TCNQ as a volatilizable morphology modulator enables enhanced performance in non-fullerene organic solar cells†
Runnan
Yu
 ab,
Huifeng
Yao
ab,
Huifeng
Yao
 *a,
Ling
Hong
a,
Mengyuan
Gao
*a,
Ling
Hong
a,
Mengyuan
Gao
 c,
Long
Ye
c,
Long
Ye
 c and
Jianhui
Hou
c and
Jianhui
Hou
 a
a
aState Key Laboratory of Polymer Physics and Chemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: yaohf@iccas.ac.cn
bBeijing Advanced Innovation Center for Soft Matter Science and Engineering, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China
cSchool of Materials Science and Engineering, Tianjin University, Tianjin 300350, P. R. China
First published on 25th November 2019
Abstract
TCNQ is applied in the non-fullerene-based active layer to fully investigate its effect on the film morphology and photovoltaic performance. Facilitated by the introduction and volatilization of TCNQ after thermal annealing, optimized film morphology with a better phase separation structure is achieved, leading to higher power conversion efficiency.
Organic solar cells (OSCs) based on bulk-heterojunction (BHJ) photoactive layers offer a promising next-generation technology for alternative energy applications and attract substantial attention.1–5 For pursuing higher efficiency in OSCs, tremendous research efforts have been dedicated to the design of polymeric or small molecular donors and various kinds of effective acceptors.6,7 In past decades, due to the unrivaled electron acceptor properties, fullerene derivatives have been the prevailing electron acceptors for BHJ OSCs. However, the intrinsic drawbacks of fullerenes, including weak absorption, poor tunability, and morphological instability, hamper the further improvement of fullerene-based OSCs and thus, researchers have recently focused on the development of non-fullerene (NF) acceptors, which have exhibited excellent properties and potentials.8–14 Besides, multiple strategies have been developed to further improve the photovoltaic performances of BHJ OSCs by manipulating the nano-morphology of active layers, such as introducing functional third components,15–17 applying solvent or solid additives,18–27 utilizing post-treatments and so on.28–31 Inspired by the innovation of materials and in-depth understanding of morphology manipulations, the power, and conversion efficiencies (PCEs) have been constantly boosted for the state-of-the-art devices.32–35
Tetracyanoquinodimethane (TCNQ) with the low-lying lowest unoccupied molecular orbital (LUMO) energy level is well-known as a powerful acceptor with a strong electron affinity. TCNQ and its derivatives have been successfully applied as a dopant to mediate the charge injection and transport process in optoelectronic devices, leading to increased mobility and reduced injection barriers.36–43 In the BHJ OSCs, TCNQ doped Leucocrystalviolet was applied as the electron transport layer in the P3HT:PCBM-based OSCs and the PCE increased by 14% compared to that of the control device with only an Al cathode.44 Besides, ultra-low concentration of TCNQ derivative (F4-TCNQ) was doped into the active layers to enhance the photoconductivity and consequent PCEs in BHJ OSCs.39–41 On the other hand, TCNQ acts as the electron trap in the donor:PCBM-based active layers due to its stronger electron capture ability and the lower LUMO energy level than that of PCBM. When increasing the concentration of TCNQ in the active layers, more free carriers will be trapped, resulting in weakened charge transport capacity and serious recombination losses. Taking advantage of this point, TCNQ was introduced as an electron trap to investigate the recombination mechanism in OSCs.45 However, there is still less research regarding investigating the influence of TCNQ on the nano-morphology in OSCs, specifically for the NF-based OSCs.
In this contribution, TCNQ is applied as a volatilizable additive into the J52:IEICO-based active layer (Fig. 1a) to systematically investigate the effect of TCNQ on the photovoltaic, electrical and morphological properties of NF-based OSCs. After optimizing the devices by regulating the amount of TCNQ and annealing conditions, we find that adding a very small level (0.1 wt%) of TCNQ significantly reduces the photovoltaic performance of as-cast devices, which can be regarded as trap-limited devices. However, the annealed device by introducing a substantial amount of TCNQ (12 wt%) yields an optimal PCE of 8.2%, enhanced by about 26% compared with the annealed device without applying TCNQ. The completely different effect on the photovoltaic performances between the as-cast and annealed devices suggests the volatility of TCNQ, which is further verified by different measurements. Comprehensive optoelectrical and morphological characterizations reveal that the introduction and volatilization of TCNQ improve the intermolecular interaction of NF acceptors and facilitate the formation of better morphology structure, contributing to the charge generation and transport process in the BHJ active layer. This work provides a comprehensive understanding of the role of TCNQ in the morphology modulation of NF-based OSCs.
