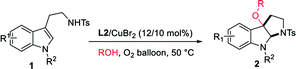Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed aerobic oxidative radical alkoxycyclization of tryptamines to access 3-alkoxypyrroloindolines†
Wei Wang‡
 a,
Jun-Rong Song‡
a,
Jun-Rong Song‡ b,
Zhi-Yao Li
b,
Zhi-Yao Li a,
Ting Zhongb,
Qin Chib,
Hai Ren
a,
Ting Zhongb,
Qin Chib,
Hai Ren *b and
Wei-Dong Pan*ab
*b and
Wei-Dong Pan*ab
aSchool of Pharmaceutical Sciences, Guizhou University, Huaxi Avenue South, Guiyang 550025, P. R. China. E-mail: wdpan@163.com
bState Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, Guiyang 550014, China. E-mail: renh@gzcnp.cn
First published on 19th May 2021
Abstract
We report a copper-catalyzed alkoxycyclization of tryptamine derivatives with O2 as the sole oxidant, leading to a variety of C3a-alkoxypyrroloindolines in good yields with high diastereoselectivities. This reaction involves an interesting double catalytic cycle in which copper-catalyzed carboamination cyclization is favored to form the C-3 radical pyrrolidinoindoline intermediate, then a copper-catalytic radical alkoxylation reaction proceeds smoothly.
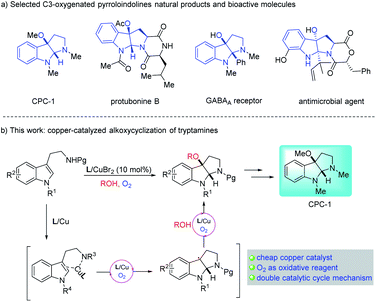
Pyrrolidino[2,3-b]indoline is an important heterocyclic core skeleton that exists in numerous biologically active natural products and pharmaceutical molecules.1 Cyclotryptamine type molecules which are oxygenated at the C3a position are especially outstanding due to their prominent bioactivity profiles,2 various applications in biological probes3 and chiral catalysts.4
As direct access to these complex products, the development of C3a-oxygenation/cyclization reactions of tryptamine or tryptophan derivatives has attracted extensive interest from synthetic chemists. Recently, some remarkable efforts have contributed to the one-step assembly of 3-hydroxyl,5 acetoxyl,6 peroxyl7 and other oxygenated8 pyrroloindolines through oxidative cyclization of tryptophan substrates. However, by utilizing a similar strategy, the direct synthesis of 3-alkoxyl pyrroloindolines remains less developed. In 2020, Zhong et al.9 reported the first example of alkoxycyclization of tryptamine derivatives using molecular iodine catalyst with tert-butyl hydroperoxide as the oxidant. None of the other studies, like using transition-metal catalysts, have been described yet.
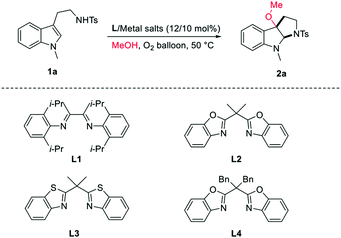
Copper salts, which are inexpensive and easily accessible, have been widely used in organic synthesis as catalysts. Copper(II)-promoted radical intramolecular carboamination of alkene has proven to be an effective means toward the synthesis of N-fused heterocycles.10 Recent reports have utilized this strategy toward the cyclization and radical alkylation, aromatization and aminooxygenation of alkene.10 However, due to the difficulty in homolytic breakage of the oxygen–hydrogen bond in alcohols with a high bond dissociation energy (BDE is ca.105 kcal mol−1),11 the related direct cyclization and radical alkoxylation of carbon–carbon double bond with copper catalysts is still unknown. Inspired by the relevant research of copper-catalyzed radical alkoxylation reaction,12 we assume that if the catalytic carboamination and radical alkoxylation tandem reaction could be realized by a single copper catalyst, which will represent as a new effective protocol for the direct construction of alkoxyl-containing N-fused heterocycles. Herein, we report an oxazoline/copper-catalyzed cascade carboamination alkoxylation of substituted tryptamine under mild eco-friendly O2 oxidation conditions, which facilitate the construction of the 3-alkoxyl pyrroloindolinese motif in good yield with good to excellent levels of diastereoselectivity (Scheme 1).
