Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of Co2FeGe Heusler alloy nanoparticles and catalysis for selective hydrogenation of propyne†
Takayuki Kojima‡§
 *ab,
Yuki Nakayac,
Hyungwon Hamc,
Satoshi Kameoka
*ab,
Yuki Nakayac,
Hyungwon Hamc,
Satoshi Kameoka b and
Shinya Furukawa‡
b and
Shinya Furukawa‡ *c
*c
aFrontier Research Institute for Interdisciplinary Sciences, Tohoku University, Sendai, Japan
bInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Japan
cInstitute for Catalysis, Hokkaido University, Sapporo, Japan. E-mail: furukawa@cat.hokudai.ac.jp
First published on 19th May 2021
Abstract
Although intermetallic compounds are attracting attention of catalysis researchers, ternary intermetallic catalysts have scarcely been investigated due the difficulty of synthesizing supported nanoparticles. In this study, we successfully synthesized SiO2 supported Co2FeGe Heuslar alloy nanoparticles. This catalyst exhibited high catalytic performance for selective hydrogenation of propyne by nano-sizing.
An alloy is a solid mixture of two or more metallic elements. It is typically categorized into a solid solution and an intermetallic compound. In the former, different metal atoms randomly occupy lattice points, and the available range of chemical compositions is wide. In the latter, different metal atoms occupy specific lattice points, forming an ordered structure, and chemical compositions available are restricted to integer ratios. Thus, intermetallic compounds have unique electronic structures and unique atomic ordered surfaces, which are completely different from those of pure metals and solid solutions, resulting in novel catalytic properties.1–6
Along with a recent increasing interest in intermetallic catalysts, many binary compounds have been investigated as catalysts thus far; however, ternary compounds have scarcely been reported as catalysts. The number of possible elemental sets forming intermetallic compounds is much larger in ternary systems than binary ones.7 In addition, novel properties originating from synergy among different elements are more likely in ternary than binary systems; for example, in the La(Co or Ru)Si catalyst for ammonia synthesis, the hydrogen storage ability, the electride property, and the electron transfer from La to the active element (Co or Ru) are believed to play key roles.8,9 Therefore, the discovery of various new catalysts is expected in ternary systems.
Heusler alloys are a group of ternary intermetallic compounds described by X2YZ with L21 structure (body-centered cubic basis) typically consisting of 8–12, 3–8, and 13–15 group elements for X, Y, and Z, respectively. This intermetallic group is popular as magnetic, thermoelectric, shape memory and topological materials while we have opened its new function as catalysts.10–14 For selective hydrogenation of alkynes, Co2FeGe Heusler alloy showed intrinsically high alkene selectivity; that is, it selectively hydrogenated alkynes but hardly hydrogenated alkenes even for hydrogenation of alkene reactants without alkynes.11 In addition, the systematic control of catalytic properties by elemental substitution (Co2MnxFe1−xGayGe1−y) was demonstrated. However, these catalysts were unsupported micron-sized powders with low surface areas (<0.1 m2 g−1) synthesized by metallurgical process (arc melting, annealing, crushing), which were far from being of practical use. Thus, synthesis of Co2FeGe nanoparticles on solid supports, the standard form of catalysts assuring high activity per material cost, is desired.
To synthesize supported intermetallic nanoparticles, much effort is required to optimize the synthesis conditions, especially in ternary systems. For Heusler alloys, supported nanoparticles with sufficient quality (small average size with sharp distribution of sizes, small second phases, ordering into L21 structure) have been reported only for Co2FeGa15–18 and Cu2NiSn,19 the former of which was not for catalysts but mainly for magnetic materials. Thus, synthesizing supported Co2FeGe nanoparticles with excellent catalytic properties for selective hydrogenation of alkynes is challenging. Nevertheless, we have achieved the synthesis of a variety of supported intermetallic nanoparticles,4,6 including those using three elements; for example, Pt3Fe1−xMx (M = Co, Ni, Cu, Zn, Ga, In, Sn, Pb)20 and PtGa with deposition of Pb, In, or Sn.21
In what follows, we report the synthesis of SiO2 supported Co2FeGe nanoparticles and its catalytic properties for selective hydrogenation of propyne (C3H4). The Co-based catalysts were prepared by the pore-filling (co-)impregnation method using SiO2 as the support. Co(NO3)3·6H2O (Wako, 98%), Fe(NO3)3·9H2O (Sigma-Aldrich, 98%), (NH4)2GeF6 (Aldrich, 99.9%) were used as the metal precursors, and the Co loading was adjusted at 3 wt%. The metal precursors were precisely weighed and dissolved together in deionized water so that the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge atomic ratio was 1.8
Ge atomic ratio was 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A mixed aqueous solution of metal precursors was added dropwise to ground dried silica gel (CARiACT G-6, Fuji Silysia, SBET = 673 m2 g−1) so that the solutions just filled the pores of the silica gel (volume of solution: 1.6 mL per gram of silica). The mixtures were sealed with a piece of plastic film and kept overnight at room temperature, followed by freeze-drying under vacuum at 0 °C and further drying overnight in an oven at 90 °C. The resulting powder was calcined in air at 500 °C for 1 h, then reduced under flowing H2 (0.1 MPa, 50 mL min−1) at 800 °C for 1 h.