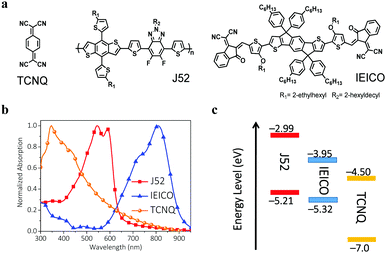
UV-vis absorption spectra of J52 and IEICO films are shown in Fig. 1b. The main absorption of J52 is found at 400–650 nm and that of IEICO is in the spectral range from 600 to 900 nm. It can be seen that the donor and acceptor materials show complementary absorption spectra. Since TCNQ shows strong crystallinity in the film state, we measure the absorption spectra of TCNQ in tetrahydrofuran solution, which shows a broad absorption in the range from 300 to 500 nm with a peak at 350 nm. The energy levels of J52, IEICO and TCNQ are diagrammed in Fig. 1c. The highest occupied molecular orbital (HOMO) and LUMO energy levels of J52 are −5.21 and −2.99 eV, respectively. The LUMO energy level of TCNQ is −4.5 eV, which is much lower than that of the IEICO (−3.95 eV). Besides, photoluminescence (PL) quenching is recorded to infer details about charge transfer between the electron donor and acceptor. As depicted in Fig. S1 (ESI†), the pristine J52 film shows an emission peak centered at 663 nm when excited at 535 nm. Notably, the PL spectrum of J52 with only 12 wt% TCNQ shows more complete quenching of the pristine J52 film when compared with the J52:IEICO blend film, implying that more effective photo-induced charge transfer occurs in the J52:TCNQ film due to the lower LUMO energy level and stronger electron affinity of TCNQ.
To fully understand the effect of TCNQ on the photovoltaic performances of NF-OSCs, J52:IEICO-based devices processed with different contents of TCNQ without thermal annealing were fabricated and evaluated under simulated AM 1.5G irradiation (100 mW cm−2). We used chlorobenzene as the processing solvent and kept the weight ratio of J52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IEICO as 1
IEICO as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
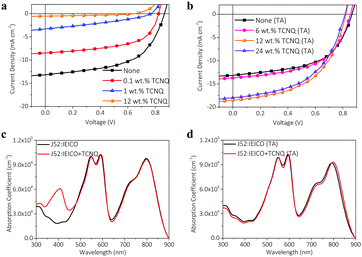
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. Besides, various concentrations of TCNQ dissolved in THF were prepared and added to the active layer blend solutions, respectively, and the film thicknesses of all the active layers were kept around 100 nm. After minutely optimizing the amount of TCNQ, we choose a part of typical devices and summarize the corresponding photovoltaic parameters in Table 1. Fig. 2a exhibits the J−V curves of the devices without thermal annealing and it is obvious to see that the introduction of 0.1 wt% TCNQ to the J52:IEICO blend lowers the PCE of devices from 6.30 to 3.96%. With the increase of TCNQ content, the open-circuit voltage (Voc), short-circuit current density (Jsc) and fill factor (FF) values sharply decrease in the unannealed devices, which can be regarded as trap-limited cells. On this account, the PCE of the unannealed device even drops to 0.01%, when adding 12 wt% TCNQ into the J52:IEICO blend.
