapy; the consumption of GSH deeply destroys the intracellular redox environment, thus further improving the production efficiency of ROS. An in vitro experiment explored the synergistic therapeutic effect of MSNsCaO2-ICG@LA-mediated CDT/PDT. The concentration of Mn2+ was measured at different time points, and showed the blood circulation curve of MSNs@CaO2-ICG@LA in mice. Because the EPR effect is conducive to the accumulation of MSNs@CaO2-ICG@LA in the tumor tissue, the brightness of the tumor site of the MSN-treated mice was higher than that of the control group, indicating that MSNs could be degraded into Mn2+ by GSH in vivo. It also illustrates the excellent thermal response characteristics of the nano-system, and the combined therapy of this nano-system enhances the ablation ability of the tumor. Compared with the nanometer platform designed by Zhang's research group in 2017, this topic also combined with MRI, which can realize the integration of diagnosis and treatment in the process of therapy, and provides excellent value for tumor treatment and further clinical application.
From the point of view of re-injecting oxygen into the infarcted myocardium to protect cardiomyocytes and reconstruct cardiac function in acute myocardial infarction treatment, Hao's team developed a nano-system named CMHP.142 This nano-system is formed by forming CaO2 in a mesoporous silica nano-platform and then assembling the thermosensitive materials eicosane and polyethylene glycol. In an in vivo experiment, pathomorphological analysis showed that there was significant left ventricular wall thinning in the myocardial infarction group, while less in the CMHPv + US group. Therefore, the survival rate of cardiomyocytes under hypoxia was significantly increased, and infarcted myocardial tissue injury was reduced to a minimum.
2.4 Water splitting
For tumor tissue, hypoxia hinders the effect of PDT treatment. PDT methods to alleviate hypoxia have been constantly explored, with the use of oxygen produced by photosynthesis to alleviate tumor microenvironment hypoxia,50,143,144 but the process of this method is tedious. The preparation of molecular hydrogen and oxygen can be accomplished by decomposing water under the action of solar energy. This method is a promising way of obtaining renewable energy storage,145,146 so, using water in the human body as a direct resource, catalytic cracking of water to produce oxygen will be more convenient and direct. It may also be used as a lasting method of providing O2. Inspired by this, the photolysis strategy of water in organisms has been used to generate O2 in the body and promote ROS production or other ways to improve PDT efficiency. Among many water separation materials, carbon nitride (C3N4) has attracted much attention because of its adjustable band gap and position, and low-permeability to light such as ultraviolet absorption has a great effect on the body,147 so C3N4 can be modified to drive the decomposition of water under highly permeating red light (>600 nm). This makes C3N4 suitable for in vivo treatment.148,149 In 2015, Kang's research team designed a metal-free carbon nanodot–carbon nitride (C3N4) nanocomposite, and verified this nanocomposite had an impressive performance. After ensuring the amount of composite catalyst in water, the optimum CDots/C3N4 ratio is 4.8 × 10−3 gCDots/gcatalyst, with CDots–C3N4 composites at λ = 420 ± 20 nm and λ = 580 ± 15 nm. The QEs that can decompose water into H2 and O2 are 16% and 6.3%, respectively. For the photocatalyst g-C3N4, it has the characteristics of rich resources and low cost,150,151 and the most important thing is that the preparation is relatively simple,152,153 so it is convenient for the application of this new catalyst. Some studies have proved that g-C3N4 has great potential as a photosensitizer to alleviate tumor hypoxia.154,155 Under weak light (20 mW cm−2), it can produce ROS and kill tumor cells. In addition, carbon points (CdS) have the advantages of unique photoelectron transfer and luminescence properties. This study also made Yang's team think of using the combination of g-C3N4 and CdS to form an excellent performance PDT photosensitizer: (i) 2H2O → H2O2 + H2; (ii) 2H2O2 → 2H2O + O2.153,156 They integrated dual-mode photodynamic therapy (Dual-PDT) into one system for multiple functions.157 First, the core–shell structured upconversion nanoparticles were attached on graphitic-phase g-C3N4 nanosheets (one photosensitizer), and then by way of in situ growth, as-prepared nanocomposite and carbon dots were assembled in ZIF-8 metal–organic frameworks, and in the end, a dual photosensitizer mixing system for PDT was realized by stepwise water splitting. In in vivo experiments, the tumor growth rate of a UCNPS-g-C3N4@ZIF-8 complex culture was significantly different with or without light, indicating that the production of oxygen under light conditions alleviated tumor hypoxia and increased the therapeutic effect. This research group also reported a new multi-functional nano-platform named UCNPs@BiOCl, which is based on the integration of NaGdF4:Yb,Tm@NaGdF4 UCNPs and bismuth oxyhalide (BiOCl) sheets, for 980 nm near-infrared light-triggered PDT.158 With the same hydrolysis strategy, the loaded UCNPs can convert near infrared light into UV/Vis region emissions, which drives the pure water pyrolysis of BiOCl sheets, and the resulting reactive oxygen species are used to kill the tumor cells.A kind of multifunctional nanocomposite (PCCN) based on C3N4 was adopted to solve the problem of hypoxia restricting the efficacy of PDT.159 The nanomaterial PCCN was designed and synthesized, which contains protoporphyrin photosensitizer IX (PpIX), a polyethylene glycol chain connector and an RGD sequence Arg–Gly–Asp motif, and was prepared by the accumulation of π–π bond between C3N4 and PpIX. PCCN uses RGD targeting and the EPR effect to reach tumor tissue, where it accumulates. PCCN produces O2 by cracking water, which is irradiated by 630 nm laser. Photosensitizers can also redistribute the energy and transfer it to the resulting O2 and produce cytotoxic 1O2. In addition, PCCN can also down-regulate the expression of hypoxia-related proteins, thus inhibiting tumor metastasis and treating tumors in two modes: ablation and inhibition of tumor metastasis. Similarly, Zhang's team (the same team as the last report) continued to develop their hydrolyzed oxygen project.160 Taking advantage of the advantage of Ru(II) complex as a photosensitizer for two-photon photodynamic excitation,161,162 a composite nanoparticle (FCRH) was designed, which was composed of iron-doped carbon nitride (Fe–C3N4), Ru(Bpy)32+ and hyperbranched conjugated copolymer (HOP) with a polyethylene glycol arm. By π–π bond stacking on Ru(Bpy)32+, photogenerated electrons are injected into Fe–C3N4 from Ru(Bpy)32+ to promote charge localization, which can more effectively separate the charge and prevent electron–hole pair recombination, thus improving the photocatalytic activity and O2 production.7,163 In in vitro experiments, after accumulating nanocomposites by enhancing EPR, FCRH has been proved to alleviate tumor hypoxia, thus strengthening the anti-tumor effect of PDT.159,164,165 Under 800 nm excitation light irradiation, there was apparent oxygen production, and the EPR spectrum confirmed the production of 1O2. After 24 hours of intravenous injection of IR780-labeled nanocomposite material, the apparent tumor contrast IR780 fluorescence gradually increased and then decreased after 36 hours, confirming its excellent tumor-specific accumulation ability in vivo. In the treatment experiment, the tumor volume of the FCRH combined with irradiation group was suppressed entirely within 14 days, while the inhibitory effect of FCRH on 4T1 tumors was negligible under the condition of no light; this shows the same excellent therapeutic effect. In the same way as in the previous work, photocatalytic materials were developed from TiO2, C3N4 and bismuth halide flakes. NIR radiation is also adopted, and the near-infrared light has the characteristics of low toxicity and vigorous penetration. The energy produced after excitation promotes the catalytic decomposition of H2O to O2 and improves the PDT efficiency.157,166 The above studies have played a leading role in using the hydrolysis of water in the body to prepare O2 to alleviate the hypoxia of tumor tissues, especially in the further improvement of the strategy in the later stages, with the use of high-penetration red light for water cleavage, which is suitable for in vivo treatment. Also, the publications about water splitting were summaried in Table 5.
| Application | Catalyst | O2 source | PSs | Excitation requirements (excitation wavelength, power density, time) | ROS species | In vitro (cell line) | In vivo (tumor-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: water splitting (Ws); mediating the photosensitizer (BiOCl); protoporphyrin IX (PpIX); ruthenium(II) complex (Ru(bpy)32+); hypoxemia after acute myocardial infarction (AMI); the phase change of heneicosane (PCH). | ||||||||
| PDT | CaO2 | Water splitting | RB | 980 nm, 2 W cm−2, 5 s | 1O2 | HT29 | HT29 | 137 |
| PDT | CaO2 | Water splitting | MB | In vitro: 660 nm, 30 mW cm−2, 6 min | 1O2 | 4T1 | 4T1 | 140 |
| In vivo: 658 nm, 280 mW cm−2, 10 min | ||||||||
| PDT/PTT | CaO2 | CaO2/B1/NH4HCO3 | aza-BODIPY | In vitro: 730 nm, 500 mW cm−2, 5 min | 1O2 | HeLa | HeLa | 139 |
| In vivo: 730 nm, 100 mW cm−2, 5 min | ||||||||
| CDT/PDT | CaO2 | Water splitting | ICG | In vitro (vivo): 808 nm, 0.64 W cm−2, 10 min | 1O2, ˙OH | MCF-7 and A549 | MCF-7 | 141 |
| US + CMHP | US | Water splitting | NO | 1.0 MHz, 0.6 W cm−2, 30 s | NO | H9c2 | NO | 142 |
| PDT | C3N4 | Ws | UCNPs | In vitro: 980 nm, 0.5 W cm−2, 5 min | ˙O2−, 1O2 | L929 | U14 | 157 |
| In vivo: 980 nm, 0.5 W cm−2, 10 min | ||||||||
| PDT | C3N4 | Water splitting | BiOCl | In vitro (vivo): 980 nm, 0.5 W cm−2, 10 min | OH˙, ˙O2− | HeLa | U14 | 158 |
| PDT | C3N4 | PCCN | PpIX | In vitro: 630 nm He–Ne, 80 mW cm−2, 10 min | 1O2 | 4T1 and MCF-7 | 4T1 | 159 |
| In vivo: 630 nm He–Ne, 40 mW cm−2, 5 min | ||||||||
| PDT | Fe–C3N4 | Water splitting | Ru(bpy)32+ | In vitro: 800 nm, 2.7 W, 3 min | 1O2 | 4T1 | 4T1 | 160 |
| In vivo: 800 nm, 2.7 W, 5 min | ||||||||
3. Improve tumor hypoxia through various oxygen delivery carriers
The culprit in hypoxia is the formation of abnormal blood vessels, often forming malformed blood vessels and poor blood flow.38,167–173 In tumor tissue this will lead to a decrease in the average level of tissue partial pressure of oxygen, which often occurs during vascular disease,174,175 lung disease, and cancer. Normal cell metabolism or treatment (PDT, drug therapy, etc.) needs oxygen, and hypoxia will seriously affect its therapeutic effect. Therefore, increasing the concentration of O2 by accelerating the blood flow pathway will be a new way to improve therapeutic efficacy.3.1 Accelerate the blood flow
The former study found that changing the blood temperature (40–42 °C) can increase the blood flow speed,176,177 and the blood will continue to carry oxygen, so the acceleration of blood flow speed will also alleviate hypoxia.178–183 The common method of increasing blood temperature is the hyperthermia produced by PTT;184–187 PTT absorbing light through the NIR region can accelerate the blood flow velocity of patients with a solid tumor, and thus improve the oxygenation level of the cancer and reduce the treatment resistance related to hypoxia. It converts light energy into heat energy by exciting light to irradiate photosensitizers. There are many kinds of photosensitizer, including small molecule fluorescent probes,188–193 nanomaterials,194,195 and so on. Therefore, the use of a mild heating method for tumor treatment is an effective way to alleviate hypoxia and promote PDT outcomes.The staining experiment of HIF-1α confirmed that the tumor's hypoxia state was obviously improved.167,197 Immunofluorescence in the LM + NIR treatment group was weak, indicating that HIF-1α was expressed at a low level in tumor tissues, and the hypoxia tolerance catalyzed by GOX may be alleviated. It was further proved that blood flow accelerated, and hypoxia was relieved during PTT treatment. Feng et al.198 prepared a liposome system containing photosensitizer Ce6 and a photothermal agent (DIR). An NIR light-activated liposome Ce6 preparation was constructed, and HCe6 and DIR molecules were co-encapsulated in PEG shell liposome bilayers. Under NIR laser irradiation at 785 nm, the fluorescence of hCe6 in dir-hCe6-liposomes will be awakened. Irradiated with 660 nm LED light at 2 mW cm−2, the function of PDT can be triggered by stimulating PS and Ce6 to produce a mild photothermal heating, which can accelerate the circulation of blood flow, lighten tumor hypoxia and promote the therapeutic effect of PDT. At the same time, it has shown great potential in skin protection and is an excellent class of activable photosensitizer.199,200In vivo experiments show that this liposome system can significantly improve tumor blood flow and alleviate tumor hypoxia.
