Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Anion exchange-induced single-molecule dispersion of cobalt porphyrins in a cationic porous organic polymer for enhanced electrochemical CO2 reduction via secondary-coordination sphere interactions
Jia-Kang
Tang
a,
Chen-Yuan
Zhu
a,
Tian-Wen
Jiang
a,
Lei
Wei
b,
Hui
Wang
a,
Ke
Yu
c,
Chun-Lei
Yang
a,
Yue-Biao
Zhang
b,
Chen
Chen
c,
Zhan-Ting
Li
a,
Dan-Wei
Zhang
*a and
Li-Ming
Zhang
*a
aDepartment of Chemistry, Fudan University, Shanghai, 200438, China. E-mail: zhangdw@fudan.edu.cn; zhanglm@fudan.edu.cn
bDepartment of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210, China
cDepartment of Chemistry, Tsinghua University, Beijing, 100084, China
First published on 28th September 2020
Abstract
Correction for ‘Anion exchange-induced single-molecule dispersion of cobalt porphyrins in a cationic porous organic polymer for enhanced electrochemical CO2 reduction via secondary-coordination sphere interactions’ by Jia-Kang Tang et al., J. Mater. Chem. A, 2020, DOI: 10.1039/d0ta07068h.
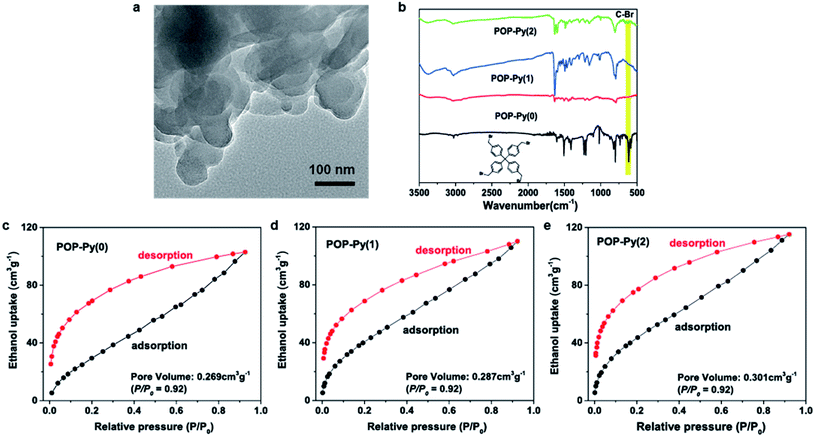
The authors regret an error in Fig. 2a in the published article. In Fig. 2a, the TEM image of POP-Py(0) was presented as the same as that of POP-Py(0)/CoTCPP. The error was due to the same image being repetitively imported in the graphics software in the revised version. The authors confirm that the error has no effect on the conclusions of this paper. Furthermore, the authors state that the raw data of the TEM image shown in Fig. 2 are available from the first author (J. T.) and/or the corresponding authors (D. Z. and L. Z.) upon request. The corrected version of Fig. 2 is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2020 |