1.2. Besides, various concentrations of TCNQ dissolved in THF were prepared and added to the active layer blend solutions, respectively, and the film thicknesses of all the active layers were kept around 100 nm. After minutely optimizing the amount of TCNQ, we choose a part of typical devices and summarize the corresponding photovoltaic parameters in Table 1. Fig. 2a exhibits the J−V curves of the devices without thermal annealing and it is obvious to see that the introduction of 0.1 wt% TCNQ to the J52:IEICO blend lowers the PCE of devices from 6.30 to 3.96%. With the increase of TCNQ content, the open-circuit voltage (Voc), short-circuit current density (Jsc) and fill factor (FF) values sharply decrease in the unannealed devices, which can be regarded as trap-limited cells. On this account, the PCE of the unannealed device even drops to 0.01%, when adding 12 wt% TCNQ into the J52:IEICO blend.
| TCNQ wt% | Thermal annealing | V oc [V] | J sc [mA cm−2] | FF | PCE [%] |
|---|---|---|---|---|---|
| 0 | w/o | 0.866 | 13.18 | 0.552 | 6.30 |
| 0.1 | w/o | 0.823 | 8.47 | 0.568 | 3.96 |
| 1 | w/o | 0.766 | 3.28 | 0.320 | 0.82 |
| 12 | w/o | 0.650 | 0.51 | 0.302 | 0.01 |
| 0 | 150 °C | 0.867 | 14.23 | 0.530 | 6.53 |
| 0.1 | 150 °C | 0.869 | 14.09 | 0.538 | 6.54 |
| 1 | 150 °C | 0.869 | 14.00 | 0.538 | 6.55 |
| 6 | 150 °C | 0.865 | 14.25 | 0.532 | 6.56 |
| 12 | 150 °C | 0.827 | 18.57 | 0.534 | 8.20 |
| 24 | 150 °C | 0.816 | 17.98 | 0.515 | 7.56 |
Then we fabricated the annealed devices processed with different contents of TCNQ under the same preparation conditions and the typical J–V curves are shown in Fig. 2b. Compared with the as-cast device (PCE of 6.30%), the annealed device without adding any TCNQ exhibits a Voc of 0.867 V, a Jsc of 14.23 mA cm−2 and a FF of 0.530, achieving a slightly higher PCE of 6.53%, which is comparable to our previously published results.46 Different from the devices without thermal annealing, the annealed devices with 0.1, 1 and 6 wt% TCNQ achieve comparable PCEs (around 6.5%) with the control devices. More strikingly, after adding 12 wt% TCNQ, the Jsc value of the annealed J52:IEICO-based device remarkably increases from 14.23 to 18.57 mA cm−2, while the Voc of the device slightly decreases to 0.827 V. Accordingly, the annealed device processed with TCNQ yields a best PCE of 8.20%, which is much higher than that of the control device. Meanwhile, this result is higher than those devices processed with solvent additives, such as 1,8-diiodooctane (DIO) and chloronaphthalene (CN) (Table S1, ESI†). Continuously increasing the content of TCNQ, the device still shows an improved PCE of 7.56% for the devices with 24 wt% TCNQ, benefiting from the enhanced Jsc value. The improved current values indicate that introducing certain amounts of TCNQ to the J52:IEICO blend might be favorable for the charge extraction and transport process in the annealed devices. On the whole, adding a small amount of TCNQ has little effect on the device performances, but increasing the amounts of TCNQ leads to a significant enhancement of PCE in the annealed devices. By optimizing the thermal annealing temperatures and times of the TNCQ-processed devices (Table S2, ESI†), the devices annealed at 150 °C for 10 min obtain the best performance, while low temperature or less time annealing results in worse photovoltaic performance.
Apparently, the TCNQ-processed devices with or without thermal annealing demonstrate completely different photovoltaic performances. To explore the underlying reason, we measured the absorption spectra of J52:IEICO blend films treated with different conditions, including as-cast or annealed at 150 °C for 10 min and with or without 12 wt% TCNQ. Fig. 2c exhibits the absorption spectra of J52:IEICO and J52:IEICO + TCNQ blend films without thermal annealing. In comparison with the as-cast J52:IEICO blend film, except for the appearance of the characteristic absorption peak of TCNQ, scarcely any changes occur in the absorption region corresponding to J52 or IEICO for the J52:IEICO + TCNQ blend films (as-cast). By comparing the absorption spectra of two thermally annealed films with or without TCNQ (Fig. 2d), it can be seen that the characteristic absorption peak of TCNQ is completely absent after treatment with thermal annealing, signifying that TCNQ volatilizes from the blend films in the heating process. The absorption overlap from 300 to 500 nm between the two annealed films provides evidence for the complete volatilization of TCNQ after thermal annealing. On the other hand, the maximum absorption wavelength corresponding to IEICO in the J52:IEICO + TCNQ (TA) film red-shifts about 8 nm compared with the J52:IEICO (TA) film, which suggests that much stronger π–π intermolecular interactions occur among the IEICO molecules with the synergistic effect of adding TCNQ and thermal annealing.