Nanoparticles containing Pt(IV) and dihydro porphyrin e6 and loaded with UCNPs were designed and developed, and can convert near-infrared light of 980 nm into emission light of 365 nm and 660 nm (Fig. 10a).201 When UCPP decomposition is triggered by 980 nm laser irradiation, O2 can be produced to compensate for consumption in the process of PDT, releasing active Pt(II) for synergistic photochemotherapy. The nano-system does not need to be accompanied by catalytic substances, such as MnO2 or catalase,57,202–204 and could solve the limitation of hypoxia PDT treatment. In an in vitro experiment, L929 cells were carefully studied by CLSM to reverse PDT-induced hypoxia. An ROS probe was used to detect ROS under hypoxia and normoxic conditions. Whether under normoxic or anoxic conditions, UCPP-treated L929 cells presented bright green fluorescence, indicating that UCPP could indeed produce O2 inside the cells and provide enough O2 for PDT to conquer PDT-induced hypoxia (Fig. 10b). In an in vivo experiment, two kinds of orthotopic superficial carcinoma, B16 and MDA-MB-231 tumor models, and two subcutaneous tumor models, HeLa and HCT116, were used to evaluate the anticancer activity. When B16, HCT116 and MDA-MB-231 tumor-bearing mice were treated with UCPP nanoparticles and then given 980 nm laser irradiation, all xenografts disappeared only after laser irradiation (Fig. 10c). The remarkable anti-tumor result in vivo was achieved by reasonably designed UCPP. The UCPP can generate O2 and Pt(II) under NIR irradiation, thereby alleviating the state of hypoxia and achieving synergistic PDT-chemotherapy.
 | ||
| Fig. 10 (a) Schematic illustration of UCPP nanoparticles. (b) CLSM imaging of different nano-systems under normoxic and anoxic conditions. (c) The change of various cancer cells (HCT116, MDA-MB-231, and B16) in tumor-bearing mice with different treatments. Reproduced from ref. 201 with permission from Springer Nature Limited, copyright 2018. | ||
 | ||
| Fig. 11 (a) Schematic diagram of the therapeutic process of oxygen produced by Bi2S3@BiNRs nanoparticles irradiated by near-infrared laser to promote ROS production. (b) After different treatments, the tumor growth curve of mice with or without laser irradiation. Reproduced from ref. 209 with permission from Wiley, copyright 2020. | ||
| Application | PS | O2 source | O2 level detection | Excitation requirements (excitation wavelength, power density, time) | ROS species | In vitro (cell lines) | In vivo (tumor-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: indicator ([Ru(dpp)3]Cl2); chlorin e6 (Ce6); oxygen dissolving meter (ODM); increase blood flow (IBF); pimonidazole hydrochloride (Hypoxyprobe™). | ||||||||
| PTT | NO | IBF | NO | In vitro: 808 nm, 1.0 W cm−2, 15 min | H2O2 | 4T1 | 4T1 | 196 |
| In vivo: 808 nm, 1.0 W cm−2, 5 min | ||||||||
| PDT/PTT | Chlorin e6 | IBF | Hypoxyprobe™ | In vitro: 785 nm, 1.0 W cm−2, 10 min | 1O2 | 4T1 | 4T1 | 198 |
| In vivo: 785 (660) nm, 2 W cm−2, 20 min (1 h) | ||||||||
| PDT | Chlorin e6 | UCPP | ODM | In vitro: 909 nm, 0.85 W cm−2, 5 min | 1O2 | L929 | HeLa and HCT116 | 201 |
| In vivo: 909 nm, 0.85 W cm−2, 10 min | ||||||||
| PTT | NO | IBF | NO | In vitro: 808 nm, 1.0 W cm−2, 10 min | Free radicals | HUVEC and MDA-MB-231 | BEL-7402 | 207 |
| In vivo: 808 nm, 0.3 W cm−2, 10 min | ||||||||
| PDT | Bi2S3 | Bi2S3 + water | [Ru(dpp)3]Cl2 | In vitro: 808 nm, 0.5 W cm−2, 10 min | O2˙−, OH˙ | 4T1 and 3T3 | 4T1 | 209 |
| In vivo: 808 nm, 0.5 W cm−2, 5 min | ||||||||
3.2 Perfluorocarbons
PFCs are a kind of organic fluorine compound. The reason why PFCs can dissolve relatively large volumes of gas is due to the weak interactions (van der Waals force) and they are clinically used as an artificial blood substitute.211,212 When the oxygen pressure in PFCs is lower than 760 Torr (1 atm) at room temperature (25 °C),213 the amount of dissolved oxygen per 100 mL liquid (40–50 mL) is twice as much as that in normal human blood.214 Because of its high O2-generating capacity,215–217 at a determined partial pressure of oxygen, it can have a higher amount of oxygen relative to the tumor matrix at a given partial oxygen pressure. From 1988, Henderson and his co-workers began using PFCs to enhance PDT by injecting porphyrin photosensitizers containing PFC nanoemulsions.218 In addition, nano-carriers containing PFC can be used as a US imaging agent and high intensity focused ultrasound (HIFU) agent.219,220 Gasification is a new PFC application technology that can significantly improve oxygenation and lung function in oleic acid-induced lung injury.221After applying this nanomaterials to mice, the tumor's oxygenation was enhanced, which could significantly improve the efficacy of PDT and radiotherapy. The introduction of a fluorinated chain segment increased the solubility of PS, increased the local O2 concentration of polystyrene in the ground state (3O2) and excited state (1O2), and improved the O2 diffusivity. Additionally, PFC emulsions have been approved by the FDA.222 Inspired by this, PFCs bring oxygen molecules into auxiliary PDT in different ways, which is a possible new method to alleviate hypoxia.
The designed nanoparticles get the most out of the O2 and longer 1O2 lifetime of PFCs to significantly utilize the loaded functional photosensitizer to perfect the outcome of PDT.212,223 In in vitro experiments, PDT was performed on LIP (IR780) and LIP (IR780 + PFH) under different hypoxia conditions (including 0.1, 1, 7, and 21 kPa O2). The LIP (IR780 + PFH) group could significantly increase O2 production under both hypoxia and normoxic conditions, and the PFC could successfully promote the production of 1O2 under hypoxic conditions. In an in vivo PDT experiment, they first measured the 1O2 production of LIP (IR780) and LIP (IR780 + PFH). In order to prevent the extra effect caused by the photothermal effect, which affects the reliability of the experimental results, they separated the irradiation times, and enough time was provided to restore the original temperature, so the method of a one-minute interval was adopted for two consecutive laser irradiations. In an in vivo experiment, the therapeutic effect of intratumoral injection was studied first, and the tumor volume was also tracked with or without laser irradiation. Through the study of tumor accumulation imaging and ultrasound imaging of IR780, the mice were irradiated by a 808 nm laser 24 hours after intravenous injection, and the tumor volume irradiated with light (808 nm, 10 s, 2 W cm−2) was about 4 times smaller than that of mice treated with IR780 alone. Tang et al.224 adopted the Oxy-PDT strategy, loading a hydrophobic NIR photosensitizer into PFC nano-droplets as an effective photodynamic nano-drug. PFH was selected as the core of the PFC. During laser irradiation, the generation of 1O2 in the PFC phase will be faster. In this way, when the tumor is in a state of hypoxia, the photodynamic effect is good. The model of a hypoxic CT26 subcutaneous tumor was established and confirmed in vivo. All in all, it shows that the photosensitizer loaded into perfluorinated carbon nanodroplets can successfully treat hypoxic tumors resistant to traditional PDT. Using the same method, a PCF nano-emulsion containing fluorinated porphyrin, using the PFC nanoemulsion to transport O2 and PS simultaneously, was designed and synthesized.225 The efficacy of PDT was determined by emulsion containing photosensitizer in cellulose. The uptake of nano-emulsion by human malignant melanoma (A375) cells was first verified by a co-localization test. Under laser irradiation, cell death was indicated by NucGreen™ fluorescence and confirmed again by flow cytometry analysis.226 It was determined that the use of fluorine-containing soluble photosensitizer is the most promising method for PDT with a PFC nano-emulsion. Besides, a reversible addition-fragmentation chain transfer (RAFT) polymerization technique was used, so plenty of amphiphilic/fluorine random copolymers with different contents of perfluorocarbon (perfluorooctyl) were prepared.227 The higher PFC content in the copolymers can produce ROS more effectively, thus improving PDT efficiency in vitro. They found that an increase in the PFC amount can carry more oxygen and therefore produce more ROS. The determination of 1O2 by 630 nm laser illumination (F-polymer (P6-F59%)-HB, H-polymer (P2-H54%)-HB) proved that the addition of PFC effectively promoted the production of 1O2.
Plasma gold metal nanostructures were combined with the semiconductors of Cu2O, ASC was loaded into O2 PFH droplets, and then LIP (ASC/PFH) nanocomposites were formed by coating liposomes (LIP).228 At the same time, due to the high O2 capacity of PFH, under a certain oxygen partial pressure, its high oxygen content can be maintained for a certain period of time and is higher than that of the tumor matrix.229 A high quantum yield of 1O2 was evaluated that was nearly 0.71, which was produced by the mechanism of plasmon-induced resonance energy transfer (PIRET) that produced, from addition of Au to Cu2O, Au@SiO2@Cu2O under NIR irradiation for ten minutes. In an in vitro experiment, the 1O2 production capacity of ASC added to Cu2O, Au@SiO2, and Au@Cu2O was evaluated by a DPBF probe, and 670 nm (0.48 W cm−2) laser was used for irradiation for 10 minutes. In addition, the real-time oxygen-carrying capacity of PFH is as high as 168 mg L−1, so it can be proved that it has good oxygen-carrying capacity. The antitumor activity of LIP (ASC/PFH) in vivo was studied, and a significant therapeutic effect in the LIP (ASC/PFH) group was shown when the laser irradiation was applied for 10 minutes every time. An H&E staining assay showed that LIP (ASC/PFH) had good biocompatibility.
Recently, a well-targeted oxygen self-sufficiency and tumor-penetration nano-platform, including IR780-loaded pH-sensitive fluorocarbon functionalized nanoparticles (SFNs) and target peptide-iRGD, has been developed (Fig. 12a).230 Through the real-time monitoring of the dissolved oxygen, a special study was carried out on the loading and release behavior of oxygen. The potential application of SFNs as oxygen storage was also studied, and the excellent O2 loading performance and the capacity of high release were verified. An in vivo PA system and HIF-1α staining analysis were used to evaluate the hypoxia state of the tumors after the use of IR780@O2-SFNs/iRGD, IR780@O2-SFNs and IR780-SHN systems. The most significant change in the FL intensity of HbO2 was in the IR780@O2-SFNs/iRGD group (50.1%), indicating that IR780@O2-SFNs/iRGD can transport O2 and PS from the hydrophobic core to the deep and anoxic areas of the solid tumor, obviously improving the oxygenation of the tumor. A biodegradable hollow MoSe2/Fe3O4 nano-heterostructure was used as a PDT enhancer for multimode imaging (CT/MR/IR) and synergistic anti-tumor therapy, and hence the nanoparticle O2@PFC@MF-2@PEG/Dox was designed (Fig. 12b).231 Due to effective photoinduced charge separation, MoSe2/Fe3O4 can produce twice as much ROS as a single MoSe2. On this basis, the use of a new hollow structure provides enough space for the PFC and O2 carrier, and O2@PFC@MF-2 can effectively overcome the anoxic microenvironment and further lead to more than 3 times the production of ROS. The anticancer drug doxorubicin (Dox) was assembled into an MF-2@PEG nanomaterial for chemotherapy so that the final O2@PFC@MF-2@PEG/Dox realized the function of multimodal imaging and treatment. With MoSe2/Fe3O4 as the PDT agent, the production ability of ROS was detected by a DCFH-DA probe, and after 808 nm laser irradiation, MoSe2/Fe3O4 nanocomposites showed enhanced emission and ROS production. In order to evaluate the antitumor effect of this nano-system, the mice were classified into five groups for different treatment; the tumor volume of the O2@PFC@MF-2@PEG + NIR and O2@PFC@MF-2@PEG/Dox + NIR groups was significantly reduced, and the O2@PFC@MF-2@PEG/Dox + NIR group showed the most significant tumor inhibitory effect due to the multimodal therapy (chemotherapy, PTT and PDT).
 | ||
| Fig. 12 (a) Structure and O2 self-sufficient mechanism of an IR780@O2-SFNs/iRGD nano platform. Reproduced from ref. 230 with permission from the American Chemical Society, copyright 2019. (b) The routine process of ROS production by nanoparticles under laser irradiation. Reproduced from ref. 231 with permission from the American Chemical Society, copyright 2019. (c) Schematic diagram of an O2 delivery system for tumor imaging guidance with cooperative PDT/PTT. Reproduced from ref. 234 with permission from Dove Press Ltd, copyright 2020. | ||
According to the advantages that IR780 can be preferentially accumulated in mitochondria and can be modified as a mitochondrial targeting agent,232,233 Chen et al.234 designed an O2 delivery system based on a mitochondrial liquid PFC, which was created for imaging-guided synergistic PDT/PTT of tumors (Fig. 12c). The nanoparticles can target mitochondria. Mitochondrial organelles and ROS production,235 redox state regulation and apoptosis-mediated cell death regulation play an essential role. Mitochondria are sensitive to hyperthermia and ROS, and finally induce tumor cell apoptosis by destroying the balance of mitochondrial ROS in cancer treatment; the most essential factor of applied IR780 as a PS is that it can significantly excite the triplet state of the PS and produce 1O2.236 So it is feasible to use a targeted mitochondrial strategy for tumor therapy. In the study of photothermal and photodynamic properties, PBS, IRP NPs and IR780 were irradiated by NIR irradiation for 5 minutes, and the temperature of the latter two groups increased to 60 °C. The temperature increased with the increase of concentration. SOSG was used to confirm the production of 1O2in vitro according to the fluorescence intensity. This method can verify the potential of IRP/O2NPs as a photosensitizer. The fluorescence intensity of 5 µM SOSG containing IRP/O2NPs (20 mg mL−1) increased sharply with the extension of irradiation time (2 W cm−2, 5 s), and increased by 4 times within 80 s. In an in vivo treatment experiment, synergistic therapy with IRP/O2NPs (PDT/PTT), through different ways, such as the generation of ROS, damage caused by mitochondrial hyperthermic and other methods, both achieved a significant anti-tumor effect, and had good biocompatibility and minimal systemic side effects. Nanoparticles (PFH@HSC) with a PFH core, hyaluronic acid (HA) and Ce6 shell were designed.237 The photoactivation property of PFH@HSC is through destroying the structure through a “redox response” and activating it from “OFF” to “ON”; after targeting anoxic tumor tissue, laser irradiation is carried out to release Ce6 and O2, and finally the production of more singlet oxygen boosts the efficacy of PDT. Due to the excellent imaging performance of Ce6, PFH@HSC can monitor tumor aggregation by fluorescence and PA imaging after intravenous administration into tumor-bearing mice. PFH@HSC nanoparticles showed a contrast ability for PA imaging related to the Ce6 concentration, which confirmed that PA imaging could be used to analyze the aggregation of PFH@HSC nanoparticles in tumors. They showed that PFH@HSC nanoparticles could improve the hypoxia inside the tumor by intravenous injection of PFH@HSC, using a hypoxia probe to examine both groups’ tumor sections for immunofluorescence staining. The anti-tumor effect of PFH@HSC nanoparticles on mice with MDA-MB-231 tumors was evaluated in vivo, and the redox activated and oxygen-enriched PFH@HSC nanoparticles showed the most effective tumor growth inhibition.
