Temperature-regulated reversible transformation of spinel-to-oxyhydroxide active species for electrocatalytic water oxidation†
Abstract
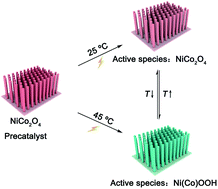
The electrocatalytic oxygen evolution reaction (OER) is a strong electrooxidation process and usually drives the self-oxidation of the anode material. Thus, exploring a facile and efficient strategy to regulate the active species of electrocatalytic materials during the OER process is critical, but it still remains a challenge. Here, the active species of NiCo2O4 for the OER is found to be NiCo2O4 at room temperature, and Ni(Co) oxyhydroxides at 45 °C. In situ Raman and online X-ray absorption spectroscopy data reveal that the reversibly mutual transformation of the active species between NiCo2O4 and oxyhydroxides can be induced by regulating the temperature. Density functional theory calculations suggest that NiOOH has high activity and different rate-limiting steps toward the OER, compared with NiCo2O4. Both the temperature-induced change in the active species and accelerated kinetics lead to the improved activity of NiCo2O4 at 45 °C. This work may provide a new insight into both understanding and regulating the active species of OER materials.


 Please wait while we load your content...
Please wait while we load your content...