Thermogravimetry analysis (TGA) is applied to further verify the volatilization of TCNQ under thermal annealing. First, TCNQ is heated from 30 to 400 °C at a rate of 10 °C min−1 as plotted in Fig. S2a (ESI†). It can be found that TCNQ begins to volatilize from the crucible at about 220 °C and then recrystallizes onto the top of the substrates without any residuals in the crucible (Fig. S3, ESI†), implying the weight loss is caused by volatilization rather than decomposition. According to the device fabrication conditions, we further examine the volatilization process of TCNQ under 200 °C. We carry out another test that the heating rate is kept as 10 °C min−1 and the temperature is held for 1 h at every 25 °C from 100 to 200 °C, respectively. As shown in Fig. S2b (ESI†), a clear weight loss appears at 150 °C, suggesting that TCNQ can volatilize from the active layer under thermal annealing at such temperature. Since the specific surface-area of dispersive TCNQ in the active layer film (about 100 nm) is much larger than in the solid bulks, the volatilization of TCNQ in the device fabrication process is much faster than that in the TGA measurements. More importantly, the poor photovoltaic performance of the unannealed OSC device with a very low content of TCNQ (0.1 wt%) specifically proves that the residuals of TCNQ in the active layer are far less than 0.1 wt% after thermal annealing under 150 °C. As shown in Fig. S4 (ESI†), the absorption overlap between the J52:IEICO blend solution and the dissolved solution of the annealed J52:IEICO + 12 wt% TCNQ film steadily proves the complete volatilization of TCNQ after annealing.
The improved photovoltaic performances encourage us to investigate the detailed electrical and morphological properties in the annealed J52:IEICO-based devices to elucidate the role of TCNQ. Firstly, the external quantum efficiency (EQE) is measured to study the enhancement in Jsc. As shown in Fig. 3a, the TCNQ device shows a broad photoresponse in the range from 300 to 900 nm, which has a similar profile with that of the control device and much higher values. The calculated Jsc values of the control and TCNQ devices are 13.76 and 18.35 mA cm−2, respectively, which are in good agreement with the results achieved from J–V measurements. To study the contribution of exciton dissociation and charge extraction for achieving such a high EQE value, the internal quantum efficiency (IQE) spectrum is calculated based on the reflective absorption spectra and displayed in Fig. S5 (ESI†). The IQE curve of the control device is barely close to 60%, while that of the TCNQ device remains near and even over 80% in the entire range of absorption, indicating that more charge carriers are collected by the electrodes.
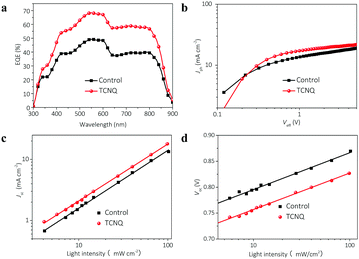 | ||
| Fig. 3 (a) EQE curves and (b) photocurrent versus effective voltage of the control and TCNQ devices. (c) Jsc and (d) Vocversus light intensity for the control and TCNQ devices. | ||
The space-charge limited current method is applied to estimate the charge transport capacities of annealed blend films to reveal the underlying reason for improved photocurrent and overall EQE.47,48 The measured electron mobility of the TCNQ film is 2.4 × 10−4 cm2 V−1 s−1, which is much higher than that of the control film (7.8 × 10−5 cm2 V−1 s−1). However, the corresponding hole mobilities of the two films reveal similar levels of 1.1 × 10−4 cm2 V−1 s−1 for the control film and 1.2 × 10−4 cm2 V−1 s−1 for the TCNQ film (Fig. S6, ESI†). In other words, the application of TCNQ facilitates the charge transport process in the J52:IEICO-based active layer, specifically for electron transport. To further estimate the charge dissociation and collection process, we study the dependence of photocurrent density (Jph) on the effective voltage (Veff) and the exciton dissociation efficiency (Pdiss) in the control and TCNQ devices.49 Here, Jph is defined as the difference between the current densities under illumination and in the dark. Veff is equal to V0 − V and V0 represents the voltage where Jph = 0 and V is the applied voltage. As shown in Fig. 3b, the calculated Pdiss for the TCNQ device is 88%, which is much higher than that of the control device (77%). The enhanced Pdiss implies that a more efficient exciton dissociation and collection process occurs in the TCNQ device.