Recently, PDT as an in situ vaccine can be used to increase the response rate of PD-1/PD-L1 antibodies.238 Hence, a bionic nanoemulsion expressing PD-1 membrane camouflage and applied to synergistic photodynamic immunotherapy for hypoxic breast tumors was developed. The perfluorocarbons in the nanoemulsion can provide oxygen and thus act as a PDT “energy source” against hypoxic tumors. The realization of the co-delivery function of photosensitizer and PD-1 protein by the nanoemulsion confirmed the synergistic therapeutic effect of PDT and immunotherapy, and the synergistic therapeutic completely inhibited the growth of primary and distal subcutaneous 4T1 tumors. Also, the publications about PFC as an O2 source were summaried in Table 7.
| Application | PSs | O2 source | Excitation requirements (excitation wavelength, power density, time) | ROS species | In vitro (cell lines) | In vivo (tumour-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: perfluorohexane (PFH); perfluorocarbon (PFC); chlorin e6 (Ce6); adriamycin (Dox); sinoporphyrin sodium (DVDMS). | |||||||
| PDT | IR780 | PFH | In vitro (vivo): 808 nm, 2 W cm−2, 20 s | 1O2 | MCF-7 and CT26 | CT26 | 229 |
| PDT | IR780 | PFH | In vitro (vivo): 808 nm, 2 W cm−2, 10 s | 1O2 | CT26 | CT26 | 224 |
| PDT | Fluorous porphyrin 1,2 | PFH | 420 nm, 8.5 mW cm−2, 30 min | 1O2 | A375, MCF7, and HEK293 | NO | 225 |
| PDT | Hypocrollin B | PFC | In vitro: 630 nm, 20 mW cm−2, 300 s | 1O2 | H-1299 | NO | 227 |
| PDT | Ce6 | PFH | In vitro (vivo): 670 nm, 0.48 W cm−2, 10 min | 1O2 | MCF-7 | MCF-7 | 228 |
| PDT | IR780 | Fluorinated nanoplatform | In vitro: 808 nm, 2 W cm−2, 200 s | 1O2 | 4T1 | 4T1 | 230 |
| In vivo: 808 nm, 2 W cm−2, 5 min | |||||||
| PDT/PTT | MoSe2/Fe3O4, Dox | PFC | In vitro (vivo): 808 nm, 0, 0.5, 1, 1.5 W cm−1, 10 min | ˙OH | HepG2 | H22 | 231 |
| PDT/PTT | IR780, ICG | PFC | In vitro: 808 nm, 2.0 W cm−2, 5 min | 1O2 | 4T1 | 4T1 | 234 |
| In vivo: 808 nm, 1.0 W cm−2, 5 min | |||||||
| PDT | Ce6 | PFH | In vitro: 660 nm, 100 mW cm−2, 5 min | 1O2 + ROS | MDA-MB-231 | MDA-MB-231 | 237 |
| In vivo: 660 nm, 100 mW cm−2, 10 min | |||||||
| PDT | DVDMS | Biomimetic nanoemulsion | In vitro: 635 nm, 200 mW cm−2, 5 min | 1O2 | 4T1 | 4T1 | 238 |
| In vivo: 635 nm, 200 mW cm−2, 30 min | |||||||
3.3 Red blood cells or hemoglobin
Red blood cells (RBC) are the staple oxygen carriers of all the vertebrates, providing enough O2 to various tissues and organs through blood transport.239 Each red blood cell contains a lot of hemoglobin molecules (Hb),240 which is a metal protein that binds to oxygen and plays a role in carrying oxygen.241 Hb is a biosafe oxygen carrier and a single Hb can bind to four oxygen molecules, so it can make good use of the ability to carry oxygen for PDT.242,243 On account of the short cycle half-life and poor stability of Hb, exposed Hb is not suitable for oxygen transport. Because of this disadvantage, people continued to explore how to make up for the shortcomings of the original poor physical properties, so there began to be the emergence of artificial red blood cell composites.244 It is reported that particles camouflaged with natural erythrocyte membranes can overcome biological obstacles and extend the time of blood circulation.245–247In a recent study, Wang et al. prepared RBC microcarriers to selectively deliver O2 into anoxic areas.248 This RBC microcarrier can surmount a series of complex biological obstacles, including transport through inflamed endothelial cells and escape from the mononuclear phagocytic system. In addition, RBC microcarriers have effective tumor accumulation capacity and can transport a mass of O2 for PDT under NIR radiation, so achieve effective solid tumor eradication. Using this strategy, a novel site-specific hypoxia probe (HP) was combined with a PDT photosensitizer (Rose Bengal, RB) to form orthogonal excitation-emission UCNPs. The author also evaluated the formation of ROS in hypoxic U87MG cells with RBC microcarriers under alternating 980 nm and 808 nm NIR irradiation with the most significant apoptosis of RBC microcarriers, indicating a large amount of O2 release. The ability of RBC microcarriers to overcome the biological barrier was also studied before the in vivo experiment, with mesoporous silicon microcarriers as the control group (cross-linked with HP, RB-modified UCNP, and FA), and the interaction between RBC microcarriers and a (HUVEC) fusion monolayer of human umbilical vein endothelial cells under inflammatory conditions was also studied. The results show that the use of erythrocyte microcarriers can effectively bypass tumor vessels and reduce immune clearance. Vascular oxygen saturation (sO2) in U87MG solid tumors was evaluated by PA imaging, and caudal vein injection of RBC microcarriers increased sO2 by 23% in half an hour. The therapeutic ability of this nano-system was evaluated in U87MG solid tumor mice. The results showed that the tumor volume decreased significantly after alternating 980 nm and 808 nm laser irradiation for two weeks. For example, Guo et al. designed an oxygen transfer biocompatible photosensitive liposome (LIH), to enhance PDT against hypoxic tumors by loading the compound with oxygen-carrier Hb and photosensitizer (Fig. 13a).249 Liposomes loaded with ICG and Hb (LIH) showed effective tumor accumulation. The effects of laser irradiation (808 nm, 1 W cm−2, 1 min) and different O2 levels on the production of 1O2 in the liposome solution were studied in vitro. LIH can produce a large amount of 1O2 under normoxic or anoxic conditions. Under the state of hypoxia, LIH can down-regulate the high expression level of HIF-1α, which proves the oxygen-carrying capacity of LIH. The PDT effect of LIH in vivo was evaluated. Mice bearing CT-26 xenografts were divided into four groups, which were treated with saline, LI + NIR irradiation, LIH or LIH + NIR irradiation, respectively. The results showed that the LIH + NIR irradiation treatment group showed the best therapeutic ability, indicating that LIH + NIR irradiation promoted cell apoptosis and inhibited proliferation.
 | ||
| Fig. 13 (a) Schematic diagram of a nano-system for relieving tumor hypoxia and enhancing PDT. Reproduced from ref. 249 with permission from Informa UK Limited, copyright 2018. (b) The P-Hb-B NPs by virtue of the carried O2 achieves PDT on cancer cells. Reproduced from ref. 253 with permission from Springer Nature Switzerland AG, copyright 2018. | ||
Although the nanoparticle drug carriers are similar. The novel nano red blood cells contain oxyhemoglobin (oxy-Hb) and the gaseous generator ammonium bicarbonate (ABC).250 The co-loading and controlled release of ICG and Dox resulted in synergistic therapeutic effects. After NIR laser irradiation, ICG in DIRAS exerts a PTT/PDT effect towards various breast cancer cells, and its PDT efficiency will be improved due to the oxygen participation of oxy-Hb. Due to the temperature rise caused by the PTT effect, ABC can be decomposed into NH3 and CO2, which further contributes to the release of Dox and plays a role in ablating tumors. In vivo experiments further evaluated the efficacy of DIRAS on PTT/PDT in 4T1 tumor-bearing mice. After irradiation, tumors treated by free ICG, ICG/RAS mixture and DIAS all showed strong green fluorescence, but the 1O2 produced by the DIAS group was the highest. Meanwhile, the fluorescence intensity of tumors treated with ICG/RAS mixture and DIRAS was significantly enhanced after laser irradiation, and the presence of oxy-Hb in RAS and DIRAS was conducive to the enhancement of PDT efficacy by ICG. In general, DIRAS had a strong efficacy of PTT/PDT in vivo. In 2018, Liu et al. prepared an artificial RBC nanomaterial (AmmRBC) for oxygen-self-supplied tumor PDT to overcome hypoxia-mediated tumor resistance.251 Biomembrane recombination technology has been used to produce biovesicles composed of erythrocyte membrane (RBCM), which encapsulates a complex formed between hemoglobin and biocompatible dopamine (PDA) (Fig. 14). AmmRBCs have demonstrated great ability for tumor accumulation and a high tolerance of PDT to extreme hypoxia in the tumor, resulting in complete ablation of the tumor. In general, as a highly biocompatible component in the human body, red blood cells have great application prospects in the adjuvant treatment of cancer with photodynamic therapy. Li's group designed a multifunctional nano-complex (BP@RB-HB) through the molecule assembly method.252 Because of the large overlap of emission spectra between Rose Bengal (RB) and BP, RB was chosen as the FRET receptor. Under two-photon laser irradiation, RB is indirectly excited by the intra-particle FRET mechanism, thus boosting the depth of treatment. In addition, Hb in nanocomposites can bring O2 into the tumor through active and passive targeting mechanisms, and improve the efficiency of PDT. Liu et al. synthesized an NIR photosensitizer – brominated 4,4-difluoro-4-bora-3a,a-diaza-s-indacene (BODIPY-Br2) with high 1O2 production efficiency and high fluorescence emission (Fig. 13b).253 NIR photosensitizer Hb-conjugated biodegradable peptides as O2 carriers were synthesized through a click reaction between polypeptides and Hb-BODIPY-Br2NPs, denoted as (p-Hb-B-NPs), and near-infrared imaging-guided PDT was achieved. The oxygen-carrying capacity and tumor tracking and treatment ability of the prepared polymer nanoparticles were verified by the study of HepG2 cancer cells. The phototoxic effect was best when the concentration of p-HB-B-NPs was 4 μM, and there was no phototoxic effect under a N2 atmosphere. In addition, it was proved that p-Hb-B-NPs also had a strong cell killing force under hypoxia and weak light irradiation (25 mW cm−2, 10 min).
 | ||
| Fig. 14 Schematic illustration of AmmRBCs for tumor therapy. Reproduced from ref. 251 with permission from Wiley, copyright 2018. | ||
Gao et al. reported an acoustically driven and magnetically navigated analog red cell (RBCM) micromotor that serves as an O2 and PS delivery platform that can actively transport O2 and PS.254 They made use of the natural cell membrane camouflage that particles display in a long circulation of blood in living organisms and prepared a new type of RBCM micromotor by wrapping Fe3O4 nanoparticles and ICG with hemoglobin. By co-incubating normal hepatocytes (L02 cells) with different concentrations of RBCM, it was proved that RBCM micromotors had no obvious toxic effect on hepatocytes, indicating that RBCM micromotors had good cytocompatibility. They also used a microfluidic chip to test the ability of the RBCM micromotor to target the predetermined tumor area. With propulsion from an ultrasonic field and navigation from an external magnetic field, the RBCM micromotor quickly entered the right-side reservoir along the microfluidic channel. The O2-carrying capacity of the RBCM micromotor was evaluated by p50. Tumor killing ability was evaluated by flow cytometry. After irradiation (808 nm, 0.35 W cm−2, 15 min), dual-oxygen-ICG-loaded RBCM nanoparticles could kill more than 75% of the cells, and an MTT assay was carried out again to confirm this result. In conclusion, the use of an RBCM micromotor as an active transmission platform can improve the anticancer effect of photodynamic therapy.
In recent years, new fluorescent materials have developed rapidly, aiming at the many advantages of conjugated polymer nanoparticles (CPNs), such as enhanced light capture ability, low cytotoxicity and excellent photostability.255,256 Inspired by these, Jiang et al. used conjugated polymer nanoparticles (CPNs) connected with hemoglobin (Hb) to prepare a PDT system that can luminesce and supply O2.257 Hb acts as a catalyst for the luminol–H2O2 chemiluminescence system and provides O2. To change the state of Hb during blood circulation, Hb-NPs were coated to form Hb-NPs@liposomes. When H2O2 and Hb exist at the same time, luminol emits blue light in the range of 375–550 nm, while NPs based on MEH-PPV have an absorption spectrum in the 400–550 nm range, so this situation meets the requirement of CRET as a donor–acceptor pair. After absorbing luminol radiation, Hb-NPs can sensitize oxygen molecules in Hb and produce ROS to kill cancer cells. The cell survival rate with S/deoxy-Hb-NPs@liposome at all concentrations was significantly higher than that with S/oxy-Hb-NPs@liposome, which confirmed the crucial role of oxygen in PDT. They studied the relationship between nanoparticle size and efficacy and found that smaller HB-NP groups produced more ROS.