1. A mixed aqueous solution of metal precursors was added dropwise to ground dried silica gel (CARiACT G-6, Fuji Silysia, SBET = 673 m2 g−1) so that the solutions just filled the pores of the silica gel (volume of solution: 1.6 mL per gram of silica). The mixtures were sealed with a piece of plastic film and kept overnight at room temperature, followed by freeze-drying under vacuum at 0 °C and further drying overnight in an oven at 90 °C. The resulting powder was calcined in air at 500 °C for 1 h, then reduced under flowing H2 (0.1 MPa, 50 mL min−1) at 800 °C for 1 h.
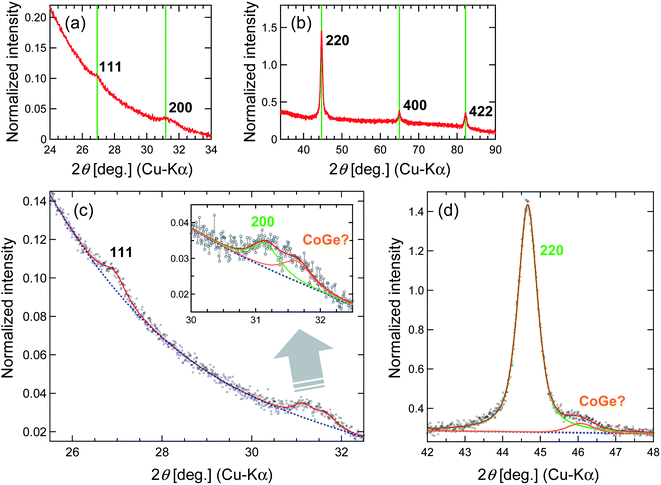
Fig. 1 shows the powder X-ray diffraction (XRD) (Rigaku Ultima IV) pattern for the synthesized Co2FeGe/SiO2. Although tiny peaks of another phase (possibly CoGe) were detected by peak fitting, as shown in Fig. 1c and d, all visible peaks in Fig. 1a and b are assigned to Co2FeGe Heusler phase (L21 structure). The peaks were broad, indicating the formation of nano-sized grains. The volume-weighted average grain size (dXRD) was roughly estimated to be 13 nm from the full width at half maximum (FWHM) of the 220 peak using the Scherrer equation:
dXRD = Kλ/W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
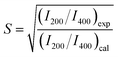 | (2) |
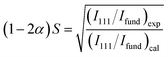 | (3) |
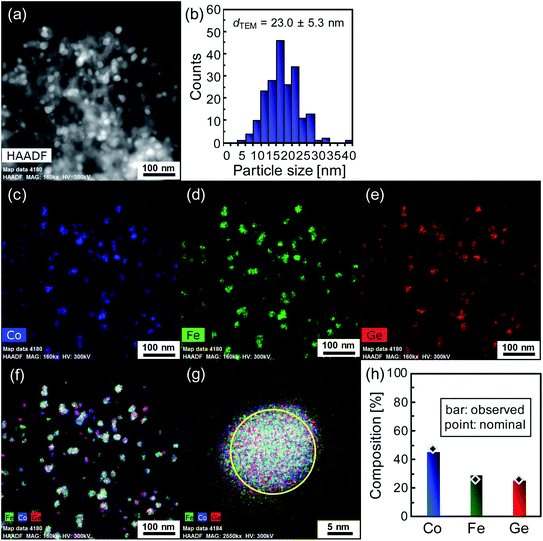
Fig. 2a shows the image obtained by high-resolution high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) (FEI Titan G2). Brighter particles with relatively uniform diameters below about 30 nm are observed on darker skeletal matter, which indicates that Co2FeGe nanoparticles are relatively homogeneously distributed on SiO2 supports. The diameters of the brighter particles were counted, as shown in Fig. 2b. The size distribution was relatively narrow. The volume-weighted average diameter (dTEM) was estimated to be 23.0 ± 5.3 nm by
| dTEM = Σnidi4/Σnidi3 | (4) |
The Co2FeGe/SiO2 was tested for catalytic reaction of the C3H4 hydrogenation using a standard flow reactor (see ref. 11 for details). Thirty mg of the catalyst was heated under H2 gas flow at 800 °C for 1 h to remove surface oxides; then, feeding of a gaseous mixture of [0.1% C3H4/40% H2/He balance] began at ambient temperature and pressure at 30 mL min−1 (20 °C, 0.1 MPa) (space velocity: about 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1). The products were analyzed by gas chromatography (Agilent 490 Micro GC with PoraPLOT Q column) after waiting 30 min at each temperature. The conversion of C3H4 and the selectivity of products were estimated by
000 h−1). The products were analyzed by gas chromatography (Agilent 490 Micro GC with PoraPLOT Q column) after waiting 30 min at each temperature. The conversion of C3H4 and the selectivity of products were estimated by
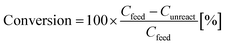 | (5) |
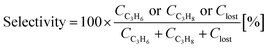 | (6) |
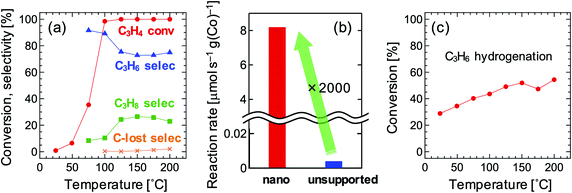
Fig. 3a shows the results of the catalytic test. The carbon lost was negligible. The C3H6 selectivity was as high as over 70% even when the C3H4 conversion was 100%. In general, strong adsorption of C3H4 prevents re-adsorption of C3H6, which suppresses the further hydrogenation of C3H6, resulting in high C3H6 selectivity when the C3H4 conversion is below 100%. Once all C3H4 is consumed, C3H6 is quickly hydrogenated; thus, the C3H6 selectivity drastically decreases once the C3H4 conversion achieves 100% in most catalysts, including pure metals29,30 and Co2FeGa11 (Fig. S1a and b†). Therefore, the Co2FeGe/SiO2 synthesized here has an intrinsic selectivity for C3H6 as well as the unsupported Co2FeGe powders synthesized metallurgically, the C3H6 selectivity of which was over 90% even when the C3H4 conversion was 100% (Fig. S2†).11 The reaction rate per weight of Co used was significantly enhanced up to as much as 2000 times by nano-sizing compared with the unsupported one, as shown in Fig. 3b. In terms of stability, a small deactivation was observed in the cooling process after heating up to 200 °C (Fig. S3†), likely due to oligomerization or coking, while the C3H6 selectivity was improved over 90%.