In our studies, the commercial easily available N-methyl tryptamine 1a was chosen as model substrate. Initially, 10 mol% of metal salt CuBr2 was used as catalyst, the 3-alkoxylation product 2a was obtained as 38% yield with 14/1 dr (Table 1, entry 1). When the diimine ligand L1 was added, only 28% yield of desired product was obtained (Table 1, entry 2). Interestingly, when the bisbenzoxazoline L2/CuBr2 was used, the yield of the reaction was obtained in 45% with 20/1 dr (Table 1, entry 3). The bisbenzothiazoline L3 and dibenzyl-modified bisbenzoxazoline L4 failed to improve the reaction (Table 1, entries 4–5). Attempts to improve the yield by further screening of copper salts were not successful (Table 1, entries 6–10). A better result was obtained by increasing the solvent of methanol resulting in 71% yield with 20/1 dr (Table 1, entry 11). When the reaction was conducted at the air atmosphere, the yield decreased greatly (Table 1, entry 12). Therefore, the employment of L2/CuBr2 (12/10 mol%) in 4 mL MeOH at 50 °C was selected as the optimal conditions for this reaction.
| Entry | Metal salts | Ligand | Yieldb (%) | Drc |
|---|---|---|---|---|
| a Carried out under oxygen atmosphere: metal salt (0.02 mmol, 10 mol%), 1a (0.2 mmol), 2 mL MeOH.b Isolated yields.c dr was determined by 1H NMR.d 4 mL methanol was used.e Air atmosphere; nr: not reaction. | ||||
| 1 | CuBr2 | — | 38 | 14/1 |
| 2 | CuBr2 | L1 | 28 | >20/1 |
| 3 | CuBr2 | L2 | 45 | >20/1 |
| 4 | CuBr2 | L3 | 24 | 13/1 |
| 5 | CuBr2 | L4 | 35 | 8/1 |
| 6 | Cu(OTf)2 | L2 | Trace | — |
| 7 | CuO | L2 | nr | — |
| 8 | Cu(OAc)2 | L2 | nr | — |
| 9 | Cu(ClO4)2 | L2 | nr | — |
| 10 | CuCl2 | L2 | 15 | 8/1 |
| 11d | CuBr2 | L2 | 71 | >20/1 |
| 12d,e | CuBr2 | L2 | 46 | >20/1 |
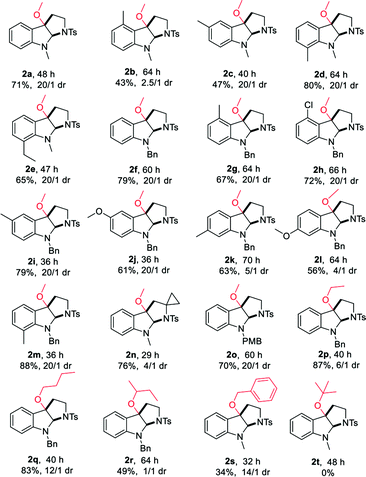
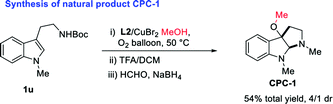
With the optimized reaction conditions in hand, we continued to investigate the substrate scope of the reaction (Table 2). Reactions of N-methyl substituted tryptamines that contain either a methyl or ethyl group at different positions of the indole ring proceeded smoothly to furnish the desired products 2a–e in good to excellent yields with moderate to excellent diastereoselectivities. Notably, the N-Bn and N-PMB substituted tryptamines were also suitable substrates for this reaction, the corresponding products 2f, 2o were obtained in 70, 79% yields with >20/1 dr. N-Bn substituted substrates that contain different functional groups at different positions of the indole ring employed the reaction conditions well, affording the desired products 2g–m in good to excellent yields with high diastereoselectivities. Furthermore, the use of other alcohols, for instance, ethanol, n-butanol, sec-butyl alcohol or benzyl alcohol allowed the cyclic alkoxylation reaction smoothly (2p–s). However, when the steric and bulky tert-butyl alcohol was employed under the optimal conditions, no trace amount of desired product was observed. The applicability of this protocol was further demonstrated by the short, rapid construction of bio-active natural product CPC-1 in a total yield of 54% with 4/1 dr from material 1u (Table 2).
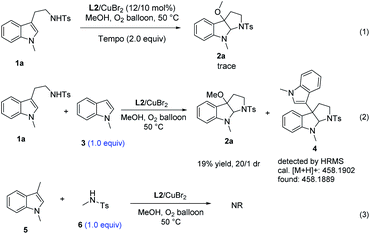
In order to gain insight into the mechanism of the methodology, several control experiments were carried out. As shown in Scheme 2, the radical scavenger, 2,2,6,6-tetramethylpiperidine1-oxyl (TEMPO), inhibited the alkoxycyclization process completely, suggesting that a radical process might be involved in this reaction (Scheme 2: eqn (1)).13 When the nucleophilic substrate 1-methyl indole was involved in the standard conditions (Scheme 2: eqn (2)), trace amount of 3-indole pyrrolidinoindoline adduct 4 was detected by HRMS, suggesting that the exposed carbocation intermediate may be the precursor for the formation of the 3-alkoxylation product. Besides, the amidyl radical addition process has been ruled out by the substrate scope investigation of 1n (Table 2) as the ring opening of cyclopropane moieties did not occur. When 1,3-dimethyl-indole (5) and N-4-dimethylbenzenesulfonamide (6) were involved in the standard conditions, the reaction did not take place (Scheme 2: eqn (3)), which indicated that this reaction proceeded via an intramolecular collaborative tandem process.
