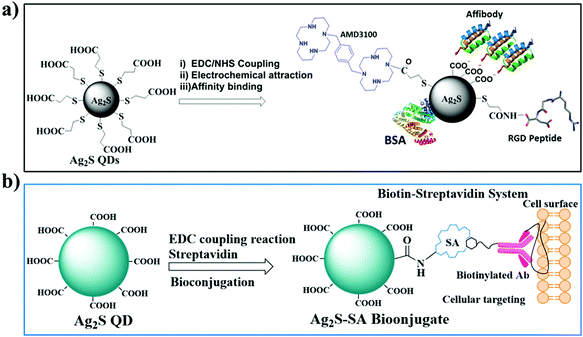
Ag2S quantum dot theragnostics
Baskaran
Purushothaman
and
Joon Myong
Song
 *
*
College of Pharmacy, Seoul National University, Seoul 08826, South Korea. E-mail: jmsong@snu.ac.kr; Fax: +82 2 871 2238; Tel: +82 2 880 7841
First published on 30th October 2020
Abstract
Silver sulfide quantum dots (Ag2S QDs) as a theragnostic agent have received much attention because they provide excellent optical and chemical properties to facilitate diagnosis and therapy simultaneously. Ag2S QDs possess brightness and photostability suitable for intense and stable bioimaging. It has been verified via in vitro and in vivo studies that Ag2S QDs do not cause serious toxicity, unlike the first generation of widely used heavy metal-based (cadmium or lead) QDs. In particular, Ag2S QDs emit in the near infrared-II region (NIR-II window) that enables deep tissue penetration and imaging. Furthermore, various chemotherapeutic agents and targeting moieties can be efficiently conjugated to the surface of Ag2S QDs due to advanced technologies in the relevant surface chemistry using covalent bonding, high affinity, and electrostatic interaction. In addition, Ag2S QDs themselves exhibit an anticancer activity based on the photothermal effect. Consequently, Ag2S QDs can function as both a therapeutic agent and an imaging agent in imaging-based diagnosis concurrently, which led to the creation of Ag2S theragnostic nanomaterials. In this review, the synthetic methods, physicochemical properties, bioconjugations, and bioapplications of Ag2S QD theragnostic nanomaterials are discussed in detail.
1. Introduction
Quantum dots (QDs) are semiconducting nanocrystals that are smaller than the exciton Bohr radius.1 The excellent optical properties of QDs have attracted much interest in the biomedical field because of their unique multiplexing imaging capabilities compared with organic dyes.2,3 However, most QDs are made from heavy metal elements such as Cd,4,5 Pb,6 and Hg.7 The bio-applications of heavy metal QDs such as PbSe, PbS, HgTe, HgS, and CdHgTe are challenging because of their potential toxicity in major organs even at low concentrations.8 Thus, many research groups have developed heavy metal free silver sulfide QDs (Ag2S QDs) as an alternative nanoprobe that do not contain Cd, Pb, or Hg heavy metals. Recently, heavy metal free Ag2S QDs have emerged as a new class of bioimaging agents9–11 because of their superior structural, physicochemical, and bio-properties, such as a high quantum yield (QY), ultra-bright photoluminescence (PL), low or non-toxicity, biocompatibility, high photostability, and tunability of emission wavelengths. In particular, Ag2S QDs have been found to extend the emission range up to the second NIR window (NIR-II, 1000–1400 nm), which permits tissue imaging with deep penetration due to the reduced tissue auto-fluorescence and absorption in the NIR-II region.12 In addition, Ag2S has an ultralow solubility product constant (Ksp = 6.3 × 10−50), which means that only the minimum amount of Ag ions enters the cells. The Ag2S QD functionalized with biomolecules, such as proteins, antibodies, peptides, DNA, or vitamins, enables multivalent binding to intracellular targets. The flexibility in terms of surface chemistry and emission wavelength of bioconjugated Ag2S QDs facilitates their use as a probe for multicolor imaging. Ag2S QDs can efficiently produce many narrow and different emission wavelength regions as a function of their composition, which can provide an opportunity to simultaneously observe many disease markers. Researchers have utilized Ag2S QDs in biological applications and evaluated their potential use as an imaging agent for diagnosis or target monitoring in numerous in vivo and ex vivo models, such as small animals, xenograft tissues, biofluids (urine and blood), and biopsy tissues. The Ag2S QDs have successfully facilitated deep tissue imaging,9 optical tracking for circulating tumor cells (CTCs),13 and drug or gene delivery.14,15Besides their function as imaging agents, recently, Ag2S QDs have been further developed as theranostic agents.16–21 Generally, theranostic agents are materials that function as both a diagnostic and a therapeutic agent. As mentioned above, Ag2S QDs have successfully been shown to have diagnostic potential with their excellent imaging properties in the NIR-II region. There have been great advancements in a variety of surface chemistries that have enabled the conjugation of various chemicals or biological materials to Ag2S QDs. As a result, various therapeutic agents and tumor targeting moieties have been conjugated to the surface of Ag2S QDs, which has led to the birth of Ag2S QD theragnostics. Real-time NIR-II tumor imaging of PEGylated Ag2S QDs had an ultrahigh spatial resolution (40 μm) to track angiogenesis progressing in a tiny tumor (2–3 mm in diameter) in vivo.22 The toxicology studies of Ag2S QDs revealed that a very low amount of Ag accumulated in the organs.23 Importantly, Ag2S QDs are non-ionizing radiation agents. These in vivo properties of Ag2S QDs, such as being non-ionizing, ultrahigh spatial resolution and intensity, and less toxicity, give them potential as a theragnostic nanomaterial eventually applicable to humans. In this review, the syntheses and applications of Ag2S QD theragnostics are intensively discussed. After providing a survey of the synthetic strategies, physicochemical properties, and biofunctionalizations of Ag2S QDs, we discuss their potential applications in numerous molecular, in vitro, in vivo, and ex vivo models and as theranostic agents.
2. Preparation of heavy metal free Ag2S QDs
Ag2S nanoparticles are prepared by mixing silver nitrate (AgNO3) and sodium sulfide (Na2S) in an aqueous solution. Here, AgNO3 is used as the Ag+ ion source, and Na2S is used as the S2− anion source for Ag2S synthesis. The Ag2S nanoparticles are formed immediately after the reaction of AgNO3 and Na2S in an aqueous solution. However, the formation depends on the concentration of sulfide ions. Accordingly, coarse-crystalline Ag2S is formed immediately due to the low solubility of the Ag2S in an aqueous solution without a complexing or stabilizing agent. Then, the addition of a complexing agent (e.g., sodium citrate, Na3Cit) to the aqueous solution promotes the formation of Ag2S QDs. Sodium citrate plays a major role in the formation of Ag2S QDs. Na3Cit is adsorbed onto the Ag2S to prevent agglomeration and serves as a stabilizing agent. In addition, Na3Cit also serves as a protective layer on the surface of the Ag2S QDs. Moreover, Na3Cit reduces the Ag+ ion to metallic silver. This is an additional advantage. However, recently, different stabilizing agents and sulfur sources have been used for the preparation of Ag2S QDs. Different sulfur sources such as hydrogen sulfide (H2S), elemental sulfur (S), sodium sulfide (Na2S), 3-mercaptopropionic acid (3-MPA), thiourea, 1-dodecanethiol (DT), and sodium thiosulfate (Na2S2O3) have been used to prepare Ag2S QDs. On the other hand, mostly AgNO3 and CH3COOAg have been used as the silver source. The Ag2S QDs can be prepared by different synthetic methods such as pyrolysis, hot-injection, hydrothermal, sonication treatment, and microwave irradiation techniques.2.1 Pyrolysis method
Pyrolysis is defined as the thermal decomposition of materials at high temperature under an inert atmosphere. Ag2S QDs are prepared by pyrolysis using silver and sulfur sources as precursors dissolved in organic solvents with a high boiling point (BP). Organic solvents such as ethylene glycol (BP: 197.6 °C), oleic acid (OA) (BP: 360 °C), octadecylamine (ODA) (BP: 348.8 °C), octadecene (BP: 315 °C), and 1-octylamine (BP: 176 °C) are used separately or in a mixture. Capping ligands like 2-mercaptoethanol (ME), 1-dodecanethiol (DDT), 3-mercaptopropionic acid (3-MPA), or 11-mercaptoundecanoic acid (MUA) are generally used to prepare Ag2S QDs of uniform size by the pyrolysis method. When the silver and sulfur sources in organic solvents of high BPs are heated at high temperatures, these precursors are decomposed into silver and sulfur ions. These ions interact to form Ag2S QDs. The coated capping agents control the growth of these Ag2S QDs. As a result, a uniform size distribution of Ag2S QDs can be achieved.Fig. 1a shows the preparation of Ag2S QDs using the pyrolysis method. Du et al. prepared the first single-crystalline, monodisperse Ag2S QDs with diameters of 10.2 ± 0.4 nm using a silver diethyldithiocarbamate (Ag(DDTC)) precursor,24 for which the prepared Ag2S QDs produced NIR-II emissions at 1058 nm under 785 nm excitation. These Ag2S QDs were obtained by the pyrolysis of Ag(DDTC) in a mixture of oleic acid, octadecylamine, and 1-octadecane. This method has been considered an effective way to produce size- and shape-controlled Ag2S nanocrystals. Zhang et al. prepared both hydrophobic and hydrophilic Ag2S QDs using the pyrolysis method.9 The hydrophobic Ag2S QDs were prepared by mixing a solution of Ag(DDTC) with the surface capping ligand 1-dodecanethiol (DDT). Then, the solution was heated at a high temperature under an inert atmosphere to obtain DDT-Ag2S QDs. A ligand exchange reaction of the DT-Ag2S QDs with a surfactant dihydrolipoic acid (DHLA) produced hydrophilic carboxylic acid functionalized DHLA-Ag2S QDs. The carboxyl groups of the DHLA ligands on the Ag2S QD surfaces were functionalized with a cyclic arginine–glycine–aspartic acid (RGD) peptide for targeted cell imaging. Hong et al. also synthesized biocompatible, highly fluorescent NIR-II 6PEG-Ag2S QDs using the pyrolysis method.25 Briefly, hydrophobic Ag2S QDs were synthesized by the pyrolysis of Ag(DDTC) with the surface capping ligand DT. The hydrophobic DT capped Ag2S QDs were replaced with the surfactant DHLA by ligand exchange to yield hydrophilic Ag2S QDs. Then, the amine functionalized 6-arm PEG was reacted with the DHLA-Ag2S QDs using the EDC/NHS coupling reaction, and consequently, highly water-soluble 6PEG-Ag2S QDs were obtained. The prepared 6PEG-Ag2S QDs were stable for 1 year in PBS, and the fluorescence QY was determined as approximately 15.5%. The NIR II emissive 6PEG-Ag2S QDs were used for imaging deep inner organs in mice.