To reveal the reason for the lower C3H6 selectivity of the Co2FeGe/SiO2 than that of the unsupported Co2FeGe, the catalytic test for C3H6 hydrogenation was conducted in the same manner as the C3H4 hydrogenation as shown in Fig. 3c. A certain amount of C3H6 was converted to C3H8, whereas it was scarcely converted by the unsupported one.11 This means the presence of the sites that further hydrogenate C3H6 in the C3H4 hydrogenation. For ordinary catalysts, the conversion is larger for the C3H6 hydrogenation than C3H4 hydrogenation at a lower temperature region in these reaction conditions11 (Fig. S1†). The larger conversion of C3H6 than C3H4 at ≤75 °C for the Co2FeGe/SiO2 (Fig. 3a and c) thus also indicates the presence of non-selective sites for the C3H4 hydrogenation. An anomaly at 175 °C in Fig. 3c is likely a result of conflict between the acceleration of reaction and the deceleration of C3H6 adsorption along with increasing temperature, which is often observed.11
Taking into account the origin of the high alkene selectivity that inactive Ge atoms shrink the size of active ensembles and thereby prevent the re-adsorption of alkene molecules, which is indicated from electronic structures,11 two candidates are considered for the non-selective sites. One is a monometallic Co ensemble. In this impregnation synthesis, a part of the particles likely have chemical compositions that deviate from the target value. In particles with excess Co, monometallic Co ensembles should form, which is active for hydrogenation but not selective, as indicated by the tests using Co/SiO2 (Fig. S1a and c†). Actually, Co2FeGe/SiO2 catalysts preliminary prepared with the atomic ratio of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe:
Fe:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge = 2
Ge = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 loaded exhibited a poor selectivity (Fig. S4a†) in contrast to the present catalyst (loaded Co
1 loaded exhibited a poor selectivity (Fig. S4a†) in contrast to the present catalyst (loaded Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge = 1.8
Ge = 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the former showed an extra peak in the temperature programmed CO desorption profile as well as the pure Co in addition to the peaks for the unsupported Co2FeGe (Fig. S4b†).31 The other candidate for non-selective sites is a specific site formed by nano-sizing, such as the corner and the edge. These low-coordinated sites are generally active but in different environments from the terrace sites; thus, they can be non-selective. A tiny amount of the second phase CoGe is unlikely as the candidate because a high ethylene selectivity in selective hydrogenation of acetylene by CoGe has been reported.32
1), and the former showed an extra peak in the temperature programmed CO desorption profile as well as the pure Co in addition to the peaks for the unsupported Co2FeGe (Fig. S4b†).31 The other candidate for non-selective sites is a specific site formed by nano-sizing, such as the corner and the edge. These low-coordinated sites are generally active but in different environments from the terrace sites; thus, they can be non-selective. A tiny amount of the second phase CoGe is unlikely as the candidate because a high ethylene selectivity in selective hydrogenation of acetylene by CoGe has been reported.32
Although it cannot be concluded whether the Co ensembles or the specific sites dominated the reduction of selectivity, the selectivity will increase up to the value for the unsupported one if the non-selective sites are identified and removed. Actually, the C3H6 selectivity was improved along with the deactivation (Fig. S3†), indicating that the non-selective sites were killed by carbon deposition due to oligomerization or coking. Nevertheless, over 70% for C3H6 selectivity at 100% conversion of C3H4 is high enough for the condition under abundant hydrogen (C3H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1
H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400). This high selectivity was also confirmed in the C3H4 hydrogenation in the presence of abundant C3H6 (Fig. S5†). These results indicate that the excellent catalytic properties of intermetallic micro-powders can be conserved in their nanoparticles. The reaction rate of C3H4 per surface area of Co2FeGe was roughly estimated to be 1.2 × 10−7 mol s−1 m−2 at 75 °C by assuming the 23 nm-spheres with density of 8.66 g cm−3 (estimated by XRD for the unsupported one and using atomic weights). The value for the unsupported one was 4.1 × 10−8 mol s−1 m−2 at 75 °C. Although the estimation was very approximate, it can at least be said that the reaction rate did not decrease, or rather, it seems that the reaction rate somewhat increased by nano-sizing. This fact also assures the conservation of intrinsic catalytic properties after nano-sizing, while which increases the surface energy, enhancing the adsorption of reactant species, thereby possibly increasing the reaction rate.
400). This high selectivity was also confirmed in the C3H4 hydrogenation in the presence of abundant C3H6 (Fig. S5†). These results indicate that the excellent catalytic properties of intermetallic micro-powders can be conserved in their nanoparticles. The reaction rate of C3H4 per surface area of Co2FeGe was roughly estimated to be 1.2 × 10−7 mol s−1 m−2 at 75 °C by assuming the 23 nm-spheres with density of 8.66 g cm−3 (estimated by XRD for the unsupported one and using atomic weights). The value for the unsupported one was 4.1 × 10−8 mol s−1 m−2 at 75 °C. Although the estimation was very approximate, it can at least be said that the reaction rate did not decrease, or rather, it seems that the reaction rate somewhat increased by nano-sizing. This fact also assures the conservation of intrinsic catalytic properties after nano-sizing, while which increases the surface energy, enhancing the adsorption of reactant species, thereby possibly increasing the reaction rate.
The catalytic performance is compared with those of other supported intermetallic catalysts reported (Table 1). Since Ni is a typical catalyst for hydrogenation, 3d-transition-metal-based intermetallic catalysts reported for selective hydrogenation of alkynes are mostly Ni-based. Although the performance cannot be exactly compared, because the reported catalysts were tested for selective hydrogenation of acetylene (C2H2) with different reaction conditions, the selectivity of Co2FeGe/SiO2 seems to be at the same level as those of the reported catalysts. By comparing a specific reaction rate per weight of Ni or Co at 100 °C, the activity of Co2FeGe/SiO2 also seems to be at the same level as those of the reported catalysts.
| Catalyst | Ni(Co) wt% | Amount [mg] | C2H2(C3H4) flow rate [mL min−1] | C2H2(C3H4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4(C3H6) C2H4(C3H6)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He(Ar, N2) He(Ar, N2) |
GHSV [mL g−1 h−1] | Conv. [%] | Selec. [%] | Temp. [°C] | Specific rate [mLC2H2(C3H4) min−1 gNi(Co)−1] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ni3Ge/MCM-41 | 3.2 | 16 | 3.9 | 3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.1 17.1 |
107520 | 30 | 85 | 250 | 2340 | 33 |
| NiGa/Mg/Al-LDH | 10 | 50 | 1.2 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106.8 106.8 |
144000 | 94 | 81 | 220 | 226 | 34 |
| 26 | 82 | 100 | 62 | |||||||
| 1.2 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82.8 82.8 |
144000 | 72 | 75 | 186 | 173 | ||||
| 7 | 87 | 93 | 17 | |||||||
| Ni3Ga/MgAl2O4 | 2 | 100 | 0.33 | 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.7 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.3 33.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.67 26.67 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
91 | 77 | 200 | 152 | 35 |
| 9 | 96 | 100 | 15 | |||||||
| Co2FeGe/SiO2 | 3 | 30 | 0.03 | 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0:17.97 0:17.97 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
98 | 89 | 100 | 33 | This work |
| 3 | 60 | 0.03 | 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.97 14.97 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
98 | 77 | 100 | 16 |
Conclusions
We have successfully synthesized the SiO2 supported Co2FeGe nanoparticles by the co-impregnation method. The XRD indicated that the sample was almost a single phase of a highly ordered Heusler structure. The HAADF-STEM with EDX indicated that Co2FeGe nanoparticles with the average size of 23 nm were relatively homogeneously distributed on SiO2 supports. This nano-sizing enhanced the reaction rate per weight for C3H4 hydrogenation by 2000 times compared with the unsupported powders, while conserving high C3H6 selectivity. This study proves that even ternary intermetallic compounds can be downsized into supported nanoparticles with conserving intrinsic catalytic properties. In future, supported nanoparticles of various ternary intermetallic compounds including Heusler alloys would be developed not only as catalysts but also as other functional materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the technical staffs of faculty of engineering, Hokkaido University for HAADF-STEM/EDX analysis. This work was conducted under the Cooperative Research Program of Institute for Catalysis, Hokkaido University (Grant #20B1006 and #21A1004). A part of this work was supported by JSPS KAKENHI grant number JP19H02452, 17H04965, and 20H02517, and the Sumitomo Electric Industries Group CSR Foundation.References
- A. P. Tsai, S. Kameoka and Y. Ishii, J. Phys. Soc. Jpn., 2004, 73, 3270–3273 CrossRef CAS.
- M. Armbrüster, R. Schlögl and Y. Grin, Sci. Technol. Adv. Mater., 2014, 15, 034803 CrossRef PubMed.
- A. P. Tsai, S. Kameoka, K. Nozawa, M. Shimoda and Y. Ishii, Acc. Chem. Res., 2017, 50, 2879–2885 CrossRef CAS PubMed.
- S. Furukawa and T. Komatsu, ACS Catal., 2017, 7, 735–765 CrossRef CAS.
- M. Armbrüster, Sci. Technol. Adv. Mater., 2020, 21, 303–322 CrossRef PubMed.
- S. Furukawa, T. Komatsu and K. Shimizu, J. Mater. Chem. A, 2020, 8, 15620–15645 RSC.
- J. Dshemuchadse and W. Steurer, Acta Crystallogr., 2015, A71, 335–345 CrossRef PubMed.
- Y. Gong, J. Wu, M. Kitano, J. Wang, T. N. Ye, J. Li, Y. Kobayashi, K. Kishida, H. Abe, Y. Niwa, H. Yang, T. Tada and H. Hosono, Nat. Catal., 2018, 1, 178–185 CrossRef CAS.
- J. Wu, J. Li, Y. Gong, M. Kitano, T. Inoshita and H. Hosono, Angew. Chem., Int. Ed., 2019, 58, 825–829 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka and A.-P. Tsai, ACS Omega, 2017, 2, 147–153 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka, S. Fujii, S. Ueda and A.-P. Tsai, Sci. Adv., 2018, 4, eaat6063 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka and A.-P. Tsai, Sci. Technol. Adv. Mater., 2019, 20, 445–455 CrossRef CAS.
- T. Kojima, S. Kameoka and A.-P. Tsai, ACS Omega, 2019, 4, 21666–21674 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka and A.-P. Tsai, KONA Powder Part. J., 2021, 38, 110–121 CrossRef.