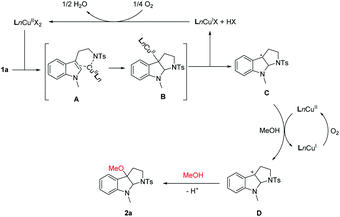
Combining with the previous reports about copper-catalyzed carboamination,10 alkoxylation12 of alkene, a possible reaction pathway is proposed in Scheme 3. Initially, a ligand–exchange reaction of Cu(II) species with substrate 1a proceeds to form the chelation intermediate A. Subsequent nitrogen intramolecular addition–cyclization forms the C3a Cu(II) pyrrolidinoindoline intermediate B, Then, homolytic cleavage of carbon–Cu(II) bond to generate the Cu(I) species and C3a radical intermediate C. The C3a radical could be oxidized by CuII species to generate the C3a cation intermediate D. Subsequent nucleophilic attack of alcohol delivers the product 2a. Meanwhile, CuII complex was produced in situ through the reaction of Ln–CuI complex with O2 on the basis of the previous reports,14 completing the catalytic cycle.
In conclusion, we have successfully developed copper-catalyzed alkoxycyclization of tryptamine under mild O2 oxidation conditions, affording C3a-alkoxylation pyrrolidinoindolines in good yields with high diastereoselectivities. This protocol was proved practicable and useful by the rapid concise total synthesis of natural product CPC-1. Mechanistic studies illustrated that the copper-catalyzed carboamination cyclization was favored to form the C-3 radical pyrrolidinoindoline intermediate, then a copper-catalyzed radical alkoxylation reaction proceeded to deliver the desired product. The extension of the present catalytic protocol to other useful reactions and biological evaluation of these products are undergoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the NSFC (22061010), Guizhou Province Science and Technology Foundation, China (QKHJC[2019]1214), CAS “Light of West China” Program, Guizhou Provincial Thousands of Innovative and Entrepreneurial Talents program (GZQ202006080) for the financial support.Notes and references
- (a) C. R. Jamison, J. J. Badillo, J. M. Lipshultz, R. J. Comito and D. W. C. MacMillan, Nat. Chem., 2017, 9, 1165–1169 CrossRef CAS PubMed; (b) F. L. Cherblanc, K. L. Chapman, J. Reid, A. J. Borg, S. Sundriyal, L. Alcazar-Fuoli, E. Bignell, M. Demetriades, C. J. Schofield, P. A. DiMaggio Jr, R. Brown and M. J. Fuchter, J. Med. Chem., 2013, 56, 8616–8625 CrossRef CAS PubMed; (c) S. Tadano, Y. Sugimachi, M. Sumimoto, S. Tsukamoto and H. Ishikawa, Chem, 2016, 22, 1277–1291 CrossRef CAS; (d) F.-Q. Wang, Q.-Y. Tong, H.-R. Ma, H.-F. Xu, S. Hu, W. Ma, Y.-B. Xue, J.-J. Liu, J.-P. Wang, H.-P. Song, J.-W. Zhang, G. Zhang and Y.-H. Zhang, Sci. Rep., 2015, 5, 9294 CrossRef CAS PubMed.
- (a) J. Han, S.-T. Niu, Y. Liu, L. Gan, T. Wang, C.-D. Lu and T. Yuan, Org. Chem. Front., 2018, 5, 586–589 RSC; (b) A. E. M. Blom, J. Y. Su, L. M. Repka, S. E. Reisman and D. A. Dougherty, ACS Med. Chem. Lett., 2020, 11, 2204–2211 CrossRef CAS PubMed; (c) U. L. Sang, Y. Asami, D. Lee, J. H. Jang, J. S. Ahn and H. Oh, J. Nat. Prod., 2011, 74, 1284–1287 CrossRef PubMed; (d) J. Xu and R. Tong, Green Chem., 2017, 19, 2952–2956 RSC.
- (a) S. Lepthien, M. G. Hoesl, L. Merkel and N. Budisa, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16095–16100 CrossRef CAS PubMed; (b) W. Zhong, J. P. Gallivan, Y. Zhang, L. Li, H. A. Lester and D. A. Dougherty, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 12088–12093 CrossRef CAS PubMed.
- K. Ishihara, M. Fushimi and M. Akakura, Acc. Chem. Res., 2007, 40, 1049–1055 CrossRef CAS PubMed.