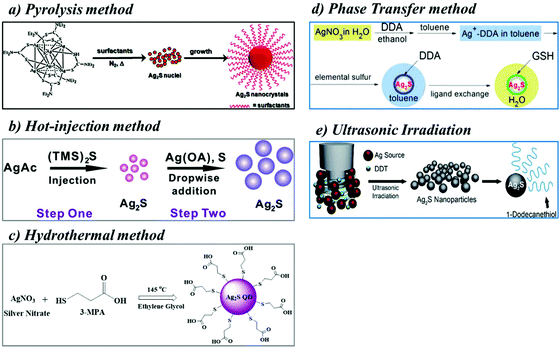 | ||
| Fig. 1 Schematics of the preparation of heavy metal free Ag2S QDs; (a) pyrolysis method, reproduced from ref. 24 with permission from [American Chemical Society], copyright [2010]. (b) Hot-injection method, reproduced from ref. 27 with permission from [American Chemical Society], copyright [2012]. (c) Hydrothermal method, reproduced from ref. 10 with permission from [Elsevier], copyright [2012]. (d) Phase transfer method, reproduced from ref. 32 with permission from [Elsevier], copyright [2014]. (e) Ultrasonic irradiation, reproduced from ref. 34 with permission from [Royal Society of Chemistry], copyright [2018]. | ||
2.2 The hot-injection method
The hot injection method is the most common approach to synthesize colloidal QDs of uniform size.26 It involves supersaturation and subsequent nucleation by rapid injection of one or more precursor solutions into hot solvents containing silver precursors (silver acetate (AgAc) and silver nitrate (AgNO3)). During the addition of the precursors such as (bis(trimethylsilyl) sulfide ((TMS)2S) and sulfur into the hot solvent, the silver and sulfur ions react immediately and nucleation occurs. Once nucleation occurs, the color of the solution turns bright yellowish-brown. The color change in the reaction mixture is evidence of the nucleation, and then, the homogeneous growth of nanocrystals occurs in the reaction mixture. Finally, the hot reaction solvent is cooled to room temperature and purified by washing with organic solvents, and the subsequent centrifugation provides the Ag2S QDs. Surfactants (e.g., 3-mercaptopropionic acid (3-MPA) or 11-mercaptoundecanoic acid (MUA)) or capping agents (e.g., GSH) are also used during the synthetic process to prevent aggregation and to improve the hydrophilicity of the QDs. The hot-injection method was an inspiring example for the synthesis of the best-quality QDs. However, the reaction temperature is uncontrollable upon the addition of the precursor solutions, which leads to poor synthetic reproducibility and thermal instability. Therefore, many different synthesis strategies have been developed, such as non-injection methods (aqueous synthesis including hydrothermal and solvothermal method, microwave irradiation, and sonication treatment), and biosynthesis.Jiang et al. prepared small- and large-sized Ag2S QDs using the hot-injection method (Fig. 1b),27 which enabled tunable emissions from 690 to 1227 nm. The emission wavelength was tuned by changing the reaction temperature. In this method, they used silver acetate (AgAc) to prepare the small-sized Ag2S QDs. Briefly, a mixture of AgAc, myristic acid (MA), 1-octylamine (OA), and 1-octadecene was added into a three neck round bottom flask. The reaction mixture was heated at high temperature under an inert atmosphere. Finally, the precursor solution (bis(trimethylsilyl) sulfide ((TMS)2S) dissolved in TOP) was injected quickly into the reaction mixture under vigorous stirring to acquire the small-sized Ag2S QDs. The hot solution containing the Ag2S QDs was cooled to room temperature and purified by washing with methanol, and pure Ag2S QDs were obtained after centrifugation. Using these small-sized Ag2S QDs as a seed, large-sized Ag2S QDs were prepared. The OA and seed solution were added into a three neck round bottom flask containing toluene solvent with magnetic stirring. Finally, the silver (AgNO3 and OA dissolved in toluene) and sulfur precursors (sulfur powder dissolved in toluene) were added dropwise into the toluene solution by a syringe pump. The PL emission spectrum of the small-sized Ag2S QDs in hexane solution showed a peak at 813 nm. The TEM images of the small-sized Ag2S QDs showed spherical particles of 1.5 ± 0.4 nm in diameter. On the other hand, the large-sized Ag2S QDs were 4.6 nm in diameter, and their PL emission peak was red-shifted from 813 nm to 1227 nm. The as-prepared Ag2S QDs were dispersed in organic solvents. Then, their surface ligand was replaced with carboxylic acid (COOH) functionalized alkanethiolates such as MUA for bioimaging applications. Recently, VA Öberg et al. also synthesized Ag2S QDs using the hot-injection method.28 They used AgNO3 as the silver source and (TMS)2S as the sulfur source for the preparation of Ag2S QDs. The AgNO3 was dissolved in oleic acid and oleylamine solvent and heated to near boiling temperature. Then, (TMS)2S in octadecene solvent was injected quickly into the solution mixture. The color of the reaction solution turned black, which indicated the formation of Ag2S QDs.
2.3 Hydrothermal method
The hydrothermal method is one of the best examples for the preparation of water-soluble Ag2S QDs for biological applications. In this method, the precursors are placed into a thick-walled borosilicate tube or Teflon-lined container or stainless steel autoclave filled with water or organic solvents at a high temperature (100–300 °C) and vapor pressure (≥1 bar) for a defined time. This combination effect of high temperature and pressure provides a one-step synthetic method to produce very fine nanoparticles. The hydrothermal method has been used to produce a wide range of nanoparticles including quantum dots. In this process, the reaction conditions such as the temperature, pressure, concentration of the precursors, stabilizing agents, and reaction time may affect the formation of nanoparticles. Hydrophilic ligands or surfactants have been used to functionalize the surface of QDs, which are suitable for in vitro and in vivo bio-applications. Small hydrophilic thiols such as 2-mercaptoethanol (ME), thioglycolic acid (TGA), 3-MPA, or MUA are generally used to replace hydrophobic capping agents like trioctylphosphine (TOP). The capping agent serves as a surface stabilizing agent for QDs with good emission efficiencies.However, these Ag2S QDs are not water-soluble and dispersed only in nonpolar organic solvents (e.g., cyclohexane, toluene, and hexane). To make hydrophilic Ag2S QDs, many groups attempted to fabricate water-soluble Ag2S QDs. For example, Hocaoglu et al. reported a one-step synthesis of 2-MPA-coated aqueous Ag2S QDs by a hydrothermal method with emission wavelengths from 780 to 950 nm for in vitro imaging.29 Typically, the 2-MPA was dissolved with deionized (DI) water, and then, the pH was adjusted to 7.5 with sodium hydroxide and acetic acid. Then, AgNO3 was added, and the pH was adjusted to 7.5, and the reaction solution was heated at the defined temperature. Subsequently, the Na2S aqueous solution was added slowly, and the reaction mixture was stirred vigorously at 5000 rpm. The color of the solution turned from yellow to dark brown, which confirmed the formation of Ag2S QDs. In addition, they also investigated the toxicity of the prepared Ag2S QDs. Those QDs had low cytotoxicity in mouse embryo fibroblast NIH/3T3 cells even at high concentrations (600 μg mL−1). A similar one-step aqueous method was also reported in the literature. Jiang et al. synthesized the first water-soluble carboxylic acid terminated Ag2S QDs for NIR imaging by the hydrothermal method using AgNO3 as the silver source and 3-MPA as the capping ligand and sulfur source and ethylene glycol as the solvent (Fig. 1c).10 The aqueous Ag2S QDs exhibited bright photoluminescence and excellent photostability. This was the first report for the synthesis of water-soluble Ag2S QDs for in vivo imaging. Until now, there have been many water-soluble NIR emissive Ag2S QDs that have been synthesized by different synthetic methods.
Gui et al. fabricated polymer functionalized Ag2S QDs using the aqueous synthesis method with sizes ranging from 2.6 to 3.7 nm in diameter.30 A multidentate polymer (poly(acrylic acid)-graft-cysteamine-graft-ethylenediamine) was used as the capping reagent for the fabrication of Ag2S QDs. The sulfur powder and hydrazine hydrate (N2H4·H2O) were dissolved in DI water, producing sulfur containing a hydrazine (S-N2H4·H2O) complex as the sulfur source. Subsequently, a fixed molar ratio of the multidentate polymer and AgNO3 was mixed in water under an N2 atmosphere to produce supramolecular hydrogels. Next, the aqueous sulfur source (S-N2H4·H2O) was injected, and then, the reaction mixture was stirred at 95 °C. After cooling the reaction solution, acetone was added to form the Ag2S QDs as a precipitate. The precipitated QDs were collected by centrifugation, washed with ethanol, and redispersed in water. Their PL emission was tuned across a wide range from deep red to NIR-II emission (687–1096 nm) by changing the ratios of their precursors (AgNO3 and S-N2H4·H2O) and multidentate polymers, making them an ideal candidate for multicolor imaging.
2.4 Microwave-assisted synthesis of QDs
Microwave-assisted synthesis is the science of applying microwave energy to a chemical reaction as a heating source. Microwave irradiation is a rapid and simple synthetic technique to complete a synthesis in a short period and to enhance the product yield and purity. The microwave (MW) heating method is a cost-effective and environmentally friendly approach compared to the conventional heating method. In the conventional heating method, the chemical reactions performed on a laboratory scale generally utilize a heating mantle or hot plate, oil baths, or a refluxing condenser for high temperature reactions. These pieces of equipment in the conventional heating method transfer thermal energy to the solvent or reactant, and thus, it takes a much longer time to achieve the final temperature of the reaction. Such a heating method typically leads to thermal gradients throughout the reaction medium, which may cause serious issues in scale-up production. In contrast, microwave heating can heat the solvent or reactant (i.e., target materials) without heating the external materials (heating mantle or hot plate), which saves energy and time.The two mechanisms involved in the microwave irradiation technique include dipolar polarization and ionic conduction. In general, microwave energy commonly heats any material with moving electric charges such as polar molecules or conducting ions in a reaction medium. The dipolar polarization mechanism is involved when microwaves are applied to polar molecules such as water molecules. The polar molecules try to orient themselves by the rapidly changing alternating electric field, and thus, heat is produced by the rotation, friction, and collision of the molecules. The ionic conduction mechanism can be considered when a microwave is applied to charged particles in a solution (usually ions in a semiconducting sample). The charged particles oscillate or fluctuate through the solution from the microwave irradiation. Then, they rapidly form colloids with their neighboring molecules or atoms. As a result, the local temperature rises in the reaction sample. Thus, the microwave irradiation technique is used to produce thermal energy for chemical reactions without disturbing any external materials such as beakers and conical flasks.
Chen et al. prepared β-lactoglobulin (β-LG) capped Ag2S QDs for in vivo imaging using microwave irradiation.31 Briefly, at first, they mixed equal amounts of aqueous solutions of β-LG and AgNO3 under magnetic stirring for 5 min, and then, the pH of the solution was changed with 1 N NaOH. Then, the aqueous solution of Na2S was injected into the mixture of β-LG and AgNO3 solution followed by heating at 100 °C for 1 min under microwave irradiation. Finally, after cooling the reaction solution to room temperature, it was centrifuged and washed with PBS, and pure β-LG-Ag2S QDs were acquired. The size of the β-LG-Ag2S QDs was determined by dynamic light scattering (DLS) with an average diameter of 5.7 ± 0.93 nm. The PL spectrum of the Ag2S QDs represented a strong NIR-II fluorescence emission at 1062 nm with a relatively narrow full-width-at-half-maximum (FWHM) of 199 nm.
2.5 Phase-transfer synthesis
The phase transfer synthetic method facilitates the migration of reactants from one phase into another phase where the reaction occurs. In this method, nanoparticles are prepared in an organic medium (toluene) using water-soluble metal salts as starting materials. However, metal ions cannot be transferred directly to an organic phase from an aqueous metal salt solution with an organic solvent containing dodecylamine (DDA). The transfer of metal ions to the organic phase is possible only with the help of an organic solvent such as ethanol containing DDA. Particularly, ethanol is appropriate as an intermediate solvent because ethanol and water are miscible. This mixture provides the maximum contact between the metal ions and DDA. Yang et al. developed a general protocol to transfer metal ions from an aqueous solution into an organic phase, which greatly extended the scope of the applications of the phase transfer technology for the synthesis of inorganic nanoparticles. Zhao et al. developed highly stable, biocompatible, and NIR-II emitting glutathione (GSH) functionalized Ag2S QDs by phase-transfer synthesis (Fig. 1d), which produced PL emissions in the NIR-II window at 1045 nm under 800 nm excitation.32 Briefly, an Ag+ aqueous solution was mixed with an ethanol solution of DDA, and then, the Ag–DDA mixture was transferred into toluene to extract the Ag+ ions. The obtained Ag+ ion–DDA mixture retrieved from the toluene was dried and dissolved in oleylamine (OLA). Sulfur powder was added to the Ag+ ion-DDA mixed with the OLA solvent, and then, the mixture was heated at 150 °C for 1 h to obtain the Ag2S QDs. The DDA-coated Ag2S QDs were precipitated by the addition of a methanol solvent and redispersed in toluene. Finally, the hydrophobic DDA-capped Ag2S QDs were replaced by a ligand exchange reaction between DDA and GSH, and water-dispersed and GSH stabilized Ag2S QDs were obtained. These Ag2S QDs exhibited high photostability, low toxicity, and good biocompatibility as a NIR-II photoluminescent nanoprobe.Peng Jiang et al. also developed a phase transfer strategy to obtain Ag2S QDs.33 In this method, a novel strategy has been developed to prepare highly reactive phosphine-free chalcogenide precursors (sulphide and selenide) based on the phase transfer of anions from water to an organic medium (toluene) using didodecyldimethylammonium bromide (DDAB). In this study, they have directly prepared a sulfide precursor (DDAB-S2−) through the phase transfer of S2− from Na2S aqueous solution using DDAB as a carrier. The developed chalcogenide precursors have been successfully adapted to the preparation of many metal based chalcogenide nanocrystals (e.g. Ag2S, PbS, Ag2Se, PbSe, and CdSe) under mild conditions.