In another effort, a hybrid protein oxygen nano-carrier was uncovered, which hybridized with Hb and HSA by an intermolecular disulfide bond (C@HPOC) to enhance the resistance of PDT to tumor growth and metastasis.258 C@HPOC possesses targeted delivery of photosensitizer and O2 to the tumor, which helps to enhance the infiltration of CD8 + T cells in the tumor. C@HPOC-mediated PDT can effectively inhibit tumor cell metastasis by stimulating systemic anti-tumor immunity in the 4T1 mTNBC murine model. The killing ability of C@HPOC on the tumor was investigated in vitro, with irradiation from a 660 nm laser (0.1 W cm−2, 2 min), indicating that C@HPOC + laser exhibited high phototoxicity against 4T1 cells compared with the control groups. To investigate the tumor cell-killing effect of C@HPOC + laser in vitro, they irradiated with a 660 nm laser (2 min, 0.1 W cm−2) in different groups (including the control group); the results revealed that the C@HPOC + laser group showed significant phototoxicity. To figure out whether C@HPOC-mediated PDT can trigger the emergence of ICD, they used 4T1 cells treated with C@HPOC + laser and then evaluated CRT exposure, HMGB1 release, and ATP secretion. Finally, it was shown that C@HPOC has the effect of oxygen-boosted PDT. In general, oxygen-enhanced immunogenic PDT, induced by C@HPOC, ablates the primary tumor and effectively inhibits distant tumor and lung metastasis.
A new strategy that is a kind of bionic red blood cells (BRBCs) is prepared by the layer by layer assembly method, using Fe3O4@CuO, an oxygen donor (oxy-Hb), an anti-cancer drug (ZnPc) and a photocrosslinking acrylate modified hyaluronic acid (HA) gel shell.259 The authors used Fe3O4@CuO as a specific recruiter for oxy-Hb, and the HA gel shell can prevent oxy-Hb from releasing O2 in the blood, but when BRBCs enter the tumor and accumulate, the HA shell will be degraded by HAase, which triggers the release of O2 to alleviate the hypoxia of the tumor. ZnPc consumed O2 during PDT treatment, resulting in enhanced fluorescence of intracellular probes. The fluorescence signals of the BRBC and BRBC+ magnetic treatment groups were significantly stronger than those of other groups with HAase, indicating that BRBCs can simulate the adequate oxygen supply of erythrocytes in the PDT process in hypoxic tumors. Magnetic field-assisted tumor targeting was proved in vivo, and the accumulation of BRBCs in vivo with or without a magnetic field was studied and compared. With the same fluorescence excitation conditions, strong ZnPc fluorescence signals were detected in tumor tissues with or without a magnetic field, but the fluorescence signal intensity with BRBC + magnetism was significantly higher than that in the control group. The data show that a magnetic field can enhance tumor-targeting efficiency, and BRBCs have excellent performance in tumor targeting. Also, the publications about RBC or Hb as an O2 vehicle were summaried in Table 8.
| Application | PSs | ROS species | Excitation requirements (excitation wavelength, power density, time) | ROS indicator | In vitro (cell lines) | In vivo (tumour-bearing mice) | O2 carrier | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: 1,3-diphenylisobenzofuran (DPBF); man-made pseudo-RBCs (mmRBCs); Rose Bengal (RB); probe for reactive oxygen species (ABDA); acoustically powered and magnetically navigated red blood cell-mimicking micromotor (RBCM); hemoglobin (Hb)-linked conjugated polymer nanoparticles (CPNs); zinc phthalocyanine (ZnPc). | ||||||||
| PDT | Rose Bengal | 1O2 | In vitro: 980 nm (0–30 min) | DPBF | U87MG | U97-MG | RBC | 248 |
| In vivo: 980 nm (2 W cm−2, 15 min) & 808 nm (1.5 W cm−2, 15 min) | ||||||||
| PDT | ICG | 1O2 | In vitro: 808 nm, 1 W cm−2, 1 min | SOSG | CT-26 | S180 and CT-26 | Hb | 249 |
| In vivo: 808 nm, 1.5 W cm−2, 3 min | ||||||||
| PDT/PTT | ICG/Dox | 1O2 | In vitro (vivo): 808 nm, 2.0 W cm−2, 10 min | SOSG | 4T1, HUVECs | 4T1 and 4T1-Luc | Hb | 250 |
| PDT | MB | 1O2 | In vitro (vivo): 808 nm, 2.0 W cm−2, 10 min | DCFH-DA, COS7 | 4T1 | 4T1 | mmRBC | 251 |
| PDT | RB | 1O2 | 800 nm, 390 mW | ABDA | MCF-7 | MCF-7 | Hb | 252 |
| PDT | BODIPY | 1O2 | In vitro: 635 nm, 25 mW cm−2, 10 min | DCFH-DA | HepG2 | NO | Hb | 253 |
| PDT | ICG | 1O2 | In vitro: 808 nm, 0.35 W cm−2, 15 min | ABDA | HeLa and L02 | NO | RBCM | 254 |
| PDT | NPs | 1O2 | Chemiluminescence of luminol | DCFH | HeLa and HCT-8 | — | CPNs | 257 |
| PDT | Ce6 | 1O2 | In vitro: 660 nm, 0.1 W cm−2, 0.5 min | SOSG | 4T1 | 4T1 | Hb | 258 |
| In vivo: 660 nm, 0.1 W cm−2, 30 min | ||||||||
| PDT | ZnPc | 1O2 | In vitro: 665 nm LED, 5 min | DCFH-DA, Ru(dpp)3 Cl2 | A549 | A549 | Oxy-Hb | 259 |
| In vivo: 665 nm LED, 2 W, 30 min | ||||||||
3.4 Hyperbaric oxygen
Hyperbaric oxygen (HBO) as a clinical adjuvant therapy has been used to cure carbon monoxide poisoning, and in wound healing, soft tissue infection and other diseases.18,260,261 As an adjuvant therapy, it has many advantages, such as the ability to deliver oxygen to tissues without hemoglobin and the ability to reduce tumor interstitial fluid pressure (IFP). HBO can solve the problem of the slow diffusion rate and short diffusion distance of oxygen in tissue.262 It can effectively oxygenate or even alleviate the anoxic area of the tumor.263 If the hypoxia environment is not changed, the therapeutic effect will be reduced. For tumor tissue hypoxia, hyperoxia to compensate for oxygen depletion is a good way to improve hypoxia. Hyperbaric oxygen can provide extra oxygen in tumor tissue by adding oxygen or substances or other molecules that can produce O2. Another way is by modifying the structure of photosensitizers, making light-dependent photosensitizers into light-independent photosensitizers (Type II to Type I).At present, there are few studies on the use of HBO combined with PDT in the treatment of human squamous cell carcinoma (SCC), aiming at the advantages of HBO combined with PDT. Hence, Fang et al. proposed a method using HBO combined with 5-aminolevulinic acid PDT to inhibit the proliferation of human squamous cell lines.264 It was found that the combination of HBO and PDT could not only inhibit the proliferation of A431 cells in vitro but also down-regulate Bcl-2 to significantly enhance PDT-induced apoptosis and autophagy. Autophagy plays a survival-promoting role in apoptosis. Therefore, the combination of HBO and PDT may be a promising treatment method for human squamous cell carcinoma. The combination of HBO and ALA-PDT cooperatively up-regulated the proteins Bax and active caspase 3, and down-regulated Bcl-2 in A431 cells. Western blot tests detected the expression of apoptosis-related proteins (Bax, Bcl-2, and caspase 3), and the results indicated that the expression of Bax and caspase in A431 cells increased cooperatively. In contrast, the expression of Bcl-2 decreased, indicating that apoptosis is an intrinsic pathway of mitochondria. The authors also reduced the production of ROS in A431 cells by dual ALA-HBO treatment, which was conducive to autophagy. In general, ALA-HBO is one of the promising methods for the treatment of human squamous cell carcinoma.
Due to the hypoxia-related environment and the difficulty of photosensitizers penetrating tumor cells away from blood vessels, the therapeutic effect is limited in deep tumors. To address this problem, Yang's group proposed a therapeutic strategy of upconverting nano-sensitizers (UNPSs) and HBO to reshape the extracellular matrix to enhance PDT (Fig. 15).265 UNPSs are designed to have a Nd3+-sensitized sandwich structure, in which the up-conversion core acts as a light transducer to transfer energy to an adjacent photosensitizer to produce ROS. The hyperbaric oxygen-assisted PDT process promotes oxygen diffusion by decomposing collagen in the tumor extracellular matrix, and promotes the penetration of UNPSs to the deep tumor. They evaluated the in vitro PDT efficacy of UNPS using MTT analysis, and cell viability was significantly reduced with increasing UNPS concentration after 808 nm laser irradiation and HBO application. HBO adjuvant PDT with UNPSs can improve the efficacy of cancer treatment. HBO-assisted PDT with UNPS was used to carry out an experiment on the mouse model of xenotransplantation. Firstly, the metabolism of UNPSs was studied; after intravenous injection, fluorescence could be detected after 6 hours and disappeared after 48 hours. They classified different treatment groups, with the group exposed to the laser being exposed for 16 minutes at 24 and 48 hours after administration; by calculating the tumor volume, the therapeutic effect could be directly reflected according to the change of tumor volume. However, the UNPSs + NIR + HBO group had the most dramatic treatment effect. In short, HBO-enhanced PDT using UNPSs represents not only a promising choice for more efficient treatment of tumors, but also a new strategy. Also, the publications about HBO as an O2 source were summaried in Table 9.
 | ||
| Fig. 15 Schematic illustration of the PDT process with or without HBO; the synergistic therapy could relieve hypoxia and enhance the effect of PDT. Reproduced from ref. 265 with permission from the American Chemical Society, copyright 2018. | ||
| Application | PSs | O2 source | Excitation requirements (excitation wavelength, power density, time) | ROS indicator | In vitro (cell line) | In vivo (tumor-bearing mice) | Ref. |
|---|---|---|---|---|---|---|---|
| Abbreviations: precursors of protoporphyrin IX (ALA). | |||||||
| PDT | ALA | HBO | 630 ± 15 nm, 5 J cm−2, 8 h | Detection kit | A431 | NO | 264 |
| PDT | RB | HBO | In vivo: 808 nm, 0.75 W cm−2, 16 min | DCFH-DA | 4T1 | 4T1 | 265 |
4. Summary and outlook
PDT shows great potential in clinical research and application by improving treatment efficiency with minimal invasiveness through the precise management of laser irradiation compared with traditional oncology therapies. However, hypoxia seriously limits the efficiency of PDT and leads to tumor invasion and metastasis. Therefore, the presence of adequate molecular oxygen throughout the tumor volume is critical for effective PDT, the mechanism of using photosensitizers activated by light to produce cytotoxicity (biological reactive oxygen species).The maldistribution of blood vessels leads to highly uneven oxygen levels within the tumor, which in this case leads to the presence of severe hypoxia (deep tissue hypoxia). For drug therapy or PDT, tumor cells that lack adequate molecular oxygen are resistant to PDT in the presence of either drug therapy or photodynamic therapy. Therefore, tumor hypoxia limits the widespread clinical application of PDT to overcome the “Achilles’ heel”; so far, many strategies have been carried out to overcome hypoxia. This review paper summarizes methods for producing molecular oxygen in situ or carrying molecular oxygen into anoxic tumor areas through different carriers to improve tumor hypoxia, and this is systematically summarized and discussed. These oxygen replenishment strategies and in situ oxygen production strategies are described as follows.
• Enhance tumor oxygen delivery by utilizing hemoglobin, red blood cells, and PFCs;
• Oxygen is produced by catalyzing H2O2 with different catalysts;
• Light-driven water splitting increases in situ oxygen production;
• Changing blood flow in different ways increases oxygen transport;
• Oxygen is produced by photosynthesis near the tumor tissue.
These strategies significantly alleviate the hypoxia of the tumor microenvironment and improve the efficacy of PDT, and the relief of hypoxia can also enhance the therapeutic effect of some drugs. However, there are still several issues that need to be noted in terms of the disadvantages of the supplementary oxygen strategy as described below:
(1) Problems with low endogenous hydrogen peroxide content
For the in situ generation of O2 within tumors, the amount of endogenous H2O2 is only 50–100 μM, so this will not achieve the goal of catalyzing endogenous hydrogen peroxide in tumor tissue with exogenous catalysts (commonly used metal ions). Therefore, this also makes the oxygen supplementation approach to PDT more difficult, which is a major drawback of the supplemental oxygen strategy. On a more optimistic note, the novel strategy of delivering exogenous H2O2 into tumors and using catalysts to decompose exogenous H2O2 into O2 was used to solve the problem of low endogenous hydrogen peroxide content.
(2) Toxicity of metal catalysts
After solving the problem of a low content of endogenous hydrogen peroxide, another major disadvantage of the oxygen supplement strategy to improve the therapeutic effect of PDT is the toxicity of metal catalysts. MnO2, Pt and other catalysts are often used to catalyze hydrogen peroxide for oxygen production. In addition to catalysis, these catalysts will also produce some uncontrollable cytotoxicity, such as binding with some enzymes, resulting in enzyme inactivation. Therefore, it may bring a series of problems; moreover, metal ion metabolism is also difficult, which may bring some uncontrollable side effects.
(3) The difficulty of synthesis of oxygen-delivery materials
As a complementary strategy for exogenous oxygen delivery to the tumor system, the selection of materials should be more scientific, meet better biocompatibility, and target tumor cells. Therefore, materials with better properties need to be used. These materials include: biomaterials, such as fungi, white cell membranes, cell membranes, red blood cells and hemoglobin; and chemical materials: perfluorocarbons. For photosensitive bacteria hybridized with cyanobacteria and photosensitizers with different properties for PDT therapy of photosynthesis-enhanced tumors under light stimulation, this method of in situ generation of oxygen has been recently proposed as an effective method to alleviate tumor hypoxia, but the preparation process of cyanobacteria is tedious and takes a lot of time, and surgery is needed to implant photosensitive bacteria in biological applications. If this method leads to poor wound healing there will be large scars, so in later studies, photodynamic therapy can be used to remove scars and be combined with the treatment process, so as to shield some shortcomings. For the extraction of cell membranes, the process is also more tedious, therefore, to realize the engineering of extraction process will greatly promote the application of materials. Chemical materials can be difficult to synthesize, and some materials require multi-step synthesis, which will make it more difficult to supplement oxygen therapy strategies.