- (a) X. Deng, K. Liang, X. Tong, M. Ding, D. Li and C. Xia, Org. Lett., 2014, 16, 3276–3279 CrossRef CAS PubMed; (b) Z.-J. Liu and P.-Q. Huang, J. Org. Chem., 2019, 84, 5627–5634 CrossRef CAS PubMed.
- (a) D. Tu, L. Ma, X. Tong, X. Deng and C. Xia, Org. Lett., 2012, 14, 4830–4833 CrossRef CAS PubMed; (b) M. Z. Wang, T. X. Si, C. F. Ku, H. J. Zhang, Z. M. Li and A. S. Chan, J. Org. Chem., 2018, 84, 831–839 CrossRef PubMed.
- X. Lu, Y. Bai, Y. Li, Y. Shi, L. Li, Y. Wu and F. Zhong, Org. Lett., 2018, 20, 7937–7941 CrossRef CAS PubMed.
- (a) W. Yang, L. Huang, Y. Yu, D. Pflästerer, F. Rominger and A. S. K. Hashmi, Chem.–Eur. J., 2014, 20, 3927–3931 CrossRef CAS PubMed; (b) E. C. Gentry, L. J. Rono, M. E. Hale, R. Matsuura and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 3394–3402 CrossRef CAS PubMed; (c) K. Liang, X. Tong, T. Li, B. Shi, H. Wang, P. Yan and C. Xia, J. Org. Chem., 2018, 83, 10948–10958 CrossRef CAS.
- Y. Li, J. Guo, X. Lu and F. Zhong, Org. Biomol. Chem., 2020, 18, 32–35 RSC.
- Selected examples: (a) E. S. Sherman, P. H. Fuller, D. Kasi and S. R. Chemler, J. Org. Chem., 2007, 72, 3896–3905 CrossRef CAS PubMed; (b) W. Zeng and S. R. Chemler, J. Am. Chem. Soc., 2007, 129, 12948–12949 CrossRef CAS; (c) T. W. Liwosz and S. R. Chemler, J. Am. Chem. Soc., 2012, 134, 2020–2023 CrossRef CAS PubMed; (d) C. Qi, F. Hasenmaile, V. Gandon and D. Lebœuf, ACS Catal, 2018, 8, 1734–1739 CrossRef CAS; (e) P. Shi, J. Wang, Z. Gan, J. Zhang, R. Zeng and Y. Zhao, Chem. Commun., 2019, 55, 10523 RSC; (f) T. Wdowik, S. L. Galster, R. L. L. Carmo and S. R. Chemler, ACS Catal., 2020, 10, 8535–8541 CrossRef CAS.
- (a) K. Jia and Y. Chen, Chem. Commun., 2018, 54, 6105–6112 RSC; (b) X. Wu, M. Wang, L. Huan, D. Wang, J. Wang and C. Zhu, Angew. Chem., Int. Ed., 2018, 57, 1640–1644 CrossRef CAS PubMed; (c) H. Ren, J.-R. Song, Z.-Y. Li and W.-D. Pan, Org. Lett., 2019, 21, 6774–6778 CrossRef CAS PubMed.
- (a) H. Yi, X. Zhang, C. Qin, Z. Liao, J. Liu and A. Lei, Adv. Synth. Catal., 2014, 356, 2873–2877 CrossRef CAS; (b) X. Gao, X. Pan, J. Gao, H. Jiang, G. Yuan and Y. Li, Org. Lett., 2015, 17, 1038–1041 CrossRef CAS PubMed; (c) Z. Liao, H. Yi, Z. Li, C. Fan, X. Zhang, J. Liu, Z. Deng and A. Lei, Chem.–Asian J., 2015, 10, 96–99 CrossRef CAS PubMed; (d) C. Chatalova-Sazepin, Q. Wang, G. M. Sammis and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 5443–5446 CrossRef CAS PubMed.
- M. Wada, T. Murata, H. Oikawa and H. Oguri, Org. Biomol. Chem., 2014, 12, 298–306 RSC.
- (a) A. E. King, L. M. Huffman, A. Casitas, M. Costas, X. Ribas and S. S. Stahl, J. Am. Chem. Soc., 2010, 132, 12068–12073 CrossRef CAS PubMed; (b) C. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2010, 49, 6174–6177 CrossRef CAS PubMed; (c) Q. Li, Y. Huang, T. Chen, Y. Zhou, Q. Xu, S.-F. Yin and L.-B. Han, Org. Lett., 2014, 16, 3672–3675 CrossRef CAS PubMed; (d) I. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown and C. J. Urch, Science, 1996, 274, 2044–2046 CrossRef PubMed; (e) M. Taki, S. Teramae, S. Nagatomo, Y. Tachi, T. Kitagawa, S. Itoh and S. Fukuzumi, J. Am. Chem. Soc., 2002, 124, 6367–6377 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02679h |
| ‡ These authors contributed equally to this works. |
| This journal is © The Royal Society of Chemistry 2021 |