2.6 Sonication method
The sonication method is one of the alternative techniques to prepare QDs. During the synthetic process, ultrasound waves travel through the solution medium in alternating low and high-pressure cycles at frequencies in the range of 20–40 kHz. The low-pressure cycle creates microscopic bubbles, and high-pressure cycles collapse the microscopic bubbles. This pressure cycle produces a localized shock wave that releases tremendous mechanical and thermal energy. The growth and collapse of the microbubbles in a solvent system under the influence of ultrasonic waves is known as acoustic cavitation. Using these extreme transient conditions, a variety of nanomaterials is produced in the microbubbles. For example, Kang et al. prepared Ag2S QDs by the ultra-sonication method (Fig. 1e).34 AgNO3 was dissolved in 1-dodecanethiol and sonicated for 10 minutes to generate localized hot spots within the acoustic cavitation of collapsing bubbles during the ultrasonic irradiation (power: 50%; temperature: ∼160 °C) that produced Ag2S QDs 10 nm in size.2.7 Miscellaneous methods
Tang et al. prepared two different Ag2S QDs using high viscosity solvents.35 High viscosity solvents help to slow the reaction, which contributes to the isolation of Ag2S QDs with different emission wavelengths. The 3-MPA coated Ag2S QDs were synthesized by heating a mixture of AgNO3 and 3-MPA as precursor materials, and ethylene glycol was used as a highly viscous solvent. Ag2S QDs with a maximum emission wavelength from 500 to 820 nm were fabricated by quenching the reaction mixture at different time intervals from 10 to 25 min. The size of the obtained QDs increased as the maximum emission wavelength increased. Ag2S QDs with a NIR emission range of 840 nm to 1200 nm were prepared by using a highly viscous, heat transfer organic solvent such as Dowtherm A. Briefly, a mixture of Ag(DDC), oleic acid, and hexadecylamine was heated at 200 °C, and immediately, the color of the reaction mixture turned from colorless to brown. Then, the reaction mixture was quenched at different time points from 2 to 30 min to acquire the hydrophobic Ag2S QDs. The hydrophobic QDs were converted into hydrophilic Ag2S QDs using tetraethylorthosilicate (TEOS) for in vivo NIR-II fluorescence imaging.3. Physicochemical properties of Ag2S QDs
Many research teams have studied heavy metal QDs for in vitro and in vivo imaging, especially for high-content imaging, due to their unique optical properties.1,36–41 Organic dyes have also been used frequently for bioimaging. However, they are not very efficient for use in multicolor imaging because of their weak signal intensity, relatively short fluorescence lifetimes, broad absorption, and emission spectra. To overcome these limitations, heavy metal QDs have been developed for bioimaging. The heavy metal QDs have shown broad absorption spectra, sharp and narrow emission spectra, large Stokes shifts (>100 nm), and very small spectral overlap among emission regions. Based on these properties, such as a small spectral overlap, strong photostability, and brightness, heavy metal QDs can be ideally designed for high-content imaging at the cellular level. Particularly, simultaneous excitation of heavy metal QDs at a single excitation wavelength is very attractive for high-content imaging because the excitation wavelength does not need to be changed for each QD. However, most of the heavy metal QDs are composed of heavy metals such as Cd or Pb. Several studies have reported that heavy metal ions (Cd2+ or Pb2+) released from the QD core cause serious side effects.8,42,43 Noncadmium and nonlead QDs are highly desirable for in vitro and in vivo imaging applications because they overcome the limitations of heavy metal QDs.44 Thus, noncadmium and nonlead QDs, such as InAs and InP QDs, have also been designed for biomedical applications.45–48 However, very expensive and toxic chemicals (e.g., tris-(trimethylsilyl)phosphine ((TMS)3P), tris(trimethylsilyl)arsine (TMSi)3As, tris(trimethylgermyl) arsine (TMGe)3As, and tris(isopropyldimethylsilyl) arsine (iPrDMSi)3As) used for the synthesis of InAs and InP QDs have restricted their use in biological imaging. Therefore, the development of nontoxic dot nanoprobes such as Ag2S QDs is essential for bio-imaging applications. The solubility of Ag2S QDs is very low in physiological environments; thus, only very low amounts of Ag+ ions are released into the biological environment, thereby reducing the toxicity due to Ag metal ions. Ag2S QDs are more biocompatible than any of the other heavy-metal-based QDs. Ag2S QDs have attracted much attention recently because of their unique optical properties such as their NIR-II emission (1000–1400 nm).49,50 Ag2S QDs synthesized using different methods have a monodisperse spherical shape and diameters ranging between 2 and 10 nm. Jiang et al. demonstrated that water-soluble Ag2S QDs (QY = 13%) with different growth times provided different sizes of Ag2S QDs ((size 1.5 ± 1.1 nm (growth time 5 min) and 6.3 ± 1.7 nm (growth time 30 min)). In addition, different excitation and emission spectra were acquired by changing the molar concentrations of the silver nitrate and the growth time. Fig. 2a shows the absorption and emission spectra of Ag2S QD synthesized as a function of the silver nitrate concentration and growth time. The emission maxima of Ag2S QDs covered the range of 510 nm to 1221 nm. At a growth time of 60 min, the Ag2S QDs had a strong emission peak of 1221 nm.51 Zhang et al. prepared DT-and DHLA-functionalized hydrophilic Ag2S QDs for NIR-II imaging.9 The emission peaks of DT-Ag2S and DHLA-Ag2S were observed at ∼1160 nm and 1200 nm, respectively. However, DT-Ag2S QDs of different sizes (5.4, 7, and 10 nm) exhibited similar emission spectra (Fig. 2b). Generally, the emission spectra were variable depending on the functional material used for the preparation of the Ag2S QDs. Tang et al. prepared different sizes of Ag2S QDs by tuning the reaction time and temperature (Fig. 2c).35 Water-soluble Ag2S QDs were synthesized with a size from 1.48 to 6.12 nm, which exhibited a size-dependent emission in the range of 548 to 820 nm under a single excitation wavelength (λex = 488 nm). Ag2S/SiO2 QDs synthesized with a size from 6.55 to 9.10 nm had size-dependent emissions in the range of 877 to 1130 nm under a single excitation wavelength (λex = 785 nm).35 The Ag2S/SiO2 QDs had a high QY and high fluorescence intensity, thereby making them suitable for functionalization with biomolecules and applications in NIR imaging. QY is defined as the number of emitted photons per number of absorbed photons. Generally, the QYs of the QDs are variable depending on the compositions of the QDs. Recently, Duman et al. prepared polyethyleneimine polymer functionalized NIR-II Ag2S QDs (PEI-Ag2S NIRQDs) with a size of 2.54 nm and a very high QY (QY = 166%, pH 5.5) by direct aqueous synthesis.52 The different pH values of the PEI-Ag2S QDs led to different PL emissions under a single excitation wavelength (at pH 5.5, λex = 783 and λem = 812 nm; at pH 7.4, λex = 783 and λem = 828 nm; and at pH 5.5, λex = 783 and λem = 825 nm). The NIR-II emission spectrum of Ag2S QDs is mainly dependent on the precursor and functionalized material used for the synthesis of the Ag2S QDs.53–57 Thus, the excitation and emission spectra of Ag2S QDs mainly depend on the reaction time, pH, size, and functionalized materials.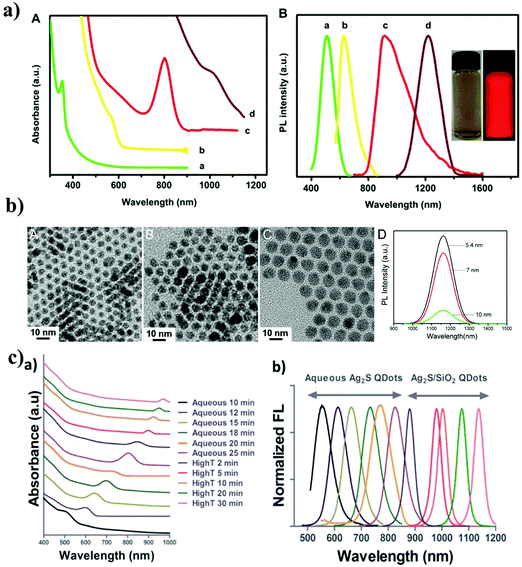 | ||
| Fig. 2 Physicochemical properties of Ag2S QDs; (a) the absorption and PL emission spectra of Ag2S QDs synthesized as a function of the silver nitrate concentration and growth time. Reproduced from ref. 10 with permission from [Elsevier], copyright [2020]. (b) TEM and PL emission spectra of DT-Ag2S QDs with different sizes (5.4, 7, and 10 nm). Reproduced from ref. 9 with permission from [American Chemical Society], copyright [2020]. (c) The absorption and PL emission spectra of Ag2S QDs and Ag2S/SiO2 QDs. Reproduced from ref. 35 with permission from [American Chemical Society], copyright [2015]. | ||
4. Biofunctionalization of heavy metal free Ag2S QDs
The biofunctionalization of heavy metal free Ag2S QDs was achieved using biomolecules such as proteins, peptides, antibodies, DNA, or other signaling molecules via covalent and noncovalent conjugation approaches. Noncovalent conjugation is defined by hydrophobic, electrostatic, and high-affinity interactions between the biomolecules and the surface of the Ag2S dot nanoprobe. Covalent conjugation of an Ag2S dot nanoprobe with biomolecules is accomplished through coupling chemistry using activated functional groups that exist at the surface of the QDs.4.1. Noncovalent conjugation approach
Noncovalent conjugation of biomolecules to the surface of Ag2S QDs is mainly based on two types of reactions: electrostatic interaction and high-affinity binding. The principle behind the electrostatic interaction is that the attraction between oppositely charged molecules, i.e., the surface of the dot nanoprobe composed of charged groups readily reacts with the oppositely charged biomolecules.58 This electrostatic interaction is a very simple and widely used noncovalent bioconjugation approach because it does not require any external chemicals and reagents. However, factors such as ionic strength, pH, and charge play an important role in the electrostatic interaction between the dot nanoprobe and the biomolecules. Bioconjugation based on high-affinity interactions is mainly dependent on the high affinity between the biomolecules and the surface of the dot nanoprobe. The most widely used high-affinity interaction is the biotin and streptavidin (SA) interaction, for which an SA-functionalized dot nanoprobe is mixed with biotinylated biomolecules.59 Biotinylation of biomolecules usually involves chemical preparation with an amine-, thiol- or carboxyl reactive biotin reagent. SA is covalently linked to dot nanoprobes containing carboxyl groups on the surface with the use of EDC. Fig. 3a shows the representative noncovalent interactions for the bioconjugation of Ag2S QDs.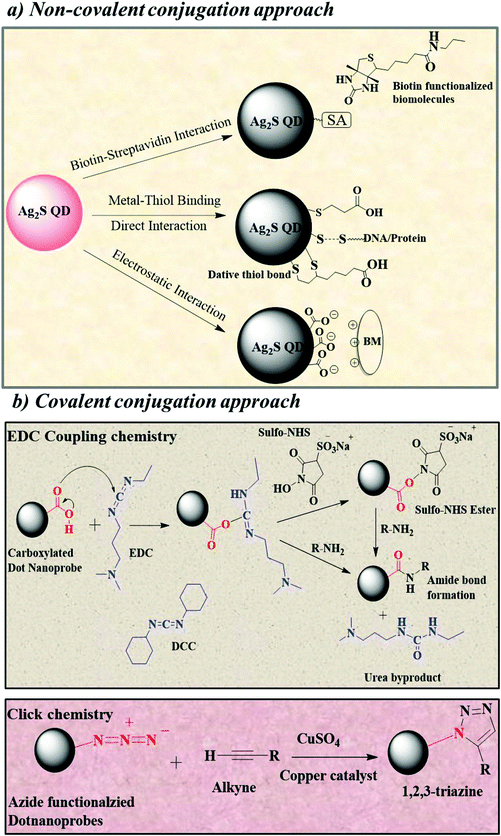 | ||
| Fig. 3 Biofunctionalization of Ag2S QDs; (a) noncovalent conjugation approach; (b) covalent conjugation approach. | ||
The electrostatic interaction method was used to prepare the affibody-functionalized Ag2S QDs utilized for photoacoustic imaging (PAI). Affibodies are scaffold proteins with a 3-helix bundle structure composed of 58 amino acids. Zhang et al. prepared the affibody-functionalized Ag2S QDs by the electrostatic interaction between the positively charged histidine in the epidermal growth factor receptor (ZEGFR:1907) and the negatively charged –carboxylic acid (–COOH) on the surface of the Ag2S QDs.60 In that study, the carboxylic acid group (3-MPA) containing the water-soluble Ag2S QDs was synthesized using a previously reported method by Jiang et al. Then, it was modified with the epidermal growth factor receptor 1 (EGFR)-targeted small protein affibody ZEGFR:1907. The resulting nanoprobe, ZEGFR:1907-Ag2S QDs, was used for targeted PAI of EGFR-overexpressed tumors. This synthetic bioconjugation evades complex chemical conjugation and is a promising method for the preparation of nanoprobes for tumor-targeted imaging.