The free charge carriers suffer from nongeminate recombination dominated by bimolecular or trap-assisted recombination.50,51 We measure Jsc and Voc as a function of light intensity (Plight) to investigate the charge recombination kinetics in such systems. Quantitatively, Jsc and Plight have a power-law dependence with a power-law exponent (S). As illustrated in Fig. 3c, both the control and TCNQ devices show a linear dependence of the current density on the Plight and the control device exhibits an S value of 0.94. Meanwhile, the TCNQ device shows a higher S value of 0.98, implying the existence of extremely low bimolecular recombination, which can be attributed to the enhanced charge transport properties. Fig. 3d shows the Vocversus Plight plots, in which the control and TCNQ devices demonstrate small and similar slopes of 1.19 kBT/q and 1.18 kBT/q, respectively (kB represents the Boltzmann's constant and T is the absolute temperature), implying that the bimolecular-dominated recombination occurs in both of the devices. These results suggest that the application of TCNQ in the J52:IEICO-based device not only benefits to suppress the bimolecular recombination but also introduces no more traps into the blend due to the total volatilization of TCNQ after thermal annealing. Furthermore, it is worth noting that compared with the control device, the TCNQ device shows relatively low Voc values under different light intensities, which may have a close relationship with the morphological change for the active layers.
Then we focus on investigating the structure details of the morphology of films processed with or without TCNQ. Firstly, X-ray diffraction (XRD) measurement is employed to study the effect of TCNQ on the crystallinity of the donor and acceptor. For comparison, the XRD pattern of the pristine TCNQ film is measured. As plotted in Fig. 4, the sharp and intense peaks of TCNQ indicate its high crystallinity. The annealed J52 film exhibits two diffraction peaks at 2-Theta of 4.5° and 25.5°, while the corresponding peaks of the film with 12 wt% TCNQ move slightly and have little change in intensities. The XRD pattern of the annealed IEICO film shows a weak diffraction peak at 2-Theta of 25.6°, corresponding to a d010-spacing value of 3.4 Å. It can be seen that the TCNQ-processed IEICO film exhibits a distinct d100 peak and a much stronger d010 diffraction peak. The introduction and volatilization of TCNQ mainly improve the crystallinity and intermolecular interaction of IEICO molecules and such a feature is beneficial to improve the charge transport properties, which agrees well with the mobility results as mentioned above. In addition, no characteristic diffraction peaks of TCNQ appear in the XRD pattern of the TCNQ-added J52 or IEICO film after annealing, which further verifies the complete volatilization of TCNQ. As shown in Fig. S7 (ESI†), we also investigated the effect of TCNQ on the absorption spectrum and morphology of the donor or acceptor, respectively, which further proves that the application of TCNQ mainly affects the intermolecular interaction and packing morphology of the acceptors.