- L. Basit, C. Wang, C. A. Jenkins, B. Balke, V. Ksenofontov, G. H. Fecher, C. Felser, E. Mugnaioli, U. Kolb, S. A. Nepijko, G. Schönhense and M. Klimenkov, J. Phys. D: Appl. Phys., 2009, 42, 084018 CrossRef.
- C. Wang, L. Basit, Y. Khalavka, Y. Guo, F. Casper, T. Gasi, V. Ksenofontov, B. Balke, G. H. Fecher, C. Sönnichsen, Y. K. Hwu, J. J. Lee and C. Felser, Chem. Mater., 2010, 22, 6575–6582 CrossRef CAS.
- C. H. Wang, Y. Z. Guo, F. Casper, B. Balke, G. H. Fecher, C. Felser and Y. Hwu, Appl. Phys. Lett., 2010, 97, 103106 CrossRef.
- C. Wang, F. Casper, Y. Guo, T. Gasi, V. Ksenofontov, B. Balke, G. H. Fecher, C. Felser, Y. K. Hwu and J. J. Lee, J. Appl. Phys., 2012, 112, 124314 CrossRef.
- S. Ernst, O. Malter, A. Schuessler, K. Braunsmann, N. Trukhan and U. Mueller, inventor; BASF, SE, assignee, US Pat., US 2019/0358613 A1, 2019 Search PubMed.
- Y. Nakaya, M. Miyazaki, S. Yamazoe, K. Shimizu and S. Furukawa, ACS Catal., 2020, 10, 5163–5172 CrossRef CAS.
- Y. Nakaya, J. Hirayama, S. Yamazoe, K. Shimizu and S. Furukawa, Nat. Commun., 2020, 11, 2838 CrossRef CAS PubMed.
- T. Ida, S. Shimazaki, H. Hibino and H. Toraya, J. Appl. Crystallogr., 2003, 36, 1107–1115 CrossRef CAS.
- S. Sasaki, KEK Report, 1989, 88–14, pp. 1–136, calculated values can be downloaded from http://www.sasakiken.net/indexe.html Search PubMed.
- N. M. Butt and J. Bashir, Acta Crystallogr., Sect. A: Found. Crystallogr., 1988, 44, 396–398 CrossRef.
- International Tables for Crystallography Vol C Mathematical, Physical and Chemical Tables, ed. E. Prince, Kluwer Academic Publishers, 3rd edn, London, 2004, p. 239 Search PubMed.
- T. Kojima, S. Kameoka and A.-P. Tsai, ACS Omega, 2018, 3, 9738 CrossRef CAS PubMed.
- P. J. Webster and K. R. A. Ziebeck, J. Phys. Chem. Solids, 1973, 34, 1647–1654 CrossRef CAS.
- B. E. Warren, X-Ray Diffraction, Dover Publications, New York, 1990, p. 208 Search PubMed.
- N. Yoshida, PhD thesis, Osaka University, Japan, 1971.
- T. Kojima, S. Fujieda, G. Kato, S. Kameoka, S. Suzuki and A. P. Tsai, Mater. Trans., 2017, 58, 776–781 CrossRef CAS.
- T. Kojima, T. Koganezaki, S. Fujii, S. Kameoka and A.-P. Tsai, Catal. Sci. Technol., 2021, 10.1039/D1CY00279A.
- T. Komatsu, M. Fukui and T. Yashima, Stud. Surf. Sci. Catal., 1996, 101, 1095–1104 CrossRef CAS.
- T. Komatsu, T. Kishi and T. Gorai, J. Catal., 2008, 259, 174–182 CrossRef CAS.
- Y. Cao, H. Zhang, S. Ji, Z. Sui, Z. Jiang, D. Wang, F. Zaera, X. Zhou, X. Duan and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 11647–11652 CrossRef CAS PubMed.
- Y. Liu, X. Liu, Q. Feng, D. He, L. Zhang, C. Lian, R. Shen, G. Zhao, Y. Ji, D. Wang, G. Zhou and Y. Li, Adv. Mater., 2016, 28, 4747–4754 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02884g |
| ‡ T. K. and S. F. equally contributed to this work. |
| § Present address: Division of Chemistry and Materials, Faculty of Textile Science and Technology, Shinshu University, Japan. E-mail: E-mail: tkojima@shinshu-u.ac.jp |
| This journal is © The Royal Society of Chemistry 2021 |