4.2. Covalent conjugation approach
This synthetic approach involves the formation of covalent bonds such as an amide bond between activated Ag2S QDs and biomolecules. The most common method for covalent conjugation is carbodiimide coupling chemistry (EDC coupling or N,N-dicyclohexylcarbodiimide (DCC) coupling). EDC or DCC coupling is related to the coupling reaction of the carbodiimide-activated carboxylic acid groups on the surface of QDs with the primary amines of biomolecules.61 The key advantage of this reaction is that it forms a zero-length amide bond (i.e., no lengthy linker species) and minimizes the size of the Ag2S QDs. The more stable NHS ester and sulfonated NHS ester (sulfo-NHS) with EDC have also been used to form amide bonds. Thus, covalent couplings have been used for the conjugation of Ag2S QDs with proteins, peptides, antibodies, and oligonucleotides. The representative covalent conjugation approach is shown in Fig. 3b.The biofunctionalization of Ag2S QDs can be accomplished by EDC/NHS or DCC/NHS coupling chemistry, electrostatic attraction, and affinity binding (Fig. 4). Proteins, peptides, and cancer cell targeting ligands such as folic acid (FA) have been functionalized with Ag2S QDs for imaging and therapy. Yang et al. first reported the bioconjugation of Ag2S QDs with bovine serum albumin (BSA) through coordination between the Ag+ ions and functional groups in the BSA.62 The surface functionalization of Ag2S QDs with BSA improves its water solubility and biocompatibility. The functionalization of Ag2S QDs with proteins is achieved through direct high-affinity binding. For example, NIR-II fluorescent Ag2S QDs functionalized with the protein β-LG were prepared for in vivo NIR-II imaging.31 In this bioconjugation method, the protein β-LG was directly encapsulated on the surface of the Ag2S QDs. β-LG was used as a capping/stabilizing agent in this bioconjugation. β-LG is another example of the biofunctionalization of Ag2S QDs by high-affinity binding between a metal surface and a protein. Tang et al. prepared peptide-functionalized Ag2S QDs with EDC/sulfo-NHS coupling chemistry for deep tissue and cell imaging.35 In this work, 3-MPA was attached to the surface of Ag2S QDs by the affinity between the metal and thiol. The carboxyl group of 3-MPA was activated with EDC/sulfo-NHS and then covalently conjugated with a cyclic arginine–glycine–aspartic acid (Arg-Gly-Asp-D-Phe-Lys, cRGDfK) peptide for NIR fluorescence imaging. Similarly, Zhang et al. also prepared Ag2S QDs coated with the triblock copolymer Pluronic F127 (PF127) functionalized with FA for photoacoustic imaging by coupling chemistry.63 The FA-conjugated PF127 was prepared by a two-step DCC/NHS covalent coupling reaction. Then, the PF127-FA was physically adsorbed over Ag2S QDs to obtain the PF127-FA@Ag2S probe. Duman et al. also prepared FA-PEG conjugated Ag2S QDs for in vitro and drug delivery applications.14 In that work, the amine functionalized Ag2S QDs were prepared using polyethyleneimine (PEI) with amino acid cysteine. Then, the FA-PEG-COOH was conjugated to the amine functionalized Ag2S QDs using EDC/NHS coupling chemistry to obtain the FA-PEG-Ag2S QDs. Recently, Asik et al. prepared PEGylated Ag2S QDs by a simple one-step hydrothermal method for targeted drug delivery. At first, they prepared the PEG conjugated Ag2S QDs using AgNO3 and Na2S salts in the presence of thiolated polyethylene glycol (MPEG-SH) and carboxylic acid functionalized MPEG-SH (CMPEG-SH). Then, the FA conjugated PEG-Ag2S QDs were prepared with EDC/NHS coupling chemistry. The folic acid conjugated PEG-Ag2S QDs targeted the folate receptor overexpressed in cancerous cells. The ability of FA-PEG-Ag2S QDs as a targeted drug delivery vehicle was demonstrated using doxorubicin (DOX). DOX, one of the most widely used chemotherapeutic drugs, is loaded onto FA-PEG fabricated Ag2QDs by the electrostatic interaction between the DOX and the FA-PEG Ag2S QDs.
5. In vitro assay using Ag2S QDs
Cai et al. developed a label-free fluorometric assay for the sensitive and selective detection of cytochrome c (Cyt c) in apoptotic cells using NIR Ag2S QDs as a sensing probe.64 Cyt c plays a crucial role in the cell death pathway. Cyt c is a hemeprotein primarily found in the inner membrane of mitochondria, and essentially, it functions in mitochondrial electron transport due to its iron content. Cyt c is released into the cytoplasm from mitochondria, which activates the caspases and the apoptotic process. Thus, the release of Cyt c is considered a key factor during the apoptotic process. Therefore, many methods have been developed to detect Cyt c. For example, label-free fluorescence detection of Cyt c was developed using carbon QDs and CdTe QDs. However, these QDs had an emission in the visible region, and it may affect the early stage detection of Cyt c. Thus, Cai et al. synthesized water-soluble NIR Ag2S QDs and utilized them as a probe for the detection of Cyt c under the condition of reduced autofluorescence, reduced absorption of biomolecules, and light scattering. The same group also developed a ratiometric fluorescence assay for the detection and bioimaging of alkaline phosphatase (ALP) in HeLa cells using 3-MPA-coated NIR Ag2S QDs and calcein.65 ALP is an enzyme that catalyzes the hydrolysis of the phosphate ester group in proteins, nucleic acids, and small molecules. A high level of ALP in humans could indicate liver disease, bone disease, or prostate cancer. Many detection methods have been developed, such as a colorimetric assay, chemiluminescence assay, electrochemistry, and surface enhanced Raman scattering (SERS). These methods are performed using ultraviolet or visible light as the excitation source, which limits their applications in bioimaging. In addition, a dye-based NIR probe was also used to detect ALP. Although a complex synthesis process was used to prepare the NIR dyes, they had poor water solubility and biocompatibility. Therefore, a fluorescence assay was developed for the detection of ALP using Ag2S QDs, which had emission in the NIR region.6. In vivo animal study with Ag2S QDs
Ag2S QDs are based on I–VI semiconductors known as NIR-II emitting probes (950–1400 nm), and they are more suitable for deep tissue imaging than NIR-I emitters (650–900 nm). Du et al. conducted the first synthesis of heavy metal free Ag2S QDs (size of ∼10 nm) with emissions in the NIR-II region.24 Zhang et al.66 demonstrated long-term in vivo biodistribution of heavy metal free Ag2S QDs functionalized with PEG, for which the experimental results showed that the PEGylated-Ag2S QDs (NIR-II fluorescence emission at ∼1200 nm) selectively accumulated in the tumor area at 2 h post-injection, and the highest tumor-to-background ratio was ∼15 at ∼10 h post-injection. Wang et al.67 fabricated BSA-functionalized heavy metal free Ag2S QDs (2.1 ± 0.3 nm), which were subsequently bioconjugated with anti-VEGF Ab for targeted in vivo cancer imaging. The in vivo results demonstrated that the fluorescent signals derived from the anti-VEGF-Ag2S QDs appeared in the tumors at 0.5 h post-injection, and the NIR fluorescence signals from the tumors could be detected for up to 24 h post-injection. Recently, Chen et al.68 synthesized NIR-II-emitting Ag2S QDs with β-LG as the capping/stabilizing agent. The β-LG-encapsulated Ag2S QDs (β-LG-Ag2S QDs) dispersed well in water and emitted stably in the NIR-II region with a peak at ∼1100 nm. The fluorescence signals from the β-LG-Ag2S QDs were detected throughout the body of a mouse at 30 s post-injection. In particular, the NIR-II fluorescence was weak in the lymphatic area at 1 h post-injection, but it gradually migrated to the groin and became more intense over the following 24 h. A lymph node within the region was visualized with a diameter of 1.25 mm. The results indicate that β-LG-Ag2S QDs could be used as a NIR-II lymphatic tracer for in vivo lymph node imaging. Fig. 5a shows the in vivo fluorescence imaging of the lymph node.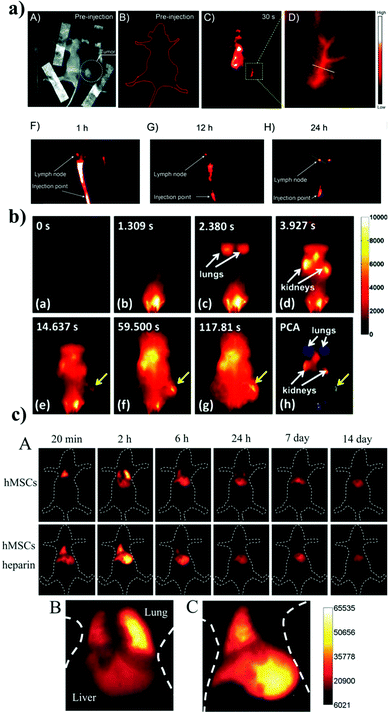 | ||
| Fig. 5 (a) The β-LG-Ag2S QDs as a NIR-II lymphatic tracer for in vivo lymph node imaging. Reproduced from ref. 68 with permission from [Royal Society of Chemistry], copyright [2016]. (b) Time course of the NIR-II fluorescence image of 6PEG-Ag2S QDs. Reproduced from ref. 25 with permission from [John Wiley and Sons], copyright [2012]. (c) In vivo tracking of intravenously injected hMSCs in mice. The time course of the in vivo NIR PL images of a healthy mouse after transplantation of Ag2S QDs-labeled hMSCs and a mouse with acute liver failure after transplantation of Ag2S QDs-labeled hMSCs in combination with heparin at different times (20 min–14 days). Reproduced from ref. 70 with permission from [John Wiley and Sons], copyright [2014]. | ||
Hong et al. prepared 6PEG-Ag2S QDs for deep inner imaging of organs in vivo (Fig. 5b).25 They studied the NIR-II fluorescence signal of 6PEG-Ag2S QDs using a 4T1 tumor-bearing mouse at a concentration of 1.34 mg ml−1. After the i.v. injection, the blood circulation of the Ag2S QDs was tracked in a real-time fluorescence image with different time intervals (0 to 210 s) under 808 nm excitation at a power dose of 0.14 W cm−2. The real-time NIR-II fluorescence image suggested that the 6PEG-Ag2S QDs first entered the heart and lungs and then the kidneys. The NIR-II fluorescence signal of the 6PEG-Ag2S QDs in the tumor region appeared initially at 15 s and continued up to 120 s (Fig. 5b). This result shows that the tumor was detected within a 2 min time period. The in vivo pharmacokinetics of 6PEG-Ag2S QDs was studied and showed an unprecedented degree of accumulation of the 6PEG-Ag2S QDs in a tumor (>10% ID per gram) through the enhanced permeability and retention (EPR) effect. Small-sized tumors in mice were detected using NIR Ag2S QDs. For example, Zhang et al. prepared albumin and metal ion chelating agent (BSA-DTPA)-functionalized paramagnetic Ag2S QDs (Ag2S@BSA-DTPAGd pQDs) for in vivo MR/fluorescence imaging of tiny tumor bearing mice.56 In that study, they utilized a tiny tumor size of 2 mm to evaluate the tumor detection capability of the Ag2S@BSA-DTPAGd pQDs. The MR images were taken at different time points (1.5, 3, and 12 h) before and after the injection of the Ag2S QDs. The tiny tumor clearly showed enhanced fluorescence at the 1.5 h time point and became weak at 3 h and disappeared at 12 h post-injection. Additionally, a tiny tumor lesion on the same tumor was distinctly observed through the in vivo fluorescence imaging with the Ag2S QDs. The tumor lesion was detected via the emission at 790 nm at the 2 h time point after the injection under the excitation of the Ag2S@BSA-DTPAGd pQDs at 670 nm. The biodistribution of Ag2S QDs in organs and tumors further revealed that the QDs could target the tiny tumors in mice. The in vivo fluorescence and computed tomography (CT) dual mode imaging in tumor bearing mice was also performed. Qin et al. prepared a nanoprobe that showed both excellent fluorescence imaging and CT contrast capabilities in a tumor. Distearoylphosphatidylethanolamine-poly(ethylene glycol)-folate (DSPE-PEG2000-FA), lecithin, and polyoxyethylene glycol monostearate and iodinated oil were used simultaneously to coat hydrophobic Ag2S QDs to make a complex nanoprobe (Ag2S-I@DSPE-PEG2000-FA) for in vivo cancer targeting and fluorescence-CT dual mode imaging. The in vivo fluorescence-CT imaging capabilities of the probe were investigated in HeLa xenograft nude mice. The nanoprobe was injected intravenously into the mice and observed using a fluorescence-CT imaging system at 5 different time points (1 min and 6, 12, 24, and 48 h). Another group studied in vivo deep tissue imaging using cRGD as a targeting agent for targeting the αvβ3 integrin receptor overexpressed in tumor cells. Tang et al. prepared the cRGDfk peptide-conjugated Ag2S QDs for deep tissue imaging.35 They chose Ag2S QDs emitting at 820 nm for the conjugation of the cRGDfk peptide to the Ag2S QDs. The biodistribution of the cRGDfk-Ag2S QDs was investigated in 4T1 tumors on the bilateral flanks of Balb/c mice. In vivo fluorescence images were taken at 1, 4, and 24 h after injection. The biodistribution data showed that the cRGDfk-Ag2S QDs were distributed all over the tissue and concentrated in the tumor at 1 and 24 h after the injection. The high tumor uptake of the QDs was also investigated. The results showed a higher uptake in the tumor relative to either the kidneys or the liver. The PEG-conjugated Ag2S QDs were also used for in vivo NIR imaging. For example, Li et al. prepared PEGylated Ag2S QDs for the in vivo imaging of the circulatory system, including lymphatic monitoring and blood flow and angiogenesis in subcutaneous xenograft tumors in mice.22 The same group also prepared PEGylated dendrimer-encapsulated Ag2S QDs (PEG-PATU Ag2S QDs) for NIR-II fluorescence imaging.69 PEG-PATU Ag2S QDs were injected into A549 tumor bearing mice for the in vivo tracking of A549 cancer cell mobility and real-time imaging of the blood circulation and vascular system of a mouse.