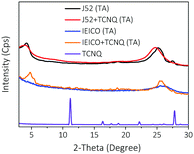 | ||
| Fig. 4 XRD patterns of J52, J52 + TCNQ, IEICO, and IEICO + TCNQ annealed films. The pristine XRD pattern of TCNQ film without thermal annealing is also displayed for comparison. | ||
Theoretical calculations have been applied to investigate the fundamental interaction between TCNQ and IEICO. First, we calculated the Hansen solubility parameters of IEICO and TCNQ according to the group additive method reported previously.52 As listed in Table S3 (ESI†), IEICO and TCNQ exhibit significant differences in polar and hydrogen bonding forces, which have a close relationship with their intermolecular electrostatic attraction. Then we applied density functional theory calculations to study the electrostatic potentials and the proposed assembled geometry structures of IEICO and TCNQ. Due to the large steric hindrance of side groups on the main conjugated chain, the intermolecular packing of IEICO molecules is highly dependent on their end-groups. As shown in Fig. S8 (ESI†), TCNQ has a complementary charge distribution with the end-groups of IEICO, suggesting that they can form a strong intermolecular interaction induced by the electrostatic force.53
Subsequently, we apply atomic force microscopy (AFM) to probe the morphological characteristics of two blend films with or without adding 12 wt% TCNQ and both of the films are thermally annealed at 150 °C for 10 min as the device fabrication conditions. As depicted in Fig. 5a and b, large aggregates and domains can be clearly observed in the J52:IEICO blend films, showing a large root-mean-square roughness (Rq) of 3.65 nm. Meanwhile, the morphology of the as-cast blend film is characterized by microscopy and AFM measurements (Fig. S9, ESI†). As we can see, after adding 12 wt% TCNQ, a large crystalline phase was clearly presented in the as-cast blend film with a large Rq value of 65.9 nm. As for the annealed blend film processed with TCNQ, it exhibits a smooth and uniform surface morphology and much finer domains with a relatively smaller roughness of 2.31 nm, which is desirable for higher exciton dissociation efficiency and Jsc for the TCNQ devices. Meanwhile, large and suitable phase separation can be observed in the transmission electron microscopy (TEM) images of control and TCNQ-processed films, respectively, suggesting the formation of better phase separation structures in TCNQ-processed films, which is consistent with the results obtained from AFM measurements.
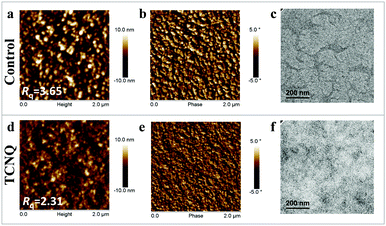 | ||
| Fig. 5 (a and d) AFM height, (b and e) phase images and (c and f) TEM images of J52:IEICO and J52:IEICO + TCNQ blend films, and both the films are treated with thermal annealing. | ||
Conclusions
In conclusion, we have comprehensively studied the effect of TCNQ on the morphology and photovoltaic performance of NF-based OSCs. TCNQ is applied in the J52:IEICO-based NF OSCs and the annealed device with a large amount of TCNQ (12 wt%) shows an enhanced PCE of 8.2%, which is much higher than that of the annealed device without adding TCNQ. However, the TCNQ-processed devices without thermal annealing demonstrate completely different photovoltaic performances, where TCNQ works as a charge trap and seriously reduces the device performance, even if the added content of TCNQ is as little as 0.1 wt%. The distinct photovoltaic performances between the as-cast and annealed devices made us aware of the volatility of TCNQ, which has been validated by the film absorption spectra and the TGA results. By a series of detailed optoelectrical and morphological analyses, we find that the introduction and volatilization of TCNQ facilitated the intermolecular interaction of the NF acceptors and modulation of the film morphology for better phase separation structure, which contributed to the charge generation and transport process in the active layer, ultimately achieving enhanced photovoltaic performances. This work demonstrates a facile application of TCNQ as a morphology modulator for improving the photovoltaic performances of NF-based OSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. H. would like to acknowledge the financial support from the National Natural Science Foundation of China (21835006, 91633301, 51961135103 and 51673201), and the Chinese Academy of Science (XDB12030200). H. Y. acknowledges the financial support from the National Natural Science Foundation of China (21805287) and the Youth Innovation Promotion Association CAS (No. 2018043). This work was supported by Beijing National Laboratory for Molecular Sciences (BNLMS-CXXM-201903). L. Y. thanks the Peiyang Scholar Program of Tianjin University for support.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS
.
- O. Inganas, Adv. Mater., 2018, 30, 1800388 CrossRef PubMed
.