(4) Excessive supplemental oxygen accelerates tumor blood vessel growth
In the human body, blood vessels can provide O2 and nutrition for the growth of all tissues, but tumors grow much faster than normal tissues, so the demand for nutrition is higher. In this state, tumor cells begin to produce growth factors that stimulate the growth of new blood vessels, resulting in abnormal vascular morphology. Abnormal vascular morphology leads to poor blood flow and reduced O2 supply. The insufficient supply of oxygen will cause cancer cells to metastasize and eventually form a malignant tumor. In addition, abnormal vascular morphology also makes anticancer drugs unable to reach the focus, which greatly reduces the therapeutic effect. For an oxygen supplementation strategy, oxygen is directly transported into the tumor tissue through hyperbaric oxygen. Oxygen supplements should be in an appropriate range, otherwise excess oxygen on the one hand increases oxygen pressure and brings a lot of side effects in the process of PDT. On the other hand, excess oxygen will promote the rapid growth of tumor blood vessels. Then the biggest problem will be how to balance the appropriate amount of oxygen to alleviate tumor tissue hypoxia and oxygen to promote tumor growth. More and more research data reveal that direct delivery of oxygen has certain limitations. Therefore, for the application of this method of oxygen supplementation there is a need to constantly explore how to monitor the partial pressure of oxygen and supplement the effective concentration of oxygen in the tumor tissue. Once mastered, it will become easy to balance the above problems.
Besides, other new strategies can be vigorously developed, including:
(1) Chemotherapy in combination with PDT
Nowadays, chemotherapy is still the main treatment for most cancers. However, during chemotherapy, cancer cells may develop resistance, which directly reduces the effectiveness of the treatment. Chemotherapy and PDT combined with adjuvant therapy can produce a synergistic effect, and the treatment results between them can complement each other; the combined effect can reduce the drug dose and systemic toxicity. At present, the most commonly used chemotherapy drugs are as follows: tipacamine (TPZ), Dox, AQ4N, platinum drugs, and so on.
(2) Antiangiogenic PDT
PDT also induces abnormal expression of VEGF, COX-2, and MMPs. The increased expression of HIF-1α in the tumor after PDT directly induces photodynamic drug resistance, with a high expression of VEGF. The combination of HIF-1α or VEGF inhibitors with PDT can prevent drug resistance and even improve therapeutic efficacy.
(3) PTT/PDT
PTT is targeted to tumor tissue by the photosensitizer. A low-power beam can be applied to tumor tissue to generate heat energy and use the heat to ablate the tumor. PDT and PTT both need to use irradiation and a photosensitizer, so the combined use of the photosensitizer can achieve dual functions simultaneously. The PTT process will also increase blood flow and alleviate the aggravation of hypoxia caused by the PDT process.
(4) Immunotherapy combined with PDT
Because an anti-tumor immune response can be initiated in the process of PDT, its effect on tumor ablation plays a very important role. Therefore, it has become a promising clinical model for cancer treatment. Multiple pathways have been reported for PDT to initiate anti-tumor immune responses, including:
◆ up-regulation of the expression of transcription factor nuclear factor-kB (NF-kB) and molecular chaperone heat shock protein 70 (HSP-70);
◆ promoting antigen presentation to cytotoxic T lymphocytes (CTL);
◆ stimulating the immune response of the host by promoting the secretion of cytokines.
Author contributions
Shuheng Qin: conceptualization, data curation, investigation, writing – review & editing; Yue Xu, Hua Li: supervision, visualization, formal analysis; Zhenwei Yuan, Haiyan Chen: supervision & guidance, validation, reviewing & editing.Conflicts of interest
The author claims that they do not have competing interests for this paper.Acknowledgements
This work was financially supported by Natural Science Foundation Committee of China (NSFC 82001891), the Post-doctoral Innovative Talent Support Program (BX20190389), China Postdoctoral science Foundation (2019M662009), and Postdoctoral Research Grant of Jiangsu Province.References
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Photodiagn. Photodyn. Ther., 2005, 2, 1–23 CrossRef CAS PubMed.
- W. M. Sharman, C. M. Allen and J. E. van Lier, in Singlet Oxygen, Uv-a, and Ozone, ed. L. Packer and H. Sies, 2000, vol. 319, pp. 376–400 Search PubMed.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, JNCI, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS PubMed.
- S. Pervaiz and M. Olivo, Clin. Exp. Pharmacol. Physiol., 2006, 33, 551–556 CrossRef CAS PubMed.
- Z. Zhou, J. Song, L. Nie and X. Chen, Chem. Soc. Rev., 2016, 45, 6597–6626 RSC.
- Z. Lv, H. J. Wei, Q. Li, X. L. Su, S. J. Liu, K. Y. Zhang, W. Lv, Q. Zhao, X. H. Li and W. Huang, Chem. Sci., 2018, 9, 502–512 RSC.
- R. C. Gilson, K. C. L. Black, D. D. Lane and S. Achilefu, Angew. Chem., Int. Ed., 2017, 56, 10717–10720 CrossRef CAS PubMed.
- C. Zhang, K. L. Zhao, W. B. Bu, D. L. Ni, Y. Y. Liu, J. W. Feng and J. L. Shi, Angew. Chem., Int. Ed., 2015, 54, 1770–1774 CrossRef CAS PubMed.
- H. Y. Ding, H. J. Yu, Y. Dong, R. H. Tian, G. Huang, D. A. Boothman, B. D. Sumer and J. M. Gao, J. Controlled Release, 2011, 156, 276–280 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed.
- M. Li, Y. Shao, J. H. Kim, Z. Pu, X. Zhao, H. Huang, T. Xiong, Y. Kang, G. Li, K. Shao, J. Fan, J. W. Foley, J. S. Kim and X. Peng, J. Am. Chem. Soc., 2020, 142, 5380–5388 CrossRef CAS PubMed.
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2018, 140, 14851–14859 CrossRef CAS PubMed.
- J. D. Chapman, C. C. Stobbe, M. R. Arnfield, R. Santus, J. Lee and M. S. McPhee, Radiat. Res., 1991, 126, 73–79 CrossRef CAS.
- J. E. Moulder and S. Rockwell, Int. J. Radiat. Oncol., Biol., Phys., 1984, 10, 695–712 CrossRef CAS.
- M. Zhou, Y. Xie, S. Xu, J. Xin, J. Wang, T. Han, R. Ting, J. Zhang and F. An, Eur. J. Med. Chem., 2020, 195, 112274 CrossRef CAS PubMed.
- P. Vaupel, M. Hoeckel and A. Mayer, Antioxid. Redox Signaling, 2007, 9, 1221–1235 CrossRef CAS PubMed.
- T. Maleki, N. Cao, S. H. Song, C. Kao, S.-C. A. Ko and B. Ziaie, IEEE Trans. Biomed. Eng., 2011, 58, 3104–3111 Search PubMed.
- I. Moen and L. E. B. Stuhr, Target. Oncol., 2012, 7, 233–242 CrossRef PubMed.
- W. Zhang, S. Li, X. Liu, C. Yang, N. Hu, L. Dou, B. Zhao, Q. Zhang, Y. Suo and J. Wang, Adv. Funct. Mater., 2018, 28, 1706375 CrossRef.
- M. R. Horsman and J. Overgaard, J. Radiat. Res., 2016, 57, I90–I98 CrossRef.
- E. B. Rankin and A. J. Giaccia, Science, 2016, 352, 175–180 CrossRef CAS PubMed.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS PubMed.
- S. O. Gollnick, B. Owczarczak and P. Maier, Lasers Surg. Med., 2006, 38, 509–515 CrossRef PubMed.
- B. Karagoz, S. Suleymanoglu, G. Uzun, O. Bilgi, S. Aydinoz, A. Haholu, O. Turken, Y. Onem and E. G. Kandemir, Basic Clin. Pharmacol. Toxicol., 2008, 102, 287–292 CrossRef CAS PubMed.
- S. Guan, L. Wang, S.-M. Xu, D. Yang, G. I. N. Waterhouse, X. Qu and S. Zhou, Chem. Sci., 2019, 10, 2336–2341 RSC.
- S. Kolemen, T. Ozdemir, D. Lee, G. M. Kim, T. Karatas, J. Yoon and E. U. Akkaya, Angew. Chem., Int. Ed., 2016, 55, 3606–3610 CrossRef CAS PubMed.
- X. Li, B.-D. Zheng, X.-H. Peng, S.-Z. Li, J.-W. Ying, Y. Zhao, J.-D. Huang and J. Yoon, Coord. Chem. Rev., 2019, 379, 147–160 CrossRef CAS.
- J. E. Moulder and S. Rockwell, Cancer Metastasis Rev., 1987, 5, 313–341 CrossRef CAS PubMed.
- J. Daruwalla and C. Christophi, World J. Surg., 2006, 30, 2112–2131 CrossRef PubMed.
- H. Cheng, J. Y. Zhu, S. Y. Li, J. Y. Zeng, Q. Lei, K. W. Chen, C. Zhang and X. Z. Zhang, Adv. Funct. Mater., 2016, 26, 7847–7860 CrossRef CAS.
- Q. Chen, L. Z. Feng, J. J. Liu, W. W. Zhu, Z. L. Dong, Y. F. Wu and Z. Liu, Adv. Mater., 2018, 30, 1 Search PubMed.
- J. Kim, H. R. Cho, H. Jeon, D. Kim, C. Song, N. Lee, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2017, 139, 10992–10995 CrossRef CAS PubMed.
- D. Zheng, B. Li, L. Xu, Q.-L. Zhang, J.-X. Fan, C.-X. Li and X.-Z. Zhang, ACS Nano, 2018, 12, 6218–6227 CrossRef CAS PubMed.
- X. Cui, Q. Zhao, Z. Huang, Y. Xiao, Y. Wan, S. Li and C.-S. Lee, Small, 2020, 16, 2004551 CrossRef CAS.
- R. Kv, T.-I. Liu, I. L. Lu, C.-C. Liu, H.-H. Chen, T.-Y. Lu, W.-H. Chiang and H.-C. Chiu, J. Controlled Release, 2020, 328, 87–99 CrossRef CAS PubMed.
- Z. Y. Yang, J. F. Wang, S. Liu, X. H. Li, L. Y. Miao, B. Yang, C. L. Zhang, J. He, S. C. Ai and W. X. Guan, Biomaterials, 2020, 229, 15 CrossRef.
- Y.-T. Fan, T.-J. Zhou, P.-F. Cui, Y.-J. He, X. Chang, L. Xing and H.-L. Jiang, Adv. Funct. Mater., 2019, 29, 1806708 CrossRef.
- R. Kumari, D. Sunil and R. S. Ningthoujam, J. Controlled Release, 2020, 319, 135–156 CrossRef CAS PubMed.
- Y. Shao, B. Liu, Z. Di, G. Zhang, L.-D. Sun, L. Li and C.-H. Yan, J. Am. Chem. Soc., 2020, 142, 3939–3946 CrossRef CAS PubMed.
- D. Wang, H. Wu, W. Q. Lim, S. Z. F. Phua, P. Xu, Q. Chen, Z. Guo and Y. Zhao, Adv. Mater., 2019, 31, 1901893 CrossRef PubMed.
- Z. Wang, T. Jia, Q. Sun, Y. Kuang, B. Liu, M. Xu, H. Zhu, F. He, S. Gai and P. Yang, Biomaterials, 2020, 228, 119569 CrossRef CAS PubMed.
- G. C. Dismukes, V. V. Klimov, S. V. Baranov, Y. N. Kozlov, J. DasGupta and A. Tyryshkin, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2170–2175 CrossRef CAS PubMed.
- R. Y. Stanier and G. Cohen-Bazire, Annu. Rev. Microbiol., 1977, 31, 225–274 CrossRef CAS PubMed.
- Z. Hosseinidoust, B. Mostaghaci, O. Yasa, B. W. Park, A. V. Singh and M. Sitti, Adv. Drug Delivery Rev., 2016, 106, 27–44 CrossRef CAS PubMed.
- T. Hasegawa, T. Matsuguchi, K. Noda, K. Tanaka, S. Kumamoto, Y. Shoyama and Y. Yoshikai, Int. Immunopharmacol., 2002, 2, 579–589 CrossRef CAS PubMed.
- K. Noda, N. Ohno, K. Tanaka, N. Kamiya, M. Okuda, T. Yadomae, K. Nomoto and Y. Shoyama, Planta Med., 1996, 62, 423–426 CrossRef CAS PubMed.
- K. Tanaka, A. Yamada, K. Noda, T. Hasegawa, M. Okuda, Y. Shoyama and K. Nomoto, Cancer Immunol. Immunother., 1998, 45, 313–320 CrossRef CAS PubMed.
- W. B. Coley, Clin. Orthop. Relat. Res., 1991, 3–11 CAS.
- S.-B. Wang, X.-H. Liu, B. Li, J.-X. Fan, J.-J. Ye, H. Cheng and X.-Z. Zhang, Adv. Funct. Mater., 2019, 29, 1904093 CrossRef.
- L. L. Liu, H. M. He, Z. Y. Luo, H. M. Zhou, R. J. Liang, H. Pan, Y. F. Ma and L. T. Cai, Adv. Funct. Mater., 2020, 30, 1910176 CrossRef CAS.