6.1 In vivo optical tracking and cytometric analysis
Chen et al.70 developed Ag2S QDs (NIR-II, 1.0–1.4 μm) and conjugated them with the Tat peptide, before using them for the labeling and tracking of human mesenchymal stem cells (thMSCs) in vivo (Fig. 5c). In vivo imaging demonstrated the distribution of the QD-labeled thMSCs in the liver and lung, and they were tracked for 14 days. The results indicated that heparin facilitated the transfer of the thMSCs from the lung to the liver, and the thMSCs were retained in the liver for a long time. These NIR-II Ag2S QDs can be used to track stem cells in living animals at high temporal and spatial resolution and have potential uses in clinical stem cell therapies.7. Ex vivo assay with Ag2S QDs
In general, semiconducting polymers, fluorescent dyes, nanoparticles, and fluorescent proteins are used as fluorescence sensors. In particular, Ag2S nanoprobes have attracted much attention for the development of biosensors due to their unique properties, including good photostability, high QY, and tunable spectral properties. Studies have shown that several dot nanoprobes are sufficiently stable for practical applications in biomedical analyses. In the following, we provide details on the applications of dot nanoprobes to biofluids (urine and blood) and biopsy tissues for the detection of pathological biomarkers and disease diagnosis.Jin et al. fabricated a novel and facile probe for CA125 with NIR-emitting Ag2S QDs as the detection platform,71 for which an Ag2S QDs/aptamer/5-FU hybrid-based NIR probe was utilized for detecting CA125 in human body fluids, such as urine, gastric juice, and serum. PEI-capped Ag2S QDs (PEI-Ag2S QDs) were developed for detecting heparin and heparinase I,72 for which the fluorescent probe was based on the fluorescence “turn-on” method according to the aggregation-induced emission enhancement characteristics of the PEI-Ag2S QDs induced by the electrostatic interaction between the PEI-Ag2SQDs and heparin. When heparinase I was present in the PEI-Ag2SQD–heparin system, the heparin was hydrolyzed into small fragments, and this de-aggregated the PEI-Ag2S QDs/heparin system to switch the fluorescence off. The PEI-Ag2S QD probe was utilized to detect the heparin and heparinase I level in serum samples.
8. Ag2S QDs as theranostic agents
8.1. Ag2S theragnostics based on chemotherapy
Chen et al. prepared tumor-targeting Ag2S QDs for cancer imaging and therapy.19 They fabricated Ag2S-cRGD and Ag2S-DOX-cRGD QDs and studied both in integrin αvβ3 over-expressing tumor cells and tumor-bearing mouse models. The in vitro fluorescence imaging was studied in U87 and MCF-7 cells. The in vivo fluorescence imaging of Ag2S-cRGD and Ag2S-DOX-cRGD was studied in MDA-MB-231 tumor-bearing mice. Fig. 6 shows the in vivo fluorescence imaging at different time points (10 min and 3 and 8 h), which shows that the Ag2S-cRGD and Ag2S-cRGD-DOX were distributed in the organs such as in the heart, liver, bladder, and intestines and the tumor, as well. However, tail vein injection of the Ag2S-cRGD-DOX into tumor bearing mice showed that the nanoprobe was mostly accumulated in the tumor compared with the other organ tissues. This result was confirmed by observing the strong red fluorescence of the nanoprobe in the peripheral and central slices of the tumor with laser scanning confocal microscopy (LSCM). It was demonstrated that the Ag2S-DOX-cRGDs possessed a better in vivo therapeutic anti-tumor efficacy compared to the DOX and Ag2S-DOX treated groups.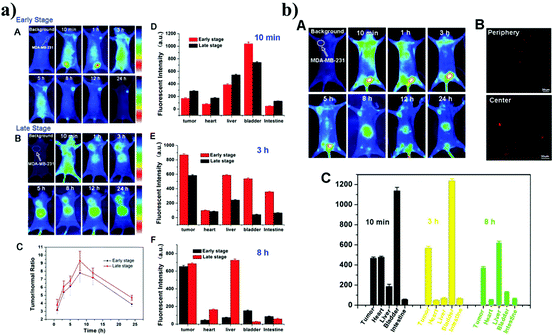 | ||
| Fig. 6 The in vivo fluorescence imaging of Ag2S-cRGD and Ag2S-cRGD-DOX at different time points (10 min and 3 and 8 h) in different MDA-MB-231 tumor-bearing mice. a) In vivo fluorescence imaging of Ag2S-cRGD; the early stage (A) and late stage (B) of tumor bearing mice; C. Relative fluorescence intensity ratios of tumor-bearing mice and fluorescence intensity of different organs with different time points (D. 10 min, E. 3 h and F. 8 h). b) A. In vivo fluorescence imaging of Ag2S-DOX-cRGD in MDA-MB-231 tumor-bearing mice; B. Strong red fluorescence of the tumor under LSCM. C. Fluorescence intensity of the tumor and organs with different time points 10 min, 3 h and 8. Reproduced from ref. 19 with permission from [Royal Society of Chemistry], copyright [2014]. | ||
Hu et al. synthesized poly(maleic anhydride-alt-1-octadecene)-polyethylene glycol (C18PMH/PEG)-functionalized Ag2S QDs (DOX@PEG-Ag2S) for simultaneous diagnosis, therapy, and real-time imaging of tumors.73 The loading of DOX onto the surface of C18PMH/PEG-coated Ag2S QDs was achieved through hydrophobic interactions between C18PMH and DOX. This nanoprobe had a high tumor targeting efficiency (8.9% ID per gram) and high drug loading capability (93 wt% of DOX to Ag2S QDs) in mice. The % ID per gram signifies the weight percentage of the injected DOX dose (ID) per gram of tissue. The DOX@C18PMH/PEG-Ag2S nanoprobes were intravenously injected into the MDA-MB-231 tumor-bearing mice. The in vivo fluorescence imaging showed that the C18PMH/PEG-Ag2S nanoprobes were more accumulated in the tumor region compared to the other organs.
Wang et al. prepared AMD3100 (AMD stands for AnorMeD)-conjugated Ag2S QDs (QD-AMD) as a theragnostic probe.16 The QD-AMD was designed to detect CXCR4 expressed in the tumor and inhibit the metastatic spread of the breast tumor (Fig. 7). AMD3100, as a CXCR4 antagonist, was approved by the US FDA. In vitro cellular imaging of QD-AMD in 4T1 and MDAMB-231 cells showed a brighter fluorescence because of the high level of CXCR4 expression compared to the in vitro cellular imaging in MCF-7 or MCF-10A cells. In vivo targeting ability of the QD-AMD was tested by NIR optical imaging of nude mouse models bearing 4T1 or MCF-7 cells.
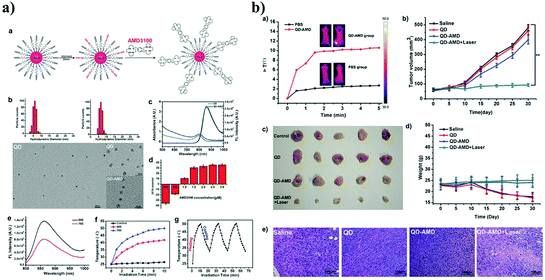 | ||
| Fig. 7 (a) Preparation and characterization of Ag2S QDs and AMD3100 functionalized Ag2S QDs. (a. Scheme, b. The DLS and TEM images of Ag2S QDs and AMD3100 functionalized Ag2S QDs. c. The absorption and emission spectrum of AMD3100 functionalized Ag2S QDs. d. Zeta potential of Ag2S QDs and AMD3100 functionalized Ag2S QDs. e. FL emission spectrum of AMD3100 functionalized Ag2S QDs with laser irradiation at 660 and 785 nm. f. Thermal changes of AMD3100 functionalized Ag2S QDs with laser irradiation at 660 and 785 nm. g. Thermal stability of AMD3100 functionalized Ag2S QDs with repeated laser heating and cooling). (b) Therapeutic efficacy of AMD3100 functionalized Ag2S QDs on different treatment groups. Reproduced from ref. 16 with permission from [John Wiley and Sons], copyright [2014]. | ||
8.2. Ag2S theragnostics based on photothermal therapy
Gao et al. prepared water-soluble aptamer43 (Apt43)-Ag2S QDs for imaging and photothermal therapy (PTT) of cancer.18 They designed the Apt43 (5′-GCA GTT GAT CCT TTG GAT ACC CTG GCC CCC CCC CCC CCC CCC C-3′), which consists of the aptamer S2.2 sequence (5′-GCA GTT GAT CCT TTG GAT ACC CTG G-3′), and an 18-cytosine (18-C). The S2.2 fragment specifically recognizes the MUC1 protein, which is overexpressed on the surface of MCF-7 cells. The imaging of MCF-7 cancer cells was performed by incubating the Apt43-Ag2S QDs at a concentration of 100 μg mL−1 for 2 h at 4 °C using a confocal laser scanning microscope (CLSM). The MCF-7 cells showed a strong red fluorescence by irradiation at 638 nm. The photothermal effect of the Apt43-Ag2S QD was evaluated by irradiation at 808 nm at a power density of 1 W cm−2. The temperature of the Apt43-Ag2S QD solution increased to ∼54 °C within 12 min, while the solution of Apt43 alone was heated to 30 °C (Fig. 8a). It has been reported that the Apt43-Ag2S QDs absorb NIR light and efficiently convert the light energy into thermal energy. Based on this optical property, the Apt43-Ag2S QDs were found to possess great potential as a photothermal therapeutic agent. The in vitro photothermal therapeutic effect of the Apt43-Ag2S QDs was tested in MCF-7 cells. The cell viability of MCF-7 cells containing the Apt43-Ag2S QDs was reported to be less than 10% after NIR irradiation for 10 min compared to the control cells without the Apt43-Ag2S QDs (Fig. 8b). Fig. 8c shows the in vivo photothermal effect of the Apt43-Ag2S QDs using MCF-7 tumor bearing BALB/c nude mice. The nude mice were treated intravenously with the Apt43-Ag2S QDs, and then, the tumor site was irradiated with a laser beam at 808 nm with a power of 1.5 W cm−2 for 3 h. The temperature increase of the tumor region to 52 °C was monitored using an IR thermal imaging instrument. The Apt43-Ag2S QDs could be bound specifically to the tumor. The in vivo PTT effect of the Apt43-Ag2S QDs was clearly observed by the reduction of the tumor size, and ablation occurred in the tumor after the laser irradiation.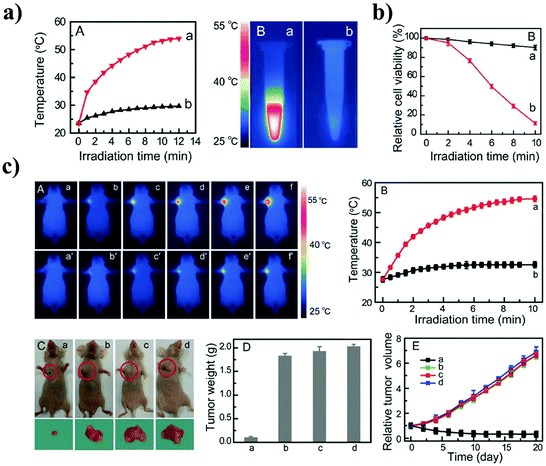 | ||
| Fig. 8 (a) Temperature–irradiation time profiles and photothermal images of Apt43-Ag2S QDs (image a) and Apt43 solution (image b). (b) The cell viability of MCF-7 cells containing Apt43-Ag2S QDs after PTT. (c) Photothermal images and temperature profiles as a function of the irradiation time for tumor sites on mice in groups. Reproduced from ref. 18 with permission from [John Wiley and Sons], copyright [2016]. | ||
Zhang et al. synthesized FA and phospholipid-functionalized Ag2S QDs (Ag2S@DSPEPEG2000-FA) for both imaging and photothermal therapy.17 They studied the toxicity of the nanoprobe using the MTT assay. HeLa and A549 cells were incubated with different concentrations of the Ag2S@DSPEPEG2000-FA. The MTT assay results showed that almost 80% of the cells survived when the silver concentration reached up to 50 μg ml−1 (Fig. 9a). The targeting abilities of the Ag2S QDs functionalized with FA (Ag2S@DP-FA) or without FA (Ag2S@DP) were compared using HeLa cells. The Ag2S@DP-FA showed a strong fluorescence intensity in the cell images, while the Ag2S@DP showed weak fluorescence. FA receptors are overexpressed in HeLa cells, and as a result, the Ag2S@DP-FA could be more accumulated in HeLa cells. The in vitro PTT effect of Ag2S@DP-FA was studied in HeLa cells incubated with Ag2S@DP-FA. A laser beam at 808 nm irradiated the cells with a laser power of 1.8 W cm−2 for 5 min. Cell death was observed with the laser irradiation. On the other hand, no laser irradiation did not cause any cell death (Fig. 9b). The in vivo PTT effect of Ag2S@DP-FA in HeLa tumor-bearing nude mice was also studied. The Ag2S@DP-FA was intravenously injected into the HeLa tumor-bearing nude mice, and the laser beam irradiated the tumor for 10 min. The temperature increased to 67 °C. The tumor cells were killed with only a 10 °C temperature increase compared to the controls (Fig. 9c).