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS
.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS
.
- K. A. Mazzio and C. K. Luscombe, Chem. Soc. Rev., 2015, 44, 78–90 RSC
.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS
.
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS
.
- C. Yan, S. Barlow, Z. Wang, H. Yan, A. K. Y. Jen, S. R. Marder and X. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef CAS
.
- G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447–3507 CrossRef CAS
.
- Y. Lin and X. Zhan, Mater. Horiz., 2014, 1, 470–488 RSC
.
- A. Wadsworth, M. Moser, A. Marks, M. S. Little, N. Gasparini, C. J. Brabec, D. Baran and I. McCulloch, Chem. Soc. Rev., 2018, 48, 1596–1625 RSC
.
- J. Hou, O. Inganäs, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119 CrossRef CAS PubMed
.
- H. Yao, D. Qian, H. Zhang, Y. Qin, B. Xu, Y. Cui, R. Yu, F. Gao and J. Hou, Chin. J. Chem., 2018, 36, 491–494 CrossRef CAS
.
- Y. Xu, H. Yao and J. Hou, Chin. J. Chem., 2019, 37, 207–215 CrossRef CAS
.
- Y. Xie, F. Yang, Y. Li, M. A. Uddin, P. Bi, B. Fan, Y. Cai, X. Hao, H. Y. Woo, W. Li, F. Liu and Y. Sun, Adv. Mater., 2018, 30, 1803045 CrossRef
.
- T. Kumari, S. M. Lee, S.-H. Kang, S. Chen and C. Yang, Energy Environ. Sci., 2017, 10, 258–265 RSC
.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS
.
- R. Yu, H. Yao, L. Hong, Y. Qin, J. Zhu, Y. Cui, S. Li and J. Hou, Nat. Commun., 2018, 9, 4645 CrossRef
.
- R. Yu, H. Yao, Z. Chen, J. Xin, L. Hong, Y. Xu, Y. Zu, W. Ma and J. Hou, Adv. Mater., 2019, 31, 1900477 CrossRef
.
- M. T. Dang and J. D. Wuest, Chem. Soc. Rev., 2013, 42, 9105–9126 RSC
.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619–3623 CrossRef CAS
.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef CAS
.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497–500 CrossRef CAS PubMed
.
- H.-C. Liao, C.-C. Ho, C.-Y. Chang, M.-H. Jao, S. B. Darling and W.-F. Su, Mater. Today, 2013, 16, 326–336 CrossRef CAS
.
- U. Vongsaysy, B. Pavageau, G. Wantz, D. M. Bassani, L. Servant and H. Aziz, Adv. Energy Mater., 2014, 4, 1300752 CrossRef
.
- S. J. Lou, J. M. Szarko, T. Xu, L. P. Yu, T. J. Marks and L. X. Chen, J. Am. Chem. Soc., 2011, 133, 20661–20663 CrossRef CAS
.
- X. Song, N. Gasparini, L. Ye, H. Yao, J. Hou, H. Ade and D. Baran, ACS Energy Lett., 2018, 3, 669–676 CrossRef CAS
.
- Z. Du, W. Chen, Y. Chen, S. Qiao, X. Bao, S. Wen, M. Sun, L. Han and R. Yang, J. Mater. Chem. A, 2014, 2, 15904–15911 RSC
.
- E. Verploegen, R. Mondal, C. J. Bettinger, S. Sok, M. F. Toney and Z. A. Bao, Adv. Funct. Mater., 2010, 20, 3519–3529 CrossRef CAS
.
- H. W. Tang, G. H. Lu, L. G. Li, J. Li, Y. Z. Wang and X. N. Yang, J. Mater. Chem., 2010, 20, 683–688 RSC
.
- L. H. Nguyen, H. Hoppe, T. Erb, S. Günes, G. Gobsch and N. S. Sariciftci, Adv. Funct. Mater., 2007, 17, 1071–1078 CrossRef CAS
.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS
.
- X. Xu, K. Feng, Z. Bi, W. Ma, G. Zhang and Q. Peng, Adv. Mater., 2019, 31, 1901872 CrossRef
.
- B. Fan, D. Zhang, M. Li, W. Zhong, Z. Zeng, L. Ying, F. Huang and Y. Cao, Sci. China: Chem., 2019, 62, 746–752 CrossRef CAS
.
- R. Yu, H. Yao, Y. Cui, L. Hong, C. He and J. Hou, Adv. Mater., 2019, 31, 1902302 CrossRef
.
- C. Liu, Z. Li, Z. Zhang, X. Zhang, L. Shen, W. Guo, L. Zhang, Y. Long and S. Ruan, Phys. Chem. Chem. Phys., 2016, 19, 245–250 RSC
.
- F. Guillain, J. Endres, L. Bourgeois, A. Kahn, L. Vignau and G. Wantz, ACS Appl. Mater. Interfaces, 2016, 8, 9262–9267 CrossRef CAS
.
- X. Han, Z. Wu and B. Sun, Org. Electron., 2013, 14, 1116–1121 CrossRef CAS
.
- H. Yan, J. G. Manion, M. Yuan, F. P. Garcia de Arquer, G. R. McKeown, S. Beaupre, M. Leclerc, E. H. Sargent and D. S. Seferos, Adv. Mater., 2016, 28, 6491–6496 CrossRef CAS
.
- Y. Zhang, H. Zhou, J. Seifter, L. Ying, A. Mikhailovsky, A. J. Heeger, G. C. Bazan and T.-Q. Nguyen, Adv. Mater., 2013, 25, 7038–7044 CrossRef CAS
.
- Y. Xiong, L. Ye, A. Gadisa, Q. Zhang, J. J. Rech, W. You and H. Ade, Adv. Funct. Mater., 2019, 29, 1806262 CrossRef
.
- H. Fan, T. Vergote, S. Xu, S. Chen, C. Yang and X. Zhu, Mater. Chem. Front., 2018, 2, 760–767 RSC
.
- Y. Karpov, T. Erdmann, M. Stamm, U. Lappan, O. Guskova, M. Malanin, I. Raguzin, T. Beryozkina, V. Bakulev, F. Günther, S. Gemming, G. Seifert, M. Hambsch, S. Mannsfeld, B. Voit and A. Kiriy, Macromolecules, 2017, 50, 914–926 CrossRef CAS
.
- M. S. Ryu and J. Jang, Sol. Energy Mater. Sol. Cells, 2011, 95, 1896–1900 CrossRef CAS
.
- M. M. Mandoc, F. B. Kooistra, J. C. Hummelen, B. de Boer and P. W. M. Blom, Appl. Phys. Lett., 2007, 91, 263505 CrossRef
.
- R. Yu, S. Zhang, H. Yao, B. Guo, S. Li, H. Zhang, M. Zhang and J. Hou, Adv. Mater., 2017, 29, 1700437 CrossRef
.
- A. Rose, Phys. Rev., 1955, 97, 1538–1544 CrossRef CAS
.
- P. Murgatro, J. Phys. D: Appl. Phys., 1970, 3, 151–156 CrossRef
.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636–4643 CrossRef CAS PubMed
.
- C. M. Proctor, M. Kuik and T.-Q. Nguyen, Prog. Polym. Sci., 2013, 38, 1941–1960 CrossRef CAS
.
- M. Lenes, M. Morana, C. J. Brabec and P. W. M. Blom, Adv. Funct. Mater., 2009, 19, 1106–1111 CrossRef CAS
.
- D. Leman, M. A. Kelly, S. Ness, S. Engmann, A. Herzing, C. Snyder, H. W. Ro, R. J. Kline, D. M. D. Longchamp and L. J. Richter, Macromolecules, 2015, 48, 383–392 CrossRef CAS
.
- J. H. Dou, Z. A. Yu, J. Zhang, Y. Q. Zheng, Z. F. Yao, Z. Tu, X. Wang, S. Huang, C. Liu, J. Sun, Y. Yi, X. Cao, Y. Gao, J. Y. Wang and J. Pei, J. Am. Chem. Soc., 2019, 141, 6561–6568 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tc04892h |
| This journal is © The Royal Society of Chemistry 2020 |