- M. Huo, L. Wang, L. Zhang, C. Wei, Y. Chen and J. Shi, Angew. Chem., Int. Ed., 2020, 59, 1906–1913 CrossRef CAS PubMed.
- Z. Zhang, J. Wang, X. Nie, T. Wen, Y. Ji, X. Wu, Y. Zhao and C. Chen, J. Am. Chem. Soc., 2014, 136, 7317–7326 CrossRef CAS PubMed.
- Z. M. Li, P. Huang, X. J. Zhang, J. Lin, S. Yang, B. Liu, F. Gao, P. Xi, Q. S. Ren and D. X. Cui, Mol. Pharm., 2010, 7, 94–104 CrossRef CAS PubMed.
- C. Lee, K. Lim, S. S. Kim, L. X. Thien, E. S. Lee, K. T. Oh, H.-G. Choi and Y. S. Youn, J. Controlled Release, 2019, 294, 77–90 CrossRef CAS PubMed.
- A. Espinosa, R. Di Corato, J. Kolosnjaj-Tabi, P. Flaud, T. Pellegrino and C. Wilhelm, ACS Nano, 2016, 10, 2436–2446 CrossRef CAS PubMed.
- W. I. Choi, J.-Y. Kim, C. Kang, C. C. Byeon, Y. H. Kim and G. Tee, ACS Nano, 2011, 5, 1995–2003 CrossRef CAS PubMed.
- H. Chen, J. Tian, W. He and Z. Guo, J. Am. Chem. Soc., 2015, 137, 1539–1547 CrossRef CAS PubMed.
- J. Zou, J. Zhu, Z. Yang, L. Li, W. Fan, L. He, W. Tang, L. Deng, J. Mu, Y. Ma, Y. Cheng, W. Huang, X. Dong and X. Chen, Angew. Chem., Int. Ed., 2020, 59, 8833–8838 CrossRef CAS PubMed.
- Z. Yu, P. Zhou, W. Pan, N. Li and B. Tang, Nat. Commun., 2018, 9, 5044 CrossRef PubMed.
- T.-J. Zhou, L. Xing, Y.-T. Fan, P.-F. Cui and H.-L. Jiang, J. Controlled Release, 2019, 307, 44–54 CrossRef CAS PubMed.
- S. Lamouille, J. Xu and R. Derynck, Nat. Rev. Mol. Cell Biol., 2014, 15, 178–196 CrossRef CAS PubMed.
- A. Dongre and R. A. Weinberg, Nat. Rev. Mol. Cell Biol., 2019, 20, 69–84 CrossRef CAS PubMed.
- H. Wang, Y. Guo, C. Wang, X. Jiang, H. Liu, A. Yuan, J. Yan, Y. Hu and J. Wu, Biomaterials, 2020, 269, 120621–120621 CrossRef PubMed.
- T. Sun, Y. Zhang, C. Zhang, H. Wang, H. Pan, J. Liu, Z. Li, L. Chen, J. Chang and W. Zhang, Front. Bioeng. Biotechnol., 2020, 8, 237 CrossRef PubMed.
- J. A. Raven, M. C. W. Evans and R. E. Korb, Photosynth. Res., 1999, 60, 111–149 CrossRef CAS.
- I. Sakurai, J.-R. Shen, J. Leng, S. Ohashi, M. Kobayashi and H. Wada, J. Biochem., 2006, 140, 201–209 CrossRef CAS PubMed.
- Y. S. Ho, Y. Xiong, W. C. Ma, A. Spector and D. S. Ho, J. Biol. Chem., 2004, 279, 32804–32812 CrossRef CAS PubMed.
- J. Li, K. Wei, S. Zuo, Y. Xu, Z. Zha, W. Ke, H. Chen and Z. Ge, Adv. Funct. Mater., 2017, 27, 1702108 CrossRef.
- J. Liu, T. Liu, P. Du, L. Zhang and J. Lei, Angew. Chem., Int. Ed., 2019, 58, 7808–7812 CrossRef CAS PubMed.
- S. Gao, P. Zheng, Z. Li, X. Feng, W. Yan, S. Chen, W. Guo, D. Liu, X. Yang, S. Wang, X.-J. Liang and J. Zhang, Biomaterials, 2018, 178, 83–94 CrossRef CAS PubMed.
- B. Wang, W. Lin, Z. Mao and C. Gao, J. Mater. Chem. B, 2018, 6, 3145–3155 RSC.
- J. Zhu, T. Xiao, J. Zhang, H. Che, Y. Shi, X. Shi and J. C. M. van Hest, ACS Nano, 2020, 14, 11225–11237 CrossRef CAS PubMed.
- P. Liu, X. Xie, X. Shi, Y. Peng, J. Ding and W. Zhou, ACS Appl. Mater. Interfaces, 2019, 11, 48261–48270 CrossRef CAS PubMed.
- J. Yin, H. Cao, H. Wang, K. Sun, Y. Li and Z. Zhang, Acta Pharm. Sin. B, 2020, 10, 2246–2257 CrossRef CAS PubMed.
- L. Deng, D. Sheng, M. Liu, L. Yang, H. Ran, P. Li, X. Cai, Y. Sun and Z. Wang, Biomater. Sci., 2020, 8, 858–870 RSC.
- J. Wang, X. Sun, W. Mao, W. Sun, J. Tang, M. Sui, Y. Shen and Z. Gu, Adv. Mater., 2013, 25, 3670–3676 CrossRef CAS PubMed.
- J. Wang, W. Mao, L. L. Lock, J. Tang, M. Sui, W. Sun, H. Cui, D. Xu and Y. Shen, ACS Nano, 2015, 9, 7195–7206 CrossRef CAS PubMed.
- R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2011, 152, 2–12 CrossRef CAS PubMed.
- C.-P. Liu, T.-H. Wu, C.-Y. Liu, K.-C. Chen, Y.-X. Chen, G.-S. Chen and S.-Y. Lin, Small, 2017, 13, 1700278 CrossRef PubMed.
- Y. Liu, Z. Wang, Y. Liu, G. Zhu, O. Jacobson, X. Fu, R. Bai, X. Lin, N. Lu, X. Yang, W. Fan, J. Song, Z. Wang, G. Yu, F. Zhang, H. Kalish, G. Niu, Z. Nie and X. Chen, ACS Nano, 2017, 11, 10539–10548 CrossRef CAS PubMed.
- D. F. Stowe and A. K. S. Camara, Antioxid. Redox Signaling, 2009, 11, 1373–1414 CrossRef CAS PubMed.
- N. Kavcic, K. Pegan, P. Vandenabeele and B. Turk, Biol. Chem., 2019, 400, 149–160 CrossRef CAS PubMed.
- J. N. Moloney and T. G. Cotter, Semin. Cell Dev. Biol., 2018, 80, 50–64 CrossRef CAS PubMed.
- M. A. O. Dawood, H. G. Abo-Al-Ela and M. T. Hasan, Fish Shellfish Immunol., 2020, 97, 268–282 CrossRef CAS PubMed.
- E. Ju, K. Dong, Z. Chen, Z. Liu, C. Liu, Y. Huang, Z. Wang, F. Pu, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2016, 55, 11467–11471 CrossRef CAS PubMed.
- H. Fan, G. Yan, Z. Zhao, X. Hu, W. Zhang, H. Liu, X. Fu, T. Fu, X.-B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2016, 55, 5477–5482 CrossRef CAS PubMed.
- J. Y. Cao and S. J. Dixon, Cell. Mol. Life Sci., 2016, 73, 2195–2209 CrossRef CAS PubMed.
- L.-S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z. Yang, W. Tang, G. Niu, H.-H. Yang and X. Chen, Angew. Chem., Int. Ed., 2018, 57, 4902–4906 CrossRef CAS PubMed.
- C. Liu, D. Wang, S. Zhang, Y. Cheng, F. Yang, Y. Xing, T. Xu, H. Dong and X. Zhang, ACS Nano, 2019, 13, 4267–4277 CrossRef CAS PubMed.
- J.-J. Hu, Q. Lei and X.-Z. Zhang, Prog. Mater. Sci., 2020, 114, 100685 CrossRef CAS.
- Y.-H. Zhang, W.-X. Qiu, M. Zhang, L. Zhang and X.-Z. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 15030–15039 CrossRef CAS PubMed.
- T. Amann, U. Maegdefrau, A. Hartmann, A. Agaimy, J. Marienhagen, T. S. Weiss, O. Stoeltzing, C. Warnecke, J. Scholmerich, P. J. Oefner, M. Kreutz, A. K. Bosserhoff and C. Hellerbrand, Am. J. Pathol., 2009, 174, 1544–1552 CrossRef CAS PubMed.
- H. Bi, Y. Dai, P. Yang, J. Xu, D. Yang, S. Gai, F. He, G. An, C. Zhong and J. Lin, Chem. Eng. J., 2019, 356, 543–553 CrossRef CAS.
- J. Liu, L. Cui and D. Losic, Acta Biomater., 2013, 9, 9243–9257 CrossRef CAS PubMed.
- X. Luan, Y.-Y. Guan, H.-J. Liu, Q. Lu, M. Zhao, D. Sun, J. F. Lovell, P. Sun, H.-Z. Chen and C. Fang, Adv. Sci., 2018, 5, 1800034 CrossRef PubMed.
- H. Zhou, J. Ge, Q. Miao, R. Zhu, L. Wen, J. Zeng and M. Gao, Bioconjugate Chem., 2020, 31, 315–331 CrossRef CAS PubMed.
- P. Liu, X. Xie, M. Liu, S. Hu, J.-S. Ding and W.-H. Zhou, Acta Pharm. Sin. B, 2021, 11, 823–834 CrossRef CAS PubMed.
- V. Hirsch, J. Salaklang, B. Rothen-Rutishauser and A. Petri-Fink, IEEE Trans. Magn., 2013, 49, 402–407 Search PubMed.
- T. L. Moore, L. Rodriguez-Lorenzo, V. Hirsch, S. Balog, D. Urban, C. Jud, B. Rothen-Rutishauser, M. Lattuada and A. Petri-Fink, Chem. Soc. Rev., 2015, 44, 6287–6305 RSC.
- C. Yang, Y. Liu, S. Su, N. Gao, J. Jing and X. Zhang, J. Mater. Chem. B, 2020, 8, 9943–9950 RSC.
- K. Yang, G. Yu, R. Tian, Z. Zhou, H. Deng, L. Li, Z. Yang, G. Zhang, D. Liu, J. Wei, L. Yue, R. Wang and X. Chen, Adv. Funct. Mater., 2021, 31, 2008078 CrossRef CAS.
- S.-B. He, H.-H. Deng, A.-L. Liu, G.-W. Li, X.-H. Lin, W. Chen and X.-H. Xia, ChemCatChem, 2014, 6, 1543–1548 CrossRef CAS.
- J. N. Rodriguez-Lopez, D. J. Lowe, J. Hernandez-Ruiz, A. N. P. Hiner, F. Garcia-Canovas and R. N. F. Thorneley, J. Am. Chem. Soc., 2001, 123, 11838–11847 CrossRef CAS PubMed.
- P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, R. S. DaCosta and X. Y. Wu, ACS Nano, 2014, 8, 3202–3212 CrossRef CAS PubMed.
- J. Chen, H. Luo, Y. Liu, W. Zhang, H. Li, T. Luo, K. Zhang, Y. Zhao and J. Liu, ACS Nano, 2017, 11, 12849–12862 CrossRef CAS PubMed.
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang, Y. Huang, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12, 651–661 CrossRef CAS PubMed.
- J. Ouyang, Y. Deng, W. Chen, Q. Xu, L. Wang, Z. Liu, F. Tang, L. Deng and Y.-N. Liu, J. Mater. Chem. B, 2018, 6, 2057–2064 RSC.
- D. He, L. Hai, X. He, X. Yang and H.-W. Li, Adv. Funct. Mater., 2017, 27, 1704089 CrossRef.
- J. Liu, Y. Liu, W. Bu, J. Bu, Y. Sun, J. Du and J. Shi, J. Am. Chem. Soc., 2014, 136, 9701–9709 CrossRef CAS PubMed.
- X.-S. Wang, J.-Y. Zeng, M.-K. Zhang, X. Zeng and X.-Z. Zhang, Adv. Funct. Mater., 2018, 28, 1801783 CrossRef.
- P. Zhang, W. Steelant, M. Kumar and M. Scholfield, J. Am. Chem. Soc., 2007, 129, 4526–4527 CrossRef CAS PubMed.
- Y. Li, X. Jian, S. Zhou, Y. Lu, C. Zhao, Z. Gao and Y.-Y. Song, ACS Appl. Mater. Interfaces, 2019, 11, 17215–17225 CrossRef CAS PubMed.
- Z. Xu, P. Sun, J. Zhang, X. Lu, L. Fan, J. Xi, J. Han and R. Guo, Chem. Eng. J., 2020, 399, 125797 CrossRef CAS.
- Z. Gao, Y. Li, Y. Zhang, K. Cheng, P. An, F. Chen, J. Chen, C. You, Q. Zhu and B. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 1963–1972 CrossRef CAS PubMed.
- Y. Peng, Y. Li, Y. Ban and W. Yang, Angew. Chem., Int. Ed., 2017, 56, 9757–9761 CrossRef CAS PubMed.
- M. Xu, S.-S. Yang and Z.-Y. Gu, Chem. – Eur. J., 2018, 24, 15131–15142 CrossRef CAS PubMed.
- P. Kanoo, G. Mostafa, R. Matsuda, S. Kitagawa and T. K. Maji, Chem. Commun., 2011, 47, 8106–8108 RSC.
- T. He, B. Ni, S. Zhang, Y. Gong, H. Wang, L. Gu, J. Zhuang, W. Hu and X. Wang, Small, 2018, 14, 1703929 CrossRef PubMed.