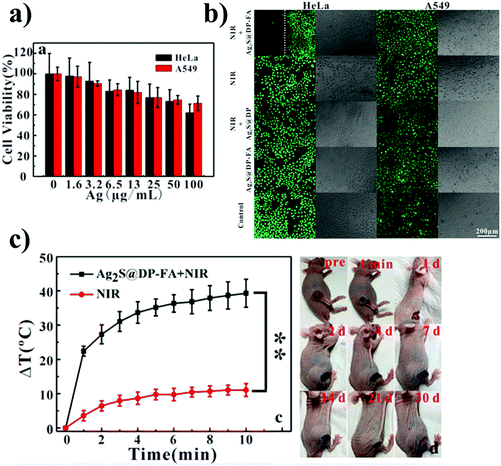 | ||
| Fig. 9 (a) MTT assay of Ag2S@DP-FA, (b) HeLa and A549 cells were incubated with a positive probe (Ag2S@DP-FA) or negative probe (Ag2S@DP) and then treated with or without NIR laser irradiation. Cells were stained with calcein-AM. (c) The temperature of the tumor evolution curves over time with laser irradiation at 808 nm and the image of tumor-bearing nude mice after treatments over time. Reproduced from ref. 17 with permission from [Springer Nature], copyright [2018]. | ||
Zhao et al. prepared Ag2S QD nanogels with genetically engineered polypeptides PC10A (Ag2S QD@PC10A) or PC10ARGD (Ag2S QD@PC10ARGD) for targeted imaging and photothermal therapy.49 The photothermal effect was studied using the Ag2S QD@PC10ARGD at various concentrations with irradiation by a laser beam at 810 nm with a power density of 2.5 W cm−2 for 8 min. The photothermal study revealed that the temperature of the Ag2S QD@PC10ARGD hybrid nanogel increased rapidly within 8 min compared to the PC10ARGD alone. The results demonstrated that the Ag2S QDs could absorb NIR light and efficiently convert light energy into thermal energy. In vitro and in vivo fluorescence imaging of the Ag2S QD@PC10ARGD hybrid nanogels was performed in HeLa and MCF-7 cells. The imaging study showed that the Ag2S QD@PC10ARGD hybrid nanogels were internalized into the HeLa cells compared to the MCF-7 cells, which may be due to their low integrin αvβ3 expression. The in vitro and in vivo PTT effect of the Ag2S QD@PC10ARGD was investigated using HeLa cells. HeLa cells incubated with the Ag2S QD@PC10ARGD were irradiated with a laser beam at 810 nm and a power density of 2.5 W cm−2 for 10 min. It was shown that the Ag2S QD@PC10ARGD successfully targeted the HeLa cells via the RGD targeting moiety. The in vivo PTT effect of the Ag2S QD@PC10ARGD was studied. The Ag2S QD@PC10ARGD hybrid nanogels were intratumorally injected into the tumor bearing mice. The tumor site was irradiated with a laser beam at 810 nm and a power density of 2.5 W cm−2. Within two minutes, the temperature of the tumor site reached 60.7 °C compared to the PBS-treated tumor that reached 37.4 °C. The in vivo results suggest that the Ag2S QD@PC10ARGD-treated tumors were effectively ablated after the laser irradiation.
Glutathione-coated Ag2S QDs (GSH-Ag2S QDs) were synthesized by Hashemkhani et al. for NIR image-guided PTT.74 The in vitro imaging study of the GSH-Ag2S QDs was performed with MCF7 and HT29 cells. The fluorescence images of the GSH-Ag2S QD-treated cells showed high intracellular uptake and lysosomal co-localization in both cell lines. The photothermal effect of the GSH-Ag2S QDs was investigated in an aqueous solution of GSH-Ag2S QDs as a function of the concentration (1–10 mg ml−1). The GSH-Ag2S QD solution was irradiated using a 795 nm laser beam at a power density of 2 W cm−2 for 20 min. The temperature increased to 45 °C within 10 min of the irradiation proving the photothermal effect of the GSH-Ag2S QDs in an aqueous solution. Furthermore, the in vitro PTT effect of the GSH-Ag2S QDs was verified by the MTT assay using MCF7 and HT29 cells. The cells were irradiated for 10 min using a 795 nm laser beam at a power of 1.82 W cm−2, and then, their cell proliferation was determined. Almost complete elimination of viable cells in the MCF7 cell line and a 60% reduction of viability in the HT29 cell line were observed. The live/dead cell-based assay confirmed that the laser beam treatment with the GSH-Ag2S QDs caused the death of the cancer cells. Annexin-V/FITC-PI staining indicated late apoptotic and necrotic cell death.
Yang et al. prepared BSA-coated Ag2S QDs for imaging and photothermal therapy.75 The in vitro photothermal effect of the BSA-Ag2S QDs was studied in 4T1 murine breast tumor cells. The 4T1 cells were incubated with the BSA-Ag2S QDs for 24 h and then irradiated by a 785 nm laser beam at a power of 1.5 W cm−2 for 3 min. Remarkable cell damage was observed with an IC50 value of 0.17 mM. In the absence of laser irradiation, there was no cell death. The in vivo photothermal effect of the BSA-Ag2S QDs was studied using 4T1 tumor-bearing mice. The BSA-Ag2S QDs were intravenously injected into the 4T1 tumor-bearing mice, and then, a 785 nm laser beam at 1.5 W cm−2 was irradiated onto the tumor for 5 min 24 h after the injection. A reduction in the tumor size was observed after 30 days. Treatment with the BSA-Ag2S QDs at a concentration of 50.0 μmol kg−1 showed effective tumor ablation without any regrowth.
8.3. Ag2S theragnostics based on sonodynamic therapy
The PDT therapy is a non-invasive cancer therapy and widely applied in clinical use. However, the cancer treatment is delaying due to the lack of deep tissue penetration of the photosensitizers. Recently, the sonodynamic therapy (SDT) has been extensively explored in cancer treatments because of the high penetration depth and low side effects. Recently, Li et al. prepared the polymeric Pluronic F-127 (PF)-modified Ag2S QDs coated on red blood cell (RBC) vesicles for image-guided SDT.76 The Ag2S QDs were used as a sonosensitizer to generate ROS under ultrasonic stimulation. In this study, the biomimetic nanoparticles (Ag2S@PF) were developed by using the RBC and Ag2S QDs to alleviate tumor hypoxia. They have performed the in vitro and in vivo study of PF-127-modified Ag2S QDs and demonstrated excellent biocompatibility and prolonged blood circulation.9. Bioconjugation of Ag2S QD theranostic agents
9.1. Bioconjugation of Ag2S QDs to therapeutic agents
The Apt43-functionalized water-soluble Ag2S QDs (Apt43-Ag2S QDs) were fabricated through coordination between the Apt43 and Ag+ ion by Gao et al.18 First, the Ag ion (AgNO3 solution) bound to the Apt43 through a coordination reaction of 18-cytosine (18-C) with the nitrogen atom and π-electrons. Then, the Apt43-Ag+ complex rapidly reacted with S2− ions from a freshly prepared Na2S solution. Finally, the formation of the Apt43-functionalized Ag2S QDs was confirmed by the color change in the reaction solution. The color changed from colorless to pale yellow then to brown. The Apt43 recognizes the MUC1 protein in MCF-7 cells. The Apt43-Ag2S QD treated cells had a lower cell viability (10%) than the untreated QDs after laser irradiation. Hu et al. prepared DOX-loaded PEG-functionalized Ag2S QDs (DOX@PEG-Ag2S) using hydrophobic–hydrophobic interactions.73 The DT-coated Ag2S QDs were prepared by a previously reported method. Then, the hydrophobic DT-coated Ag2S QDs were functionalized by PEG-grafted amphiphilic poly(maleic anhydride-alt-1-octadecene)-methoxy poly(ethyleneglycol) [C18PMH/PEG] via hydrophobic–hydrophobic interactions. Finally, the DOX was encapsulated into the PEG-grafted amphiphilic polymer functionalized Ag2S QDs coated with DT. Wang et al. prepared AMD3100-functionalized Ag2S QDs (QD-AMD) for theranostic applications.16 AMD3100 is a CXCR4 antagonist, which was covalently conjugated to carboxylic acid (3-MPA)-coated Ag2S QDs by the EDC coupling reaction. The AMD3100 of the QD-AMD was used as the therapeutic agent, while the Ag2S QDs of the QD-AMD were used as the imaging agent. Li et al. prepared polymeric Pluronic F-127-modified Ag2S QDs coated with red blood cell (RBC) vesicles for image-guided sonodynamic therapy (SDT).76 Pluronic F-127 (PF-127) is a triblock copolymer ((poly(ethylene oxide)-poly(propylene oxide)-poly (ethylene oxide), PEO-PPO-PEO). PPO is a hydrophobic unit. Briefly, the hydrophobic Ag2S QDs were encapsulated with the Pluronic F-127 polymer through hydrophobic interactions, and as a result, the PF-127-modified Ag2S QDs (Ag2S@PF) were produced. The RBC vesicles were obtained by hypotonic treatment, and the RBC vesicles were attached to the surface of the Ag2S@PF by the extrusion process. The polymeric PF-127 possessing high biocompatibility can effectively avoid protein adsorption and copolymer aggregation. In addition, the coating of this copolymer onto the hydrophobic Ag2S QDs forms hydrophilic QDs. The RBC vesicles act as a catalase in tumor cells. The RBC vesicles could significantly catalyze H2O2 in tumor cells to alleviate hypoxia. Moreover, the modification of the RBC on the surface of the Ag2S QDs could provide them with a long natural circulation, low immunogenicity, and biocompatibility.9.2. Bioconjugation of Ag2S QDs to both therapeutic agents and targeting moieties
The cyclic RGD peptide and DOX-functionalized Ag2S QDs (Ag2S-DOXcRGD) were synthesized through the acid-amine coupling reaction by Chen et al.19 They first prepared the carboxylic acid (3-MPA)-coated Ag2S QDs, and then, the DOX was covalently conjugated by using EDC and NHS coupling agents. As a result, DOX-conjugated Ag2S QDs (Ag2S-DOX) were obtained. Then, the Ag2S-DOX was again conjugated with cRGD using the EDC coupling reaction at room temperature. Finally, the unreacted reaction mixture was removed by dialysis, and pure Ag2S-DOXcRGD QDs were obtained. The cRGD peptide of the Ag2S-DOXcRGD QDs was the tumor-targeting agent. Duman et al. developed PEGylated Ag2S QDs functionalized with Cetuximab (Cet) antibody to target EGFR-overexpressed cancer cells.77 An anticancer drug, 5-fluorouracil (5FU), was loaded in the PEGylated Ag2S QDs. Fig. 10 shows the bioconjugation of the Cet antibody to 5FU loaded PEGylated Ag2S QDs. They first prepared the branched PEI and carboxylic acid-coated Ag2S QDs by a previously published method. The CDI-activated methoxy-PEG (mPEG-CDI) was conjugated to the amine groups of the branched PEI on the carboxylic acid-coated Ag2S QDs. Then, the mPEG-Ag2S QDs were conjugated with the Cet antibody. Finally, the anticancer drug 5FU was loaded into the mPEG-Ag2S-Cet QDs. The cationic PEI coating and PEGylated surface of the Ag2S QDs contributed to the electrostatic and hydrophobic interaction between the therapeutic agent 5FU and the Ag2S QDs. Zhang et al. prepared folic acid (FA)-conjugated PEGylated phospholipid-coated Ag2S QDs (Ag2S@DSPE-PEG2000-FA) by simply mixing the hydrophobic DT-capped Ag2S QDs and FA-modified PEGylated phospholipid.17 Briefly, the hydrophobic DT-capped Ag2S QDs were prepared according to the reported method. The folic acid was activated with DDC and reacted with amine containing the PEGylated phospholipid (DSPE-PEG2000-NH2) by the acid amine coupling reaction, and as a result, the PEGylated phospholipid conjugated with FA (DSPE-PEG2000-FA) was acquired. Then, the DSPE-PEG2000-FA was attached to the hydrophobic DT capped Ag2S QDs by hydrophobic–hydrophobic interactions, and finally, the Ag2S@DSPE-PEG2000-FA was formed. In this conjugation method, they used the Ag2S QDs as the PTT agent and the FA as the targeting agent for targeted cancer therapy. Multifunctional Ag2S QDs encapsulated with a polypeptide hybrid nanogel were prepared by Zhao et al.49 They used a genetically modified polypeptide PC10A and PC10ARGD that tends to form a self-assembled nano-sized hydrogel. The hydrophobic DT-capped Ag2S QDs were prepared by the pyrolysis method. Then, different concentrations of PC10A or PC10ARGD were prepared in DI water and then heated at 100 °C for 5 minutes. After cooling, PC10A or PC10ARGD nanogels were successfully formed. The Ag2S QDs were loaded in the PC10A or PC10ARGD by ultrasonic treatment so that an Ag2S QD-loaded nanogel was acquired. Briefly, the PC10ARGD was heated at 100 °C and then cooled to room temperature, and different concentrations of Ag2S QDs were mixed with the PC10ARGD. Finally, the Ag2S QDs and the PC10ARGD nanogel mixture were treated by ultrasonication for 5 min to form the Ag2S QD encapsulated-polypeptide hybrid nanogel. The Ag2S QDs were coated with the tripeptide RGD to target HeLa cells overexpressing αvβ3 integrin receptors. The PC10ARGD hydrogel consisted of both hydrophobic and hydrophilic regions. Therefore, the hydrophobic DT capped Ag2S QDs were coated with PC10ARGD through hydrophobic–hydrophobic interactions.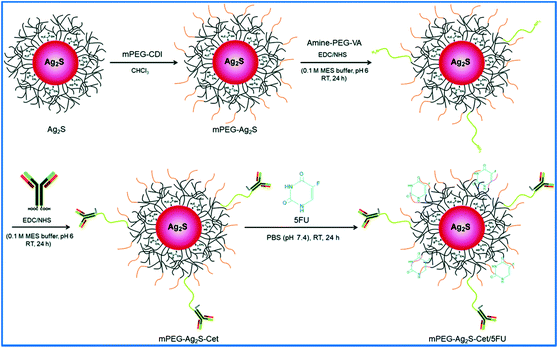 | ||
| Fig. 10 Schematic representation of Cetuximab antibody conjugated and 5-fluorouracil loaded PEGylated Ag2S QDs. Reproduced from ref. 77 with permission from [Royal Society of Chemistry], copyright [2019]. | ||
10. Biodistribution, pharmacokinetics and toxicology of Ag2S QD
The therapeutic efficacy and toxicity of nanoparticles have been studied to predict their effectiveness and side effect by using the in vivo pharmacokinetics (PK) and tissue distribution (TD). The physicochemical properties of Ag2S QDs or nanoparticles may change in the body. Therefore, the PK and TD properties of the Ag2S QDs should be addressed before wide-scale human clinical applications. Yan Zhang et al. systematically investigated the in vivo pharmacokinetics, biodistribution, and potential toxicity of PEGylated-Ag2S QDs in mice.23 In this study, they have used the PEGylated Ag2S QDs with two sets of doses (15 and 30 mg kg−1). The in vivo PK and TD of PEGylated Ag2S QDs were studied using 4T1 tumor bearing mice over a period of 2 months. They first evaluated the imaging capability of PEGylated Ag2S QDs at a dose of 15 mg kg−1. The PEGylated Ag2S QD was intravenously injected into the mouse tail vein. The NIR-II PL (1100–1700 nm region) image was collected at 24 h post-injection (p.i.). The large NIR-II PL signal of the PEGylated Ag2S QD was observed in the tumor area. This result showed a lot of accumulation of PEGylated Ag2S QDs into the tumor due to the non-specific EPR effect.The biodistribution of PEGylated Ag2S QDs was investigated at a dosage of 15 mg kg−1 using female Balb/c mouse organs. Female Balb/c mice were sacrificed at different time points (1, 2, 3, 7, 14, 28 and 60 days) after the injection of PEGylated-Ag2S QDs at a dosage of 15 mg kg−1. Various organs and tissues (heart, liver, spleen, lungs, kidneys, brain, stomach, intestine, bone, muscle, skin, blood, urine, and feces) were collected and the concentrations of Ag2S were measured using inductively coupled plasmon-mass spectrometry (ICP-MS). From the results, the Ag2S QDs were found to be widely distributed in different organs and tissues. The higher amount of Ag2S QD was accumulated in liver and spleen. Finally, most of the Ag2S QDs cleared from the mouse body after 60 days.
The pharmacokinetics of PEGylated-Ag2S QDs was evaluated in the blood over time after intravenous injection of PEGylated-Ag2S QDs into the Balb/c mice at a dosage of 15 mg kg−1. Blood was collected from the tail vein of mice at different time points p.i., and the amount of Ag2S was measured by ICP-MS at each time point. The blood circulation curve showed a blood circulation half-life of 3.66 h for PEGylated-Ag2S QDs. Also, the toxicity of PEGylated-Ag2S QDs was evaluated using serum biochemistry analysis. No obvious hepatic and renal toxicity was observed by the PEGylated-Ag2S QD treatment, even with the overloaded dosage of 30 mg kg−1. In addition, the hematoxylin and eosin (H&E) staining analysis showed that there were no evident histopathological abnormalities or lesions in the PEGylated-Ag2S QDs-treated mice at a dosage of 30 mg kg−1. Overall, the blood biochemistry, hematological analysis and histological studies suggested that the PEGylated-Ag2S QDs did not cause any significant toxicity even at higher dosage (15 and 30 mg kg−1). Javidi et al. also studied the in vivo biodistribution, pharmacokinetics, and toxicology of Ag2S QD in mice as well as rats.78 They have prepared the three different sizes of Ag2S QDs (5, 15, and 25 nm) and studied regarding their effect of injection dose, surface charge, and particle size. In addition, the acid (QDs-COOH) and amine (QDs-NH2) functionalized Ag2S QDs were prepared in similar sizes (5 nm) by capping with thioglycolic acid and cysteamine to investigate their surface charge effect on the PK and TD of Ag2S QDs. The i.v. administration of Ag2S QDs was performed in mice as well as rats. The in vivo results indicated that the Ag2S QDs were mainly accumulated in the liver and intestine regardless of particle size (5, 15, and 25 nm), injected dose (0.5, 1.0 and 4.0 mg kg−1), and surface charge. The Ag2S QDs were excreted mostly in feces.
11. Toxicity of dot nanoprobes
Detailed risk-benefit analyses are essential for QDs before they can be applied to specific diagnostic and therapeutic applications. The toxicity of QDs is assessed based on in vitro and in vivo behavior and their elemental compositions. In general, the toxicity of QDs depends on multiple factors, including their physiological characteristics (size, shape, and stability), surface properties (surface charge and surface coating), and chemical components. Another important and potential source of toxicity is the release of heavy metal ions. For instance, the release of highly toxic Cd2+, Pb2+, and Hg2+ ions from CdTe, CdSe, PbS, PbSe, and HgTe heavy metal QDs is a great concern for the research community, and it has motivated the development of toxic element-free QDs (i.e., heavy metal free Ag2S QDs).Zhang et al.79 determined the cytotoxicity of Ag2S QDs by exposing mouse fibroblast (L929) cells to Ag2S QDs at different concentrations for 72 h. The results showed that cell proliferation was dose independent, thereby suggesting that the Ag2S QDs did not affect cell proliferation. Flow cytometry analysis indicated very low cytotoxicity in terms of the induction of apoptosis and necrosis. In addition, cells treated with Ag2S QDs produced fewer ROS, and the results were not significantly different from the control. A single-cell gel electrophoresis assay (comet assay) was performed to detect the genotoxicity induced by Ag2S QDs, and the results indicated negligible genotoxicity. Recently, it was shown that the synthetic methods and surface coatings used have important effects on the toxicity profiles of QDs.80
12. Conclusions
In this review article, the fabrication, physicochemical properties, and biofunctionalization of heavy-metal-free Ag2S QDs were summarized. Ag2S QDs provide emission in the NIR-II window facilitating deep tissue imaging. Based on the remarkable optical and biological advantages inherent to Ag2S QDs in in vitro and in vivo bioapplications, the biofunctionalization of Ag2S QDs has been actively developed to enable their general use in theragnostic applications. Ag ions have a low-cellular toxicity making Ag2S QDs appropriate for use as a theragnostic agent. Various therapeutic or/and targeting agents can be functionalized onto Ag2S QDs for targeted theragnostic applications. Another attractive property of Ag2S QDs is their excellent activity as a photothermal therapeutic agent using its high photothermal conversion capability besides the chemotherapeutic activities. Ag2S QD theragnostic materials have been intensively explored as a photothermal agent and an imaging agent. In addition, Ag2S QDs possess many advantages, such as having non-ionizing radiation, ultrahigh spatial resolution, water solubility, and excellent biocompatibility. These properties inherent to Ag2S QDs have led to new theragnostic nanomaterials, which eventually could be applicable to cancer diagnosis and therapy.Data availability
This is a review manuscript and it does not contain original data.Conflicts of interest
The authors do not declare any conflict of interest.Acknowledgements
We are grateful to the Research Institute of Pharmaceutical Sciences at Seoul National University and Brain Korea 21 plus (BK 21 plus). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (2018M3A7B4071235 and 2019R1A4A2001651).References
- W. C. Chan, D. J. Maxwell, X. Gao, R. E. Bailey, M. Han and S. Nie, Curr. Opin. Biotechnol., 2002, 13, 40–46 CrossRef CAS.
- F. Wang, W. B. Tan, Y. Zhang, X. Fan and M. Wang, Nanotechnology, 2005, 17, R1 CrossRef.
- G. Farahavar, S. S. Abolmaali, N. Gholijani and F. Nejatollahi, Biomater. Sci., 2019, 7, 4000–4016 CAS.
- J. Wang, Y. Lu, F. Peng, Y. Zhong, Y. Zhou, X. Jiang, Y. Su and Y. He, Biomaterials, 2013, 34, 9509–9518 CAS.
- W. A. El-Said, J. Yoon and J.-W. Choi, Nano Convergence, 2018, 5, 1–19 CrossRef.
- M. Zhang, J. Yue, R. Cui, Z. Ma, H. Wan, F. Wang, S. Zhu, Y. Zhou, Y. Kuang and Y. Zhong, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6590–6595 CrossRef CAS.
- S. B. Hafiz, M. Scimeca, A. Sahu and D.-K. Ko, Nano Convergence, 2019, 6, 7 CrossRef.
- N. Chen, Y. He, Y. Su, X. Li, Q. Huang, H. Wang, X. Zhang, R. Tai and C. Fan, Biomaterials, 2012, 33, 1238–1244 CrossRef CAS.
- Y. Zhang, G. Hong, Y. Zhang, G. Chen, F. Li, H. Dai and Q. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS.
- P. Jiang, C.-N. Zhu, Z.-L. Zhang, Z.-Q. Tian and D.-W. Pang, Biomaterials, 2012, 33, 5130–5135 CrossRef CAS.
- A. K. Parchur, Z. Fang, J. M. Jagtap, G. Sharma, C. Hansen, S. Shafiee, W. Hu, Q. R. Miao and A. Joshi, Biomater. Sci., 2020, 8, 5133–5144 RSC.
- K. Welsher, S. P. Sherlock and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8943–8948 CrossRef CAS.
- C. Ding, C. Zhang, X. Yin, X. Cao, M. Cai and Y. Xian, Anal. Chem., 2018, 90, 6702–6709 CrossRef CAS.
- F. D. Duman, M. Erkisa, R. Khodadust, F. Ari, E. Ulukaya and H. Y. Acar, Nanomedicine, 2017, 12, 2319–2333 CrossRef CAS.
- F. D. Duman, Development of Near Infrared Emitting Cationic Ag2S Quantum Dots as Drug-Gene Delivery and Optical Imaging Agents, Koç University, 2017 Search PubMed.
- Z. Wang, Y. Ma, X. Yu, Q. Niu, Z. Han, H. Wang, T. Li, D. Fu, S. Achilefu and Z. Qian, Adv. Funct. Mater., 2018, 28, 1800732 CrossRef.
- X.-S. Zhang, Y. Xuan, X.-Q. Yang, K. Cheng, R.-Y. Zhang, C. Li, F. Tan, Y.-C. Cao, X.-L. Song and J. An, J. Nanobiotechnol., 2018, 16, 1–15 CrossRef.
- J. Gao, C. Wu, D. Deng, P. Wu and C. Cai, Adv. Healthcare Mater., 2016, 5, 2437–2449 CrossRef CAS.
- H. Chen, B. Li, M. Zhang, K. Sun, Y. Wang, K. Peng, M. Ao, Y. Guo and Y. Gu, Nanoscale, 2014, 6, 12580–12590 RSC.
- P. Navya, A. Kaphle, S. Srinivas, S. K. Bhargava, V. M. Rotello and H. K. Daima, Nano Convergence, 2019, 6, 23 CrossRef CAS.
- J. Chen, X. Zhang, R. Millican, J. E. Creutzmann, S. Martin and H.-W. Jun, Nano Convergence, 2020, 7, 1–14 CrossRef.
- C. Li, Y. Zhang, M. Wang, Y. Zhang, G. Chen, L. Li, D. Wu and Q. Wang, Biomaterials, 2014, 35, 393–400 CrossRef CAS.
- Y. Zhang, Y. Zhang, G. Hong, W. He, K. Zhou, K. Yang, F. Li, G. Chen, Z. Liu and H. Dai, Biomaterials, 2013, 34, 3639–3646 CrossRef CAS.
- Y. Du, B. Xu, T. Fu, M. Cai, F. Li, Y. Zhang and Q. Wang, J. Am. Chem. Soc., 2010, 132, 1470–1471 CrossRef CAS.
- G. Hong, J. T. Robinson, Y. Zhang, S. Diao, A. L. Antaris, Q. Wang and H. Dai, Angew. Chem., Int. Ed., 2012, 51, 9818–9821 CrossRef CAS.
- L. Sinatra, J. Pan and O. M. Bakr, Mater. Matters, 2017, 12, 3–7 CAS.
- P. Jiang, Z.-Q. Tian, C.-N. Zhu, Z.-L. Zhang and D.-W. Pang, Chem. Mater., 2012, 24, 3–5 CrossRef CAS.
- V. A. Öberg, X. Zhang, M. B. Johansson and E. M. Johansson, ChemNanoMat, 2018, 4, 1223–1230 CrossRef.
- I. Hocaoglu, M. N. Cizmeciyan, R. Erdem, C. Ozen, A. Kurt, A. Sennaroglu and H. Y. Acar, J. Mater. Chem., 2012, 22, 14674–14681 RSC.
- R. Gui, A. Wan, X. Liu, W. Yuan and H. Jin, Nanoscale, 2014, 6, 5467–5473 RSC.
- J. Chen, Y. Kong, Y. Wo, H. Fang, Y. Li, T. Zhang, Y. Dong, Y. Ge, Z. Wu and D. Zhou, J. Mater. Chem. B, 2016, 4, 6271–6278 RSC.
- Y. Zhao and Z. Song, Mater. Lett., 2014, 126, 78–80 CrossRef CAS.
- P. Jiang, D.-L. Zhu, C.-N. Zhu, Z.-L. Zhang, G.-J. Zhang and D.-W. Pang, Nanoscale, 2015, 7, 19310–19316 RSC.
- M. H. Kang, S. H. Kim, S. Jang, J. E. Lim, H. Chang, K.-j. Kong, S. Myung and J. K. Park, RSC Adv., 2018, 8, 28447–28452 RSC.
- R. Tang, J. Xue, B. Xu, D. Shen, G. P. Sudlow and S. Achilefu, ACS Nano, 2015, 9, 220–230 CrossRef CAS.
- N. Kosaka, M. Ogawa, N. Sato, P. L. Choyke and H. Kobayashi, J. Invest. Dermatol., 2009, 129, 2818–2822 CrossRef CAS.
- H. Kobayashi, Y. Hama, Y. Koyama, T. Barrett, C. A. S. Regino, Y. Urano and P. L. Choyke, Nano Lett., 2007, 7, 1711–1716 CrossRef CAS.
- D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wise and W. W. Webb, Science, 2003, 300, 1434–1436 CrossRef CAS.
- H. Kobayashi, M. Ogawa, N. Kosaka, P. L. Choyke and Y. Urano, Nanomedicine, 2009, 4, 411–419 CrossRef CAS.
- M. Han, X. Gao, J. Z. Su and S. Nie, Nat. Biotechnol., 2001, 19, 631 CrossRef CAS.
- M. J. Kim, S. Rangasamy, Y. Shim and J. M. Song, J. Nanobiotechnol., 2015, 13, 4 CrossRef.
- Y. Su, M. Hu, C. Fan, Y. He, Q. Li, W. Li, L.-h. Wang, P. Shen and Q. Huang, Biomaterials, 2010, 31, 4829–4834 CrossRef CAS.
- R. Hardman, Environ. Health Perspect., 2005, 114, 165–172 CrossRef.
- G. Xu, S. Zeng, B. Zhang, M. T. Swihart, K.-T. Yong and P. N. Prasad, Chem. Rev., 2016, 116, 12234–12327 CrossRef CAS.
- R. Xie, K. Chen, X. Chen and X. Peng, Nano Res., 2008, 1, 457–464 CrossRef CAS.
- K. T. Yong, I. Roy, R. Hu, H. Ding, H. X. Cai, J. Zhu, X. H. Zhang, E. J. Bergey and P. N. Prasad, Integr. Biol., 2010, 2, 121–129 CrossRef CAS.
- K.-T. Yong, H. Ding, I. Roy, W.-C. Law, E. J. Bergey, A. Maitra and P. N. Prasad, ACS Nano, 2009, 3, 502–510 CrossRef CAS.
- J. P. Zimmer, S.-W. Kim, S. Ohnishi, E. Tanaka, J. V. Frangioni and M. G. Bawendi, J. Am. Chem. Soc., 2006, 128, 2526–2527 CrossRef CAS.
- D.-H. Zhao, J. Yang, R.-X. Xia, M.-H. Yao, R.-M. Jin, Y.-D. Zhao and B. Liu, Chem. Commun., 2018, 54, 527–530 RSC.
- G. Hong, J. T. Robinson, Y. Zhang, S. Diao, A. L. Antaris, Q. Wang and H. Dai, Angew. Chem., Int. Ed., 2012, 51, 9818–9821 CrossRef CAS.
- P. Jiang, C. N. Zhu, Z. L. Zhang, Z. Q. Tian and D. W. Pang, Biomaterials, 2012, 33, 5130–5135 CrossRef CAS.
- F. D. Duman, I. Hocaoglu, D. G. Ozturk, D. Gozuacik, A. Kiraz and H. Y. Acar, Nanoscale, 2015, 7, 11352–11362 RSC.
- L. Tan, A. Wan and H. Li, Langmuir, 2013, 29, 15032–15042 CrossRef CAS.
- P. Miao, Y. Tang, B. Wang and F. Meng, Anal. Chem., 2016, 88, 7567–7573 CrossRef CAS.
- M.-Y. Qin, X.-Q. Yang, K. Wang, X.-S. Zhang, J.-T. Song, M.-H. Yao, D.-M. Yan, B. Liu and Y.-D. Zhao, Nanoscale, 2015, 7, 19484–19492 CAS.
- J. Zhang, G. Hao, C. Yao, J. Yu, J. Wang, W. Yang, C. Hu and B. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 16612–16621 CAS.
- H. Jin, R. Gui, J. Gong and W. Huang, Biosens. Bioelectron., 2017, 92, 378–384 CrossRef CAS.
- W.-j. Jeong, J. Bu, L. J. Kubiatowicz, S. S. Chen, Y. Kim and S. Hong, Nano Convergence, 2018, 5, 38 CrossRef.
- J.-r. Choi, D.-M. Shin, H. Song, D. Lee and K. Kim, Nano Convergence, 2016, 3, 1–13 CrossRef.
- Y. Zhang, N. Zhao, Y. Qin, F. Wu, Z. Xu, T. Lan, Z. Cheng, P. Zhao and H. Liu, Nanoscale, 2018, 10, 16581–16590 RSC.
- P. K. Bae and B. H. Chung, Nano Convergence, 2014, 1, 1–8 CrossRef.
- H.-Y. Yang, Y.-W. Zhao, Z.-Y. Zhang, H.-M. Xiong and S.-N. Yu, Nanotechnology, 2013, 24, 055706 CrossRef.
- R.-Y. Zhang, Z.-Y. Wang, X.-Q. Yang, Y. Xuan, K. Cheng, C. Li, X.-L. Song, J. An, X.-L. Hou and Y.-D. Zhao, Nanotechnology, 2018, 29, 055101 CrossRef.
- M. Cai, C. Ding, X. Cao, F. Wang, C. Zhang and Y. Xian, Anal. Chim. Acta, 2019, 1056, 153–160 CrossRef CAS.
- M. Cai, C. Ding, F. Wang, M. Ye, C. Zhang and Y. Xian, Biosens. Bioelectron., 2019, 137, 148–153 CrossRef CAS.
- Y. Zhang, Y. Zhang, G. Hong, W. He, K. Zhou, K. Yang, F. Li, G. Chen, Z. Liu, H. Dai and Q. Wang, Biomaterials, 2013, 34, 3639–3646 CrossRef CAS.
- Y. Wang and X. P. Yan, Chem. Commun., 2013, 49, 3324–3326 RSC.
- J. Chen, Y. F. Kong, Y. Wo, H. W. Fang, Y. X. Li, T. Zhang, Y. Dong, Y. S. Ge, Z. Y. Wu, D. J. Zhou and S. Y. Chen, J. Mater. Chem. B, 2016, 4, 6271–6278 RSC.
- P. Awasthi, X. An, J. Xiang, N. Kalva, Y. Shen and C. Li, Nanoscale, 2020, 12, 5678–5684 RSC.
- G. C. Chen, F. Tian, Y. Zhang, Y. J. Zhang, C. Y. Li and Q. B. Wang, Adv. Funct. Mater., 2014, 24, 2481–2488 CAS.
- H. Jin, R. J. Gui, J. Gong and W. X. Huang, Biosens. Bioelectron., 2017, 92, 378–384 CAS.
- D. Yan, Y. He, Y. L. Ge and G. W. Song, Sens. Actuators, B, 2017, 240, 863–869 CrossRef CAS.
- F. Hu, C. Li, Y. Zhang, M. Wang, D. Wu and Q. Wang, Nano Res., 2015, 8, 1637–1647 CrossRef CAS.
- M. Hashemkhani, K. Bilici, A. Muti, A. Sennaroglu and H. Y. Acar, New J. Chem., 2020, 44, 5419–5427 RSC.
- T. Yang, Y. a. Tang, L. Liu, X. Lv, Q. Wang, H. Ke, Y. Deng, H. Yang, X. Yang and G. Liu, ACS Nano, 2017, 11, 1848–1857 CrossRef CAS.
- C. Li, X.-Q. Yang, J. An, K. Cheng, X.-L. Hou, X.-S. Zhang, Y.-G. Hu, B. Liu and Y.-D. Zhao, Theranostics, 2020, 10, 867 CrossRef CAS.
- F. D. Duman, Y. Akkoc, G. Demirci, N. Bavili, A. Kiraz, D. Gozuacik and H. Y. Acar, J. Mater. Chem. B, 2019, 7, 7363–7376 RSC.
- J. Javidi, A. Haeri, F. Nowroozi and S. Dadashzadeh, Pharm. Res., 2019, 36, 46 CrossRef.
- Y. Zhang, G. S. Hong, Y. J. Zhang, G. C. Chen, F. Li, H. J. Dai and Q. B. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS.
- F. Liu, W. Ye, J. Wang, F. Song, Y. Cheng and B. Zhang, Int. J. Nanomed., 2017, 12, 5135–5148 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |