Black BiVO4: size tailored synthesis, rich oxygen vacancies, and sodium storage performance†
Abstract
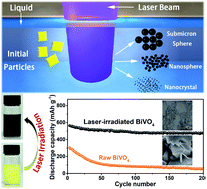
BiVO4 nanoparticles with tailored sizes and rich oxygen vacancies were facilely synthesized via pulsed laser irradiation of colloidal nanoparticles (PLICN). As anode materials for Na-ion batteries, the revolutionized sodium storage process found in sub-10 nm BiVO4 nanocrystals results in outstanding properties including extraordinary rate capability and remarkable cycling stability.
- This article is part of the themed collection: 2020 Journal of Materials Chemistry A most popular articles


 Please wait while we load your content...
Please wait while we load your content...