- G. Zhan, L. Fan, F. Zhao, Z. Huang, B. Chen, X. Yang and S.-F. Zhou, Adv. Funct. Mater., 2019, 29, 1806720 CrossRef.
- Y. Li, Z. Gao, F. Chen, C. You, H. Wu, K. Sun, P. An, K. Cheng, C. Sun, X. Zhu and B. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 30930–30935 CrossRef CAS PubMed.
- W. Zhang, J. Lu, X. Gao, P. Li, W. Zhang, Y. Ma, H. Wang and B. Tang, Angew. Chem., Int. Ed., 2018, 57, 4891–4896 CrossRef CAS PubMed.
- Y. Hao, Y. Chen, X. He, Y. Yu, R. Han, Y. Li, C. Yang, D. Hu and Z. Qian, Adv. Sci., 2020, 7, 2001853 CrossRef CAS PubMed.
- W. Yang, G. Zhu, S. Wang, G. Yu, Z. Yang, L. Lin, Z. Zhou, Y. Liu, Y. Dai, F. Zhang, Z. Shen, Y. Liu, Z. He, J. Lau, G. Niu, D. O. Kiesewetter, S. Hu and X. Chen, ACS Nano, 2019, 13, 3083–3094 CrossRef CAS PubMed.
- X. Duan, X. Yang, C. Dai, T. Tong, C. Miao and J. Zheng, Mater. Express, 2019, 9, 757–763 CrossRef CAS.
- R. Liu, M. Yu, X. Yang, C. S. Umeshappa, C. Hu, W. Yu, L. Qin, Y. Huang and H. Gao, Adv. Funct. Mater., 2019, 29, 1808462 CrossRef.
- Y. Yao, H. Zhang, Z. Wang, J. Ding, S. Wang, B. Huang, S. Ke and C. Gao, J. Mater. Chem. B, 2019, 7, 5019–5037 RSC.
- S. Z. F. Phua, C. Xue, W. Q. Lim, G. Yang, H. Chen, Y. Zhang, C. F. Wijaya, Z. Luo and Y. Zhao, Chem. Mater., 2019, 31, 3349–3358 CrossRef CAS.
- X. Xia, X. Yang, P. Huang and D. Yan, ACS Appl. Mater. Interfaces, 2020, 12, 18301–18308 CrossRef CAS PubMed.
- B. Wu, Z. Sun, J. Wu, J. Ruan, P. Zhao, K. Liu, C.-X. Zhao, J. Sheng, T. Liang and D. Chen, Angew. Chem., Int. Ed., 2021, 60, 9284–9289 CrossRef CAS PubMed.
- D. N. Hanh, B. K. Rajbhandari and A. P. Annachhatre, Environ. Technol., 2005, 26, 581–589 CrossRef CAS PubMed.
- B. S. Harrison, D. Eberli, S. J. Lee, A. Atala and J. J. Yoo, Biomaterials, 2007, 28, 4628–4634 CrossRef CAS PubMed.
- B. W. Bogan, V. Trbovic and J. R. Paterek, Chemosphere, 2003, 50, 15–21 CrossRef CAS PubMed.
- A.-C. Ndjou'ou and D. Cassidy, Chemosphere, 2006, 65, 1610–1615 CrossRef PubMed.
- S. H. Oh, C. L. Ward, A. Atala, J. J. Yoo and B. S. Harrison, Biomaterials, 2009, 30, 757–762 CrossRef CAS PubMed.
- J. Wang, Y. Zhu, H. K. Bawa, G. Ng, Y. Wu, M. Libera, H. C. van der Mei, H. J. Busscher and X. Yu, ACS Appl. Mater. Interfaces, 2011, 3, 67–73 CrossRef CAS PubMed.
- E. Pedraza, M. M. Coronel, C. A. Fraker, C. Ricordi and C. L. Stabler, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4245–4250 CrossRef CAS PubMed.
- L. Hu, P. Wang, M. Zhao, L. Liu, L. Zhou, B. Li, F. H. Albaqami, A. M. El-Toni, X. Li, Y. Xie, X. Sun and F. Zhang, Biomaterials, 2018, 163, 154–162 CrossRef CAS PubMed.
- C.-C. Huang, W.-T. Chia, M.-F. Chung, K.-J. Lin, C.-W. Hsiao, C. Jin, W.-H. Lim, C.-C. Chen and H.-W. Sung, J. Am. Chem. Soc., 2016, 138, 5222–5225 CrossRef CAS PubMed.
- Q. Yu, T. Huang, C. Liu, M. Zhao, M. Xie, G. Li, S. Liu, W. Huang and Q. Zhao, Chem. Sci., 2019, 10, 9091–9098 RSC.
- L.-H. Liu, Y.-H. Zhang, W.-X. Qiu, L. Zhang, F. Gao, B. Li, L. Xu, J.-X. Fan, Z.-H. Li and X.-Z. Zhang, Small, 2017, 13, 1701621 CrossRef PubMed.
- C. Liu, Y. Cao, Y. Cheng, D. Wang, T. Xu, L. Su, X. Zhang and H. Dong, Nat. Commun., 2020, 11, 1735 CrossRef CAS PubMed.
- H. Fu, J. Fu, S. Ma, H. Wang, S. Lv and Y. Hao, J. Mater. Chem. B, 2020, 8, 6059–6068 RSC.
- H. Zhou, L. Xia, J. Zhong, S. Xiong, X. Yi, L. Chen, R. Zhu, Q. Shi and K. Yang, Nanoscale, 2019, 11, 19823–19831 RSC.
- P. F. A. Costa, A. P. Gerola, P. C. S. Pereira, B. S. Souza, W. Caetano, H. D. Fiedler, F. Nome and N. Hioka, J. Mol. Liq., 2019, 274, 393–401 CrossRef CAS.
- H. Zhang, L. Huang, J. Zhai and S. Dong, J. Am. Chem. Soc., 2019, 141, 16416–16421 CrossRef CAS PubMed.
- R. Song, B. Luo, J. Geng, D. Song and D. Jing, Ind. Eng. Chem. Res., 2018, 57, 7846–7854 CrossRef CAS.
- G. Zhang, Z.-A. Lan and X. Wang, Chem. Sci., 2017, 8, 5261–5274 RSC.
- T. K. Fam, A. S. Klymchenko and M. Collot, Materials, 2018, 11, 1768 CrossRef PubMed.
- Y. Wang, S. Song, J. Liu, D. Liu and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 536–540 CAS.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS PubMed.
- L. Pan, S. Sun, Y. Chen, P. Wang, J. Wang, X. Zhang, J.-J. Zou and Z. L. Wang, Adv. Energy Mater., 2020, 10, 2000214 CrossRef CAS.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Science, 2015, 347, 967–970 CrossRef CAS PubMed.
- S. M. Yin, J. Y. Han, T. H. Zhou and R. Xu, Catal. Sci. Technol., 2015, 5, 5048–5061 RSC.
- H. Wan, Y. Zhang, W. Zhang and H. Zou, ACS Appl. Mater. Interfaces, 2015, 7, 9608–9618 CrossRef CAS PubMed.
- R. Chen, J. Zhang, Y. Wang, X. Chen, J. A. Zapien and C.-S. Lee, Nanoscale, 2015, 7, 17299–17305 RSC.
- C.-J. Chen, C. K. Chen, T.-H. Lu, S.-F. Hu and R.-S. Liu, RSC Adv., 2016, 6, 103160–103168 RSC.
- D. Yang, G. Yang, S. Gai, F. He, C. Li and P. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 6829–6838 CrossRef CAS PubMed.
- D. Yang, G. Yang, J. Li, S. Gai, F. He and P. Yang, J. Mater. Chem. B, 2017, 5, 4152–4161 RSC.
- D.-W. Zheng, B. Li, C.-X. Li, J.-X. Fan, Q. Lei, C. Li, Z. Xu and X.-Z. Zhang, ACS Nano, 2016, 10, 8715–8722 CrossRef CAS PubMed.
- R.-Q. Li, C. Zhang, B.-R. Xie, W.-Y. Yu, W.-X. Qiu, H. Cheng and X.-Z. Zhang, Biomaterials, 2019, 194, 84–93 CrossRef CAS PubMed.
- R. Chen, J. Zhang, J. Chelora, Y. Xiong, S. V. Kershaw, K. F. Li, P.-K. Lo, K. W. Cheah, A. L. Rogach, J. A. Zapien and C.-S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 5699–5708 CrossRef CAS PubMed.
- X. Wang, W. Lu, Z.-Y. Gu, Z. Wei and H.-C. Zhou, Chem. Commun., 2016, 52, 1926–1929 RSC.
- M. D. Brady, R. N. Sampaio, D. Wang, T. J. Meyer and G. J. Meyer, J. Am. Chem. Soc., 2017, 139, 15612–15615 CrossRef CAS PubMed.
- C. Zhang, W.-H. Chen, L.-H. Liu, W.-X. Qiu, W.-Y. Yu and X.-Z. Zhang, Adv. Funct. Mater., 2017, 27, 1700626 CrossRef.
- S. Gao, G. Wang, Z. Qin, X. Wang, G. Zhao, Q. Ma and L. Zhu, Biomaterials, 2017, 112, 324–335 CrossRef CAS PubMed.
- K. Yan, Y. Zhang, C. Mu, Q. Xu, X. Jing, D. Wang, D. Dang, L. Meng and J. Ma, Theranostics, 2020, 10, 7287–7318 CrossRef CAS PubMed.
- P. Lee, N. S. Chandel and M. C. Simon, Nat. Rev. Mol. Cell Biol., 2020, 21, 268–283 CrossRef CAS PubMed.
- G. Multhoff and P. Vaupel, in Oxygen Transport to Tissue Xli, ed. P. D. Ryu, J. C. LaManna, D. K. Harrison and S. S. Lee, 2020, vol. 1232, pp. 131–143 Search PubMed.
- M. Najafi, B. Farhood and K. Mortezaee, J. Cell. Physiol., 2019, 234, 8381–8395 CrossRef CAS PubMed.
- M. Najafi, B. Farhood, K. Mortezaee, E. Kharazinejad, J. Majidpoor and R. Ahadi, J. Cancer Res. Clin. Oncol., 2020, 146, 19–31 CrossRef PubMed.
- S. Pastorekova and R. J. Gillies, Cancer Metastasis Rev., 2019, 38, 65–77 CrossRef CAS PubMed.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- L. Yang, P. Shi, G. Zhao, J. Xu, W. Peng, J. Zhang, G. Zhang, X. Wang, Z. Dong, F. Chen and H. Cui, Signal Transduction Targeted Ther., 2020, 5, 8 CrossRef PubMed.
- C. E. Evans, K. Mattock, J. Humphries, P. Saha, A. Ahmad, M. Waltham, A. Patel, B. Modarai, L. Porter, S. Premaratne and A. Smith, Angiogenesis, 2011, 14, 119–124 CrossRef CAS PubMed.
- E. Eng, C. Holgren, S. Hubchak, P. Naaz and H. W. Schnaper, Kidney Int., 2005, 68, 695–703 CrossRef CAS PubMed.
- C. W. Song, J. G. Rhee and S. H. Levitt, Int. J. Radiat. Oncol., Biol., Phys., 1982, 8, 851–856 CrossRef CAS.
- Q. Chen, H. Chen, H. Shapiro and F. W. Hetzel, Radiat. Res., 1996, 146, 293–297 CrossRef CAS PubMed.
- M. K. Kang, M. S. Kim and J. H. Kim, Int. J. Hyperthermia, 2011, 27, 482–490 CrossRef PubMed.
- S.-Y. Lee, J.-H. Kim, Y.-H. Han and D.-H. Cho, Int. J. Hyperthermia, 2018, 34, 953–960 CrossRef PubMed.
- G. Multhoff, G. Habl and S. E. Combs, Int. J. Hyperthermia, 2016, 32, 455–463 CrossRef CAS PubMed.
- C. W. Song, H. J. Park, C. K. Lee and R. Griffin, Int. J. Hyperthermia, 2005, 21, 761–767 CrossRef CAS PubMed.
- P. W. Vaupel and D. K. Kelleher, Int. J. Hyperthermia, 2010, 26, 211–223 CrossRef CAS PubMed.
- Z. Vujaskovic, E. L. Rosen, K. L. Blackwell, E. L. Jones, D. M. Brizel, L. R. Prosnitz, T. V. Samulski and M. W. Dewhirst, Int. J. Hyperthermia, 2003, 19, 498–506 CrossRef CAS PubMed.
- R. Lima-Sousa, D. de Melo-Diogo, C. G. Alves, C. S. D. Cabral, S. P. Miguel, A. G. Mendonca and I. J. Correia, Mater. Sci. Eng., C, 2020, 117, 111294 CrossRef CAS PubMed.
- G. Ramirez-Garcia, M. Angel Honorato-Colin, E. De la Rosa, T. Lopez-Luke, S. S. Panikar, J. de Jesus Ibarra-Sanchez and V. Piazza, J. Photochem. Photobiol., A, 2019, 384, 112053 CrossRef.
- L. Hui, S. Qin and L. Yang, ACS Biomater. Sci. Eng., 2016, 2, 2127–2132 CrossRef CAS PubMed.
- K. Yue, J. Nan, X. Zhang, J. Tang and X. Zhang, Appl. Therm. Eng., 2016, 99, 1093–1100 CrossRef CAS.
- P.-L. Chen, Q.-Y. Shi, T. Chen, P. Wang, Y. Liu and L.-H. Liu, J. Mater. Sci., 2020, 55, 7843–7856 CrossRef CAS.
- P. T. M. Phuong, H. J. Won, A. I. Robby, S. G. Kim, G.-B. Im, S. H. Bhang, G. Lee and S. Y. Park, ACS Appl. Mater. Interfaces, 2020, 12, 37929–37942 CrossRef CAS PubMed.
- F. Yang, Q. Zhang, S. Huang and D. Ma, J. Mater. Chem. B, 2020, 8, 7856–7879 RSC.
- S. Lee, R. G. Thomas, M. J. Moon, H. J. Park, I.-K. Park, B.-I. Lee and Y. Y. Jeong, Sci. Rep., 2017, 7, 2108 CrossRef PubMed.
- Y. Wu, W. Zhang, D. Xu, L. Ding, R. Ma, J.-Z. Wu and J.-H. Tang, Lasers Med. Sci., 2018, 33, 1601–1608 CrossRef PubMed.
- X.-S. Zhang, Y. Xuan, X.-Q. Yang, K. Cheng, R.-Y. Zhang, C. Li, F. Tan, Y.-C. Cao, X.-L. Song, J. An, X.-L. Hou and Y.-D. Zhao, J. Nanobiotechnol., 2018, 16, 42 CrossRef PubMed.
- Y. Chen, Y. Gao, Y. Chen, L. Liu, A. Mo and Q. Peng, J. Controlled Release, 2020, 328, 251–262 CrossRef CAS PubMed.
- H.-Y. Wang, Y. Zhang, X.-H. Ren, X.-W. He, W.-Y. Li and Y.-K. Zhang, Nanoscale, 2021, 13, 886–900 RSC.
- J.-J. Hu, M.-D. Liu, F. Gao, Y. Chen, S.-Y. Peng, Z.-H. Li, H. Cheng and X.-Z. Zhang, Biomaterials, 2019, 217, 119303 CrossRef CAS PubMed.
- T. W. H. Meijer, J. H. A. M. Kaanders, P. N. Span and J. Bussink, Clin. Cancer Res., 2012, 18, 5585–5594 CrossRef CAS PubMed.
- L. Feng, D. Tao, Z. Dong, Q. Chen, Y. Chao, Z. Liu and M. Chen, Biomaterials, 2017, 127, 13–24 CrossRef CAS PubMed.
- X. Li, S. Kolemen, J. Yoon and E. U. Akkaya, Adv. Funct. Mater., 2017, 27, 1604053 CrossRef.
- X.-S. Li, M.-R. Ke, W. Huang, C.-H. Ye and J.-D. Huang, Chem. – Eur. J., 2015, 21, 3310–3317 CrossRef CAS PubMed.
- S. Xu, X. Zhu, C. Zhang, W. Huang, Y. Zhou and D. Yan, Nat. Commun., 2018, 9, 2053 CrossRef PubMed.
- Q. Chen, L. Feng, J. Liu, W. Zhu, Z. Dong, Y. Wu and Z. Liu, Adv. Mater., 2018, 30, 1870051 CrossRef.
- W. Zhu, Z. Dong, T. Fu, J. Liu, Q. Chen, Y. Li, R. Zhu, L. Xu and Z. Liu, Adv. Funct. Mater., 2016, 26, 5490–5498 CrossRef CAS.
- H. Cheng, J.-Y. Zhu, S.-Y. Li, J.-Y. Zeng, Q. Lei, K.-W. Chen, C. Zhang and X.-Z. Zhang, Adv. Funct. Mater., 2016, 26, 7847–7860 CrossRef CAS.
- Y. Yong, X. Cheng, T. Bao, M. Zu, L. Yan, W. Yin, C. Ge, D. Wang, Z. Gu and Y. Zhao, ACS Nano, 2015, 9, 12451–12463 CrossRef CAS PubMed.
- G. Song, C. Liang, H. Gong, M. Li, X. Zheng, L. Cheng, K. Yang, X. Jiang and Z. Liu, Adv. Mater., 2015, 27, 6110–6117 CrossRef CAS PubMed.
- J. Du, Z. Gu, L. Yan, Y. Yong, X. Yi, X. Zhang, J. Liu, R. Wu, C. Ge, C. Chen and Y. Zhao, Adv. Mater., 2017, 29, 1701268 CrossRef PubMed.
- J. Wang, X. Tan, X. Pang, L. Liu, F. Tan and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 24331–24338 CrossRef CAS PubMed.
- Y. Cheng, X. Kong, Y. Chang, Y. Feng, R. Zheng, X. Wu, K. Xu, X. Gao and H. Zhang, Adv. Mater., 2020, 32, 1908109 CrossRef CAS PubMed.
- Z. Cui, K. Gao, S. Ge and W. Fa, Mater. Res. Express, 2017, 4, 115032 CrossRef.
- P. Cabrales and M. Intaglietta, ASAIO J., 2013, 59, 337–354 CrossRef CAS PubMed.
- C. I. Castro and J. C. Briceno, Artif. Organs, 2010, 34, 622–634 Search PubMed.
- D. R. Spahn, Crit. Care, 1999, 3, R93–R97 CrossRef CAS PubMed.
- K. C. Lowe, Blood Rev., 1999, 13, 171–184 CrossRef CAS PubMed.
- B. A. Teicher and C. M. Rose, Science, 1984, 223, 934–936 CrossRef CAS PubMed.
- H.-Y. Lee, H.-W. Kim, J. H. Lee and S. H. Oh, Biomaterials, 2015, 53, 583–591 CrossRef CAS PubMed.
- L. S. Chin, M. Lim, T. T. Hung, C. P. Marquis and R. Amal, RSC Adv., 2014, 4, 13052–13060 RSC.
- V. H. Fingar, T. S. Mang and B. W. Henderson, Cancer Res., 1988, 48, 3350–3354 CAS.
- A. M. Vezeridis, C. d. G. Lux, S. A. Barnhill, S. Kim, Z. Wu, S. Jin, J. Lux, N. C. Gianneschi and R. F. Mattrey, J. Controlled Release, 2019, 302, 54–62 CrossRef CAS PubMed.
- M. Hubler, J. E. Souders, E. D. Shade, N. L. Polissar, J. U. Bleyl and M. P. Hlastala, Crit. Care Med., 2002, 30, 422–427 CrossRef CAS PubMed.
- D. Haufe, K. G. Dahmen, O. Tiebel, M. Huebler and T. Koch, Artif. Cells, Blood Substitutes, Biotechnol., 2011, 39, 239–246 CrossRef CAS PubMed.
- B. D. Spiess, J. Appl. Physiol., 2009, 106, 1444–1452 CrossRef CAS PubMed.
- C. Parrado, J. Nicolas, A. Juarranz and S. Gonzalez, Photochem. Photobiol. Sci., 2020, 19, 831–843 CrossRef CAS PubMed.
- X. Tang, Y. Cheng, S. Huang, F. Zhi, A. Yuan, Y. Hu and J. Wu, Biochem. Biophys. Res. Commun., 2017, 487, 483–487 CrossRef CAS PubMed.
- R. A. Day, D. A. Estabrook, J. K. Logan and E. M. Sletten, Chem. Commun., 2017, 53, 13043–13046 RSC.
- K. Kolostova, J. Spicka, R. Matkowski and V. Bobek, Am. J. Transl. Res., 2015, 7, 1203–1213 CAS.
- H. Hu, X. Yan, H. Wang, J. Tanaka, M. Wang, W. You and Z. Li, J. Mater. Chem. B, 2019, 7, 1116–1123 RSC.
- C. Liu, H. Dong, N. Wu, Y. Cao and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 6991–7002 CrossRef CAS PubMed.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Hu, Nat. Commun., 2015, 6, 8785 CrossRef CAS PubMed.
- S. Ma, J. Zhou, Y. Zhang, B. Yang, Y. He, C. Tian, X. Xu and Z. Gu, ACS Appl. Mater. Interfaces, 2019, 11, 7731–7742 CrossRef CAS PubMed.
- Y. Wang, F. Zhang, H. Lin and F. Qu, ACS Appl. Mater. Interfaces, 2019, 11, 43964–43975 CrossRef CAS PubMed.
- F. Guo, M. Yu, J. Wang, F. Tan and N. Li, RSC Adv., 2016, 6, 11070–11076 RSC.
- L. Zhang, H. Yi, J. Song, J. Huang, K. Yang, B. Tan, D. Wang, N. Yang, Z. Wang and X. Li, ACS Appl. Mater. Interfaces, 2019, 11, 9355–9366 CrossRef CAS PubMed.
- S. Chen, B. Huang, W. Pei, L. Wang, Y. Xu and C. Niu, Int. J. Nanomed., 2020, 15, 8641–8658 CrossRef CAS PubMed.
- D. C. Wallace, Nat. Rev. Cancer, 2012, 12, 685–698 CrossRef CAS PubMed.
- K. Hayashi, M. Nakamura, H. Miki, S. Ozaki, M. Abe, T. Matsumoto, T. Kori and K. Ishimura, Adv. Funct. Mater., 2014, 24, 503–513 CrossRef CAS.
- D. Hu, L. Zhong, M. Wang, H. Li, Y. Qu, Q. Liu, R. Han, L. Yuan, K. Shi, J. Peng and Z. Qian, Adv. Funct. Mater., 2019, 29, 1806199 CrossRef.
- P. Huang, Y. Zhang, Y. Liao, Q. Tang and J. Lin, Angew. Chem., Int. Ed., 2021, 60, 10647–10635 CrossRef PubMed.
- R. Liu, G. Spicer, S. Chen, H. F. Zhang, J. Yi and V. Backman, J. Biomed. Opt., 2017, 22, 025002 CrossRef PubMed.
- D. J. Singel and J. S. Stamler, Annu. Rev. Physiol., 2005, 67, 99–145 CrossRef CAS PubMed.
- J. S. O'Neill and A. B. Reddy, Nature, 2011, 469, 498–U470 CrossRef PubMed.
- S. Wang, F. Yuan, K. Chen, G. Chen, K. Tu, H. Wang and L.-Q. Wang, Biomacromolecules, 2015, 16, 2693–2700 CrossRef CAS PubMed.
- H. Yi, P. Liu, N. Sheng, P. Gong, Y. Ma and L. Cai, Nanoscale, 2016, 8, 5985–5995 RSC.
- L. Duan, X. Yan, A. Wang, Y. Jia and J. Li, ACS Nano, 2012, 6, 6897–6904 CrossRef CAS PubMed.
- C.-M. J. Hu, L. Zhang, S. Aryal, C. Cheung, R. H. Fang and L. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10980–10985 CrossRef CAS PubMed.
- N. Doshi, A. S. Zahr, S. Bhaskar, J. Lahann and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21495–21499 CrossRef CAS PubMed.
- G. Lippi, M. Franchini and G. Banfi, Int. J. Sports Med., 2010, 31, 75–76 CrossRef CAS PubMed.
- P. Wang, X. Li, C. Yao, W. Wang, M. Zhao, A. M. El-Toni and F. Zhang, Biomaterials, 2017, 125, 90–100 CrossRef CAS PubMed.
- X. Guo, J. Qua, C. Zhu, W. Li, L. Luo, J. Yang, X. Yin, Q. Li, Y. Du, D. Chen, Y. Qiu, Y. Lou and J. You, Drug Delivery, 2018, 25, 585–599 CrossRef CAS PubMed.
- G. Wan, B. Chen, L. Li, D. Wang, S. Shi, T. Zhang, Y. Wang, L. Zhang and Y. Wang, Biomaterials, 2018, 155, 25–40 CrossRef CAS PubMed.
- W.-L. Liu, T. Liu, M.-Z. Zou, W.-Y. Yu, C.-X. Li, Z.-Y. He, M.-K. Zhang, M.-D. Liu, Z.-H. Li, J. Feng and X.-Z. Zhang, Adv. Mater., 2018, 30, 1802006 CrossRef PubMed.
- H. Cao, L. Wang, Y. Yang, J. Li, Y. Qi, Y. Li, Y. Li, H. Wang and J. Li, Angew. Chem., Int. Ed., 2018, 57, 7759–7763 CrossRef CAS PubMed.
- L. Liu, T. Li, Z. Ruan and L. Yan, J. Mater. Sci., 2018, 53, 9368–9381 CrossRef CAS.
- C. Gao, Z. Lin, D. Wang, Z. Wu, H. Xie and Q. He, ACS Appl. Mater. Interfaces, 2019, 11, 23392–23400 CrossRef CAS PubMed.
- Y. Jiang and J. McNeill, Chem. Rev., 2017, 117, 838–859 CrossRef CAS PubMed.
- J. Li, C. Xie, J. Huang, Y. Jiang, Q. Miao and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 3995–3998 CrossRef CAS PubMed.
- L. Jiang, H. Bai, L. Liu, F. Lv, X. Ren and S. Wang, Angew. Chem., Int. Ed., 2019, 58, 10660–10665 CrossRef CAS PubMed.
- Z. Chen, L. Liu, R. Liang, Z. Luo, H. He, Z. Wu, H. Tian, M. Zheng, Y. Ma and L. Cai, ACS Nano, 2018, 12, 8633–8645 CrossRef CAS PubMed.
- X. Shi, Q. Zhan, X. Yan, J. Zhou, L. Zhou and S. Wei, J. Mater. Chem. B, 2020, 8, 534–545 RSC.
- N. S. Al-Waili, G. J. Butler, J. Beale, R. W. Hamilton, B. Y. Lee and P. Lucas, Med. Sci. Monit., 2005, 11, RA279–RA289 Search PubMed.
- D. Devaraj and D. Srisakthi, J. Clin. Diagn. Res., 2014, 8, 263–265 Search PubMed.
- K. Stepien, R. P. Ostrowski and E. Matyja, Med. Oncol., 2016, 33, 101 CrossRef PubMed.
- O. Thews and P. Vaupel, Strahlenther. Onkol., 2015, 191, 875–882 CrossRef PubMed.
- L.-H. Mei, G. Yang and F. Fang, Biol. Pharm. Bull., 2019, 42, 394–400 CrossRef CAS PubMed.
- J. Li, J. Huang, Y. Ao, S. Li, Y. Miao, Z. Yu, L. Zhu, X. Lan, Y. Zhu, Y. Zhang and X. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 22985–22996 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |