Enhanced carrier separation and increased electron density in 2D heavily N-doped ZnIn2S4 for photocatalytic hydrogen production†
Chun
Du
 ,
Bo
Yan
,
Zhaoyong
Lin
,
Bo
Yan
,
Zhaoyong
Lin
 and
Guowei
Yang
and
Guowei
Yang
 *
*
State Key Laboratory of Optoelectronic Materials and Technologies, Nanotechnology Research Center, School of Materials Science & Engineering, Sun Yat-sen University, Guangzhou, 510275, Guangdong, China. E-mail: stsygw@mail.sysu.edu.cn
First published on 3rd December 2019
Abstract
Element doping is an effective approach to modify photocatalysts for boosting solar H2 evolution, especially anion doping. However, there still exists controversy regarding the role of heavy doping in photocatalysis. Herein, 2D heavily N-doped ZnIn2S4 is reported based on an in-depth perspective on the role that N heteroatoms play in improving the photocatalytic properties. The electron dynamics was investigated via femtosecond transient absorption spectroscopy which revealed that the introduction of N doping in ZnIn2S4 provides a strong electron aggregation ability for improving the efficiency of charge separation. Meanwhile, the increased valence band width and the elevated conduction band, respectively, promote the mobility of holes and provide more reductive photogenerated electrons. As a result, 2D heavily N-doped ZnIn2S4 exhibited superior photocatalytic hydrogen production compared to the pristine ZnIn2S4. The optimal hydrogen evolution rate is 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 μmol g−1 h−1 under visible light irradiation. This work contributes to the improvement of photocatalytic performance in 2D ZnIn2S4 and provides an in-depth understanding of heteroatom doped photocatalysts.
086 μmol g−1 h−1 under visible light irradiation. This work contributes to the improvement of photocatalytic performance in 2D ZnIn2S4 and provides an in-depth understanding of heteroatom doped photocatalysts.
Introduction
Photocatalysis is an ideal energy conversion technology which can convert solar energy into clean hydrogen energy through water splitting.1,2 However, most photocatalysts exhibit a low photocatalytic performance due to low solar energy utilization, low carrier density and fast electron–hole recombination, which are far below the requirements of practical applications.3–6 Therefore, it is urgent to optimize photocatalysts to promote solar-to-hydrogen conversion efficiency. Currently, element doping has become the main strategy to modify photocatalysts, especially heteroatom doping.7–11 Normally, doping in photocatalysts has been reported to effectively extend the absorption range of visible light to enhance the photocatalytic performance.12–14 For instance, Ran et al. prepared P-doped g-C3N4 nanosheets with a high visible-light photocatalytic H2 generation activity.15 Lee et al. synthesized ZnS doped with Cu which shows a red shift in the visible region, leading to improved photocatalytic performance.16 Actually, element doping can regulate the electronic structure to promote charge separation and increase electron density, thereby boosting the activity. However, the role of heavy doping in photocatalysis is still ambiguous.17–19 Meanwhile, for heavily heteroatom doped photocatalysts with a slight blue shift in absorbance, the mechanism of activation has hardly been focused on.15Recently, as a layered semiconductor, ZnIn2S4 has aroused great interest due to its fascinating physical and chemical properties.20,21 In particular, hexagonal ZnIn2S4 presents a better photocatalytic performance compared to cubic ZnIn2S4, benefiting from the special electronic structure and optical properties.22,23 More importantly, electrons in hexagonal ZnIn2S4 have a higher migration ability, leading to a remarkable photocatalytic activity.24 Taking these advantages into account, the hexagonal ZnIn2S4 should be a good candidate as a photocatalyst. Nevertheless, the hexagonal ZnIn2S4 has drawbacks, for example, the narrow range of visible light absorption and the relatively rapid recombination of photo-generated electron–hole pairs, which result in low photocatalytic efficiency for practical applications. To overcome the disadvantages mentioned above, cation doping in ZnIn2S4, such as Cu, Ni, and La, has been extensively investigated.25–27 It's known that the introduction of cations can extend the light absorption range of ZnIn2S4 to enhance the performance. However, photo-excited electrons could be trapped by metal centers which could have a negative effect on the photocatalytic activity.3,28 Although anion doping is superior to cation doping, few studies about anion-doped ZnIn2S4 have been reported.
In this contribution, the hexagonal ZnIn2S4 was fabricated into a two-dimensional (2D) photocatalytic material. As is well known, 2D photocatalysts have a shorter diffusion distance for charge carriers than the bulk, which is conducive to the rapid migration of carriers to the surface, further separating the electron–hole pairs.29,30 Then, N heteroatoms were introduced into 2D ZnIn2S4 through a facile solvothermal method. Interestingly, 2D ZnIn2S4 with N shows a slight blue shift of the light absorption edge owing to the Moss–Burstein effect of heavy doping.31 This phenomenon may reduce the photocatalytic performance. However, holes can be trapped by the N dopant to suppress the recombination of photo-generated carriers. The analysis by femtosecond transient absorption spectroscopy reveals the charge carrier dynamics in the photocatalytic process. In addition, N doping can not only increase the carrier density to lead to more charge carriers participating in the reaction, but also reduce the work function, enabling electrons to escape from the surface. These results were supported by theoretical calculations. Consequently, 2D N-doped ZnIn2S4 exhibits an excellent photocatalytic performance, reaching an optimum as high as 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 μmol g−1 h−1 which is nearly 13.8-fold higher compared to that of the pristine ZnIn2S4. This work reports advances in 2D anion-doped ZnIn2S4 and reveals the possibility that heavy heteroatom doping leading to a blue shift in absorbance can improve the photocatalytic activity.
086 μmol g−1 h−1 which is nearly 13.8-fold higher compared to that of the pristine ZnIn2S4. This work reports advances in 2D anion-doped ZnIn2S4 and reveals the possibility that heavy heteroatom doping leading to a blue shift in absorbance can improve the photocatalytic activity.
Experimental section
Preparation of photocatalysts
All the products were synthesized by a one-step solvothermal method.29 In detail, 0.4 mmol Zn(CH3COO)2·2H2O, 0.8 mmol InCl3 and 1.6 mmol thioacetamide were dissolved in a 30 ml aqueous solution containing different dosages of N,N-dimethylformamide (DMF) under stirring for 0.5 h. Then, the solution was transferred into a 50 ml Teflon-lined autoclave and maintained at 180 °C for 24 h. After this, the products were obtained by washing several times and drying at 80 °C in air. According to the different dosages of DMF (0, 1, 6, 12, 15, and 18 ml) used in the synthesis, the corresponding samples are denoted as ZIS, NZIS-1, NZIS-2, NZIS-3, NZIS-4, and NZIS-5, respectively.Characterization
The phases of the samples were studied using a Rigaku D/Max-IIIA X-ray diffractometer using Cu Kα radiation (λ = 1.5406 Å, 40 kV, 20 mA) with a scanning speed of 5° min−1. The binding energies of Zn, In, S, and N atoms and the valence band (VB) were measured using an X-ray photoelectron spectrometer (XPS, Thermo Fisher Scientific, ESCALAB 250). The work functions of the as-prepared samples were investigated by UPS on the same device with a He I line (21.22 eV). The detailed microstructures and crystallographic structure were observed by transmission electron microscopy (TEM), selected area electron diffraction (SAED) and elemental mapping using a Spherical Aberration Corrected Transmission Electron Microscope (JEM-ARM200P). The thickness profile was obtained on a Bruker Multimode 8 atomic force microscope (AFM). UV-visible diffuse reflectance spectra were acquired by using a Shimadzu UV-3600 spectrophotometer. Elemental analysis (EA) was performed on an elemental analyzer (Vario EL, Germany). Photo-luminescence (PL) spectra were obtained on an FLS980 fluorescence lifetime spectrophotometer with a Xe lamp under 470 nm light, and time-resolved photoluminescence (TRPL) spectra were recorded using the same device under the excitation of a He–Cd laser with a wavelength of 472 nm. Electrochemical impedance spectroscopy (EIS) measurements and Mott–Schottky plots were both obtained on a CHI760E electrochemical workstation in Na2SO4 (0.5 M, pH = 6.8) solution with a three-electrode cell using a Pt sheet as the counter electrode and an Ag/AgCl electrode as the reference electrode. Brunauer–Emmett–Teller (BET) specific surface areas were determined using a Micromeritics ASAP 2020M system. The zeta potentials were obtained using a Litesizer nanoparticle size, zeta potential and molecular weight analyzer (EliteSizer).Calculation method
The calculations are performed in the framework of density functional theory (DFT) with the projector augmented plane-wave method, as performed using the Vienna ab initio simulation package (VASP). The generalized gradient approximation (GGA) proposed by Perdew–Burke–Ernzerhof (PBE) is selected for the exchange–correlation potential. The cut-off energy for the plane wave is set to 500 eV with spin polarization included. A unit cell for ZnIn2S4 with 14 atoms was constructed. The Gaussian smearing method was applied with a smearing parameter of 0.01 eV. The Brillouin zone was sampled using a 7 × 7 × 1 k-point mesh for optimization and a 13 × 13 × 1 k-point mesh for DOS calculations. The energy criterion is set to 10−8 eV in an iterative solution of the Kohn–Sham equation. All the structures are relaxed until the residual forces on the atoms decreased to less than 0.01 eV Å−1.Photocatalytic H2 evolution activity
Photocatalytic H2 evolution was tested in a closed gas-circulation system with a Pyrex top-irradiation photo-reactor. In a typical process, 20 mg of the photocatalyst was dispersed using a magnetic stirrer in an aqueous solution (100 ml) containing 10 ml triethanolamine (TEOA) as the electron donor. To eliminate the influence of air, the experiment was performed after degassing pre-treatment for 30 min. Then, the reaction was carried out under visible light irradiation using a 300 W Xe lamp equipped with a 400 nm cut-off filter (80 mW cm−2) for 5 h. The amount of H2 was analyzed using an online gas chromatograph with a thermal conductivity detector (SP7800, N2 carrier, Beijing Keruida Limited). To detect the wavelength dependence of the photocatalyst, the quantum efficiency (QE) for H2 evolution was analyzed with different monochromatic light (365, 420, 500, 580, and 670 nm) wavelengths. The QE can be calculated according to the equation.To evaluate the stability, the photocatalyst after the reactions was reused for cycling tests.
Femtosecond transient absorption (TA) spectroscopy experiment
TA spectra of ZIS and NZIS-3 were measured by femtosecond–nanosecond transient absorption spectrometry with a Helios (Ultrafast Systems LLC) spectrometer and a regeneratively amplified Ti:sapphire laser source (Coherent Legend, 800 nm, 150 fs, 5 mJ per pulse, and 1 kHz repetition rate). The 400 nm pump pulses were delivered by the frequency doubling portion of the 800 nm output (75%) pulse in a BaB2O4 (BBO) crystal, and the white light continuum probe light (420–780 nm) was generated by the remaining part of the 800 nm output into a sapphire window. The white light continuum was divided into probe and reference beams, which were both sent into a fiber optics-coupled multichannel spectrometer with complementary metal-oxide-semiconductor sensors and detected at 1 kHz. Meanwhile, a mechanical chopper was operated at 500 Hz to synchronize the pump repetition. During the measurements, the investigated samples were evenly dispersed in ethylene glycol and then deposited in the sample cell for TA characterization.Results and discussion
Morphology and structure
The structure of the as-prepared samples was determined from their respective XRD patterns. As shown in Fig. 1a, the diffraction peaks of all the samples are ascribed to the hexagonal ZnIn2S4 (JCPDS file no. 72-0773), demonstrating that the as-obtained samples of NZIS still maintain their original crystal structure even though they are formed in DMF solution. However, compared with those of ZIS, the relative intensities of the diffraction peaks of NZIS samples are reduced, indicating that DMF in the reaction could inhibit the growth of crystals. Meanwhile, it can clearly be seen that the peak at 47.1° corresponding to (110) shifted to higher angles. This result suggests that N atoms from DMF might be successfully incorporated into the crystal lattice or substituted for the S of ZnIn2S4 due to the smaller atomic size of N (0.74 Å) than of S (1.04 Å).32,33 | ||
| Fig. 1 (a) XRD patterns of the as-prepared photocatalysts. (b) XPS spectra of ZIS and NZIS-3 and (c) N 1s XPS spectrum of NZIS-3. | ||
To elucidate the surface chemical states of the as-prepared samples and obtain evidence for N doping in ZnIn2S4, ZIS and NZIS-3 as the representative samples were analyzed by XPS. Clearly, the specific peaks related to the N element in ZIS-3 can be observed in the full scan spectrum as compared to those in ZIS except for Zn, In and S peaks, which confirmed the presence of the N element in NZIS excluding the nitrogen adsorbed on the surface (Fig. 1b). Correspondingly, the N 1 s spectrum of NZIS-3 has two peaks at around 399.82 and 401.87 eV, attributed to the Zn–N bonds or In–N bonds and the N species, respectively (Fig. 1c).7,34–37 Meanwhile, the spectra showing the Zn LMM Auger peak and In 3d5/2 peak further confirm the existence of metal–N bonds, as shown in Fig. S1a and b.†38,39 Hence, these results reveal that N could replace S in the form of metal–N in NZIS-3, which may affect the distribution of the electrons around it.35 Furthermore, the spectra of In, Zn and S for ZIS and NZIS-3 are displayed in Fig. S1b–d.† Remarkably, the binding energies of In3d5/2 and In3d3/2 for NZIS-3 are located at 444.67 eV and 452.19 eV, which are smaller than those for ZIS (444.88 eV and 452.44 eV). As for the binding energy of Zn 2p, those for NZIS-3 (1021.70 eV and 1044.78 eV) are 0.4 eV lower than those for ZIS (1022.14 eV and 1045.17 eV). The binding energies of S 2p for NZIS-3 are both shifted to lower energies by 0.3 eV compared to those for ZIS. Due to the difference in the electronegativity of N and S, N could influence the surrounding electrons when it replaces S. Therefore, these similar phenomena of the binding energies of In, Zn and S changing to various extents can be attributed to the doping of N in NZIS. From the above results, it can be concluded that N was successfully doped into ZnIn2S4 by replacing S.
The morphologies of ZIS and NZIS-3 were characterized by TEM. Fig. S2a† shows that ZIS has an ultrathin hexagonal structure and the inset shows the SAED pattern of ZIS with two diffraction rings, which are indexed to the (218) and (1010) planes. And the corresponding elemental mapping images are displayed in Fig. S2b† for the region marked by the red square in Fig. S2a,† demonstrating uniform distribution of the S, In and Zn elements. NZIS-3(Fig. 2a) displays an irregular ultrathin sheet structure, which is different from that of ZIS. Meanwhile, the SAED image in the inset exhibits two slightly fuzzy diffraction rings owing to the low crystallinity, which is in accordance with the results of XRD. As depicted in Fig. 2b, S, In, Zn and N elements can be observed. The distribution of N intensity is the same as that of S, In and Zn. Therefore, it can be assumed that N is doped in the ZnIn2S4 and has uniform distribution. Furthermore, the thickness of ZIS and NZIS-3 was evaluated by AFM as shown in Fig. S2c, d† and 2c, d, indicating that both of them exist as two-layered nanosheets due to the bilayer with a thickness of 2.468 nm although they have different morphologies. From the above analysis, it can be concluded that N has been successfully doped into 2D ZnIn2S4. As for ZnIn2S4, the diffusion length Lex can be estimated using the following equation
 | (1) |
Optical absorption and charge separation
Photocatalytic activity is a complicated process influenced by many factors including the absorption of light and the lifetime of photo-generated electron–hole pairs.42,43Fig. 3a displays the absorbance spectra of the as-prepared samples between 200 and 800 nm. Clearly, N doping in NZIS leads to a slight blue shift in the absorption as compared to that of ZIS and the absorption capacity initially becomes weakened and then enhanced with the increasing amount of DMF in the preparation process. This phenomenon could be attributed to the Moss–Burstein effect.31 To confirm this conjecture, the N amounts of the as-prepared samples were measured by EA (Table S1†). This evidently shows that the N content in NZIS increases gradually and then decreases, which could be ascribed to excess DMF being adsorbed on the surface of the primary nanocrystal which prevents further doping of N.44 Additionally, this variation of the content of the N dopant in NZIS is the same as that of the absorption capacity. This confirms that a slight blue shift for NZIS is related to the N amount. Moreover, the optical band gap (Eg) of the as-prepared samples can be calculated according to the Kubelka–Munk function: αhν2 = A(hν − Eg).45 As shown in Fig. S3,† the Eg of ZIS is about 2.45 eV, which is consistent with the value in previous reports,21,46 whereas NZIS has larger Eg values of approximately 2.53, 2.58, 2.60, 2.58, and 2.55 eV for NZIS-1, NZIS-2, NZIS-3, NZIS-4, and NZIS-5, respectively. | ||
| Fig. 3 (a) UV-vis DRS, (b) PL spectra (c) TRPL decay spectra and (d) EIS Nyquist plots of the as-prepared photocatalysts. | ||
As is well known, efficient carrier separation is crucial to improving the photocatalytic performance. PL emission spectra are used to monitor the behavior of photo-generated electrons and holes. As depicted in Fig. 3b, a strong emission peak around 554 nm is obtained for ZIS, which is attributed to the relatively rapid recombination of electron–hole pairs, whereas NZIS-3 shows the weakest PL peak, indicating that the recombination of photo-induced electron–hole pairs is enormously restrained, implying that more electrons can participate in photocatalytic activities. Obviously, the PL intensity gradually decreased with the increase of the doped N content. This result can be ascribed to the N dopant acting as a hole trap.47,48 Therefore, it can be concluded that N doping in ZnIn2S4 is favorable for suppressing the recombination to prolong the carrier lifetime which may result in a good photocatalytic performance.
In addition, time-resolved transient photoluminescence (TRPL) decay spectra were recorded to examine the lifetime of photo-induced carriers (Fig. 3c). Clearly, the attenuation of the spectra for the NZIS samples is relatively slow with respect to that for ZIS, demonstrating that the carrier lifetime of NZIS is extended. In detail, the bi-exponential model is used to obtain the curves:
| I(t) = A1 exp(−t/τ1) + A2 exp(−t/τ2) | (2) |
The average lifetime (τA) could be evaluated using the equation46
| τA = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (3) |
Theoretical calculation
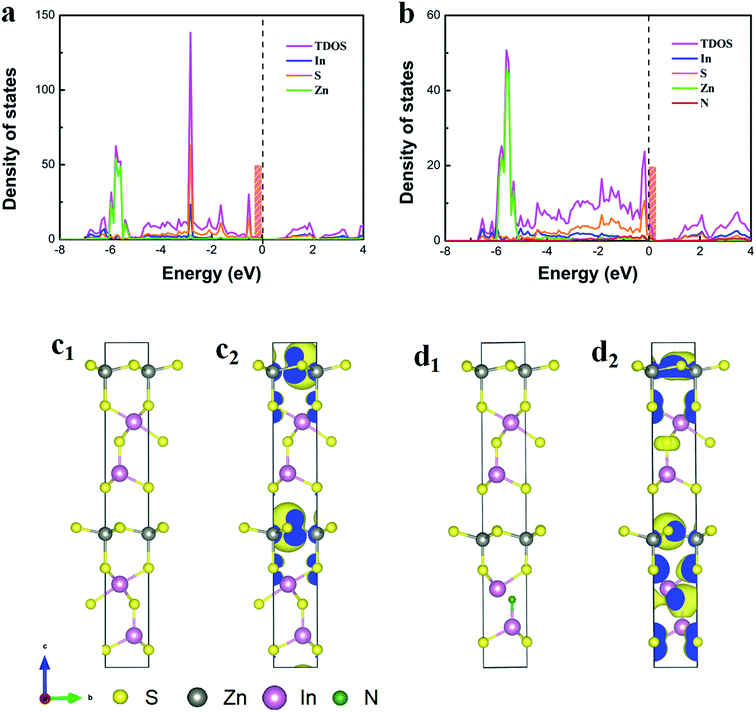
Furthermore, theoretical models of pristine ZnIn2S4 and N-doped ZnIn2S4 were constructed to understand the effect of heavy N doping on the electronic structure of ZnIn2S4. Density functional theory (DFT) calculations were carried out to reveal the electronic properties. The density of states (DOS) for 2D ZnIn2S4 and for that with N doping is, respectively, displayed in Fig. 4a and b. Compared with the pristine ZnIn2S4, N-doped ZnIn2S4 has a slightly wider band gap, which is in accordance with the results of the optical band gap, demonstrating the ideal model. Moreover, it's clear that hybridization of N-doped ZnIn2S4 results in some states passing through the Fermi level, which are considered as the electron acceptor states.49 These states near the valence band maximum (VBM) could endow N-doped ZnIn2S4 with metallic conductive character to inhibit the recombination of photo-excited electrons and holes, which strongly supports the experimental results of PL, TRPL and EIS. In addition, the DOS at the VBM of N-doped ZnIn2S4 is increased via N doping, suggesting that more charge carriers should participate in the photocatalytic activity to enhance the performance. The spatial distribution of charge density around the VBM as revealed in Fig. 4c and d shows that N doping affects the electron contribution to the orbital and increases the electron density at the VBM, which further confirm that more electrons could be easily photo-excited to the conduction band (CB).Photocatalytic activity evaluation
To verify the above results, the photocatalytic activities were analyzed under visible-light irradiation (400–800 nm). It can be found that H2 production of all the samples increased gradually with the extension of irradiation time (Fig. 5a). Among them, ZIS exhibits the lowest H2 evolution within 4 h due to the relatively rapid recombination of photo-induced carriers. However, benefiting from the introduction of the N dopant, the photocatalytic performance of NZIS is greatly improved, especially of NZIS-3. Then, the photocatalytic H2 production rates of all the samples are shown in Fig. 5b. It shows a normal distribution for the H2 evolution rate with the increasing of amount of DMF in the preparation process. The H2 evolution rate reaches an optimum value as high as 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 μmol g−1 h−1 using NZIS-3, which is approximately 13.8-fold higher with respect to the 801 μmol g−1 h−1 achieved using ZIS. Owing to the different N dopant contents, the samples of NZIS exhibit different photocatalytic efficiencies. It should be observed that the photocatalytic rate of NZIS gradually increases when the doped N concentration increases. This indicates that the N dopant is beneficial for enhancing the photocatalytic performance because of the high-efficiency separation of photo-generated electron–hole pairs for prolonging the carrier lifetime. More importantly, the 2D N-doped ZnIn2S4 shows a superior performance compared to the ZnIn2S4 with other element dopants (Table S3†), which further indicates that 2D ZnIn2S4 has been rationally modified by doping with N for favorable performance. Furthermore, the areal H2 evolution rates of all the samples can be calculated according to the results of the BET surface area and the reaction rate, as shown in Fig. S4 and Table S4.† Among them, NZIS-3 exhibits the highest areal H2 evolution rate. This motivates us to improve the performance by more rational modification by increasing the BET surface area on the basis of N doping.50,51 To understand the nature of the light response of photocatalysts, the QE of NZIS-3 was measured using various monochromatic light wavelengths. As illustrated in Fig. 5c, the QE decreases with the increase of wavelength which matches well with the optical absorption spectrum, confirming that photocatalytic H2 evolution is photo-driven. Furthermore, NZIS-3 has been investigated for photocatalytic stability by recycling for 4 cycles. Fig. 5d reveals that there is no dissimilarity in photocatalytic activity, suggesting its good stability. And then the structure of the used NZIS-3 was analyzed using the XRD patterns and XPS spectrum in Fig. 5e and f. Evidently, no changes can be found for the used sample, verifying the sufficient stability of the NZIS photocatalyst. In addition, long-term photocatalysis of NZIS-3 was carried out, as shown in Fig. S5.† It's obvious that the H2 production of NZIS-3 increases almost linearly with time even though a slight inactivation occurred after prolonged illumination, further confirming that the NZIS samples are stable. Meanwhile, the used NZIS-3 was also characterized by XPS to understand its changes with respect to the fresh sample. As plotted in Fig. S6,† there is no change in the positions of two peaks, indicating the durability of samples.
086 μmol g−1 h−1 using NZIS-3, which is approximately 13.8-fold higher with respect to the 801 μmol g−1 h−1 achieved using ZIS. Owing to the different N dopant contents, the samples of NZIS exhibit different photocatalytic efficiencies. It should be observed that the photocatalytic rate of NZIS gradually increases when the doped N concentration increases. This indicates that the N dopant is beneficial for enhancing the photocatalytic performance because of the high-efficiency separation of photo-generated electron–hole pairs for prolonging the carrier lifetime. More importantly, the 2D N-doped ZnIn2S4 shows a superior performance compared to the ZnIn2S4 with other element dopants (Table S3†), which further indicates that 2D ZnIn2S4 has been rationally modified by doping with N for favorable performance. Furthermore, the areal H2 evolution rates of all the samples can be calculated according to the results of the BET surface area and the reaction rate, as shown in Fig. S4 and Table S4.† Among them, NZIS-3 exhibits the highest areal H2 evolution rate. This motivates us to improve the performance by more rational modification by increasing the BET surface area on the basis of N doping.50,51 To understand the nature of the light response of photocatalysts, the QE of NZIS-3 was measured using various monochromatic light wavelengths. As illustrated in Fig. 5c, the QE decreases with the increase of wavelength which matches well with the optical absorption spectrum, confirming that photocatalytic H2 evolution is photo-driven. Furthermore, NZIS-3 has been investigated for photocatalytic stability by recycling for 4 cycles. Fig. 5d reveals that there is no dissimilarity in photocatalytic activity, suggesting its good stability. And then the structure of the used NZIS-3 was analyzed using the XRD patterns and XPS spectrum in Fig. 5e and f. Evidently, no changes can be found for the used sample, verifying the sufficient stability of the NZIS photocatalyst. In addition, long-term photocatalysis of NZIS-3 was carried out, as shown in Fig. S5.† It's obvious that the H2 production of NZIS-3 increases almost linearly with time even though a slight inactivation occurred after prolonged illumination, further confirming that the NZIS samples are stable. Meanwhile, the used NZIS-3 was also characterized by XPS to understand its changes with respect to the fresh sample. As plotted in Fig. S6,† there is no change in the positions of two peaks, indicating the durability of samples.
From the aforementioned analysis, it can be concluded that introduction of N into 2D ZnIn2S4 could promote the separation of the photo-generated carriers, thereby improving the photocatalytic performance. To further understand the charge carrier dynamics in the photocatalytic process, TA spectroscopy was performed to provide a deep insight into the effect of N doping. Fig. 6a and b display the representative TA kinetic traces at 496 nm for ZIS and NZIS-3 using a 400 nm pump pulse. Remarkably, ZIS exhibits a negative ground-state bleaching signal, which can reflect the dynamic behavior of electrons. The attenuation curve can be characterized using two time constants τ1 = 34.4 ps (52.9%) and τ2 = 1280 ps (47.1%), and then the average recovery time τA is estimated to be about 1.243 ns. In contrast, NZIS-3 displays a positive excited state absorption signal due to the accumulation of electrons in the new impurity state by the introduction of N doping.52,53 Similarly, the decay kinetics can be fitted using the bi-exponential model for τ1 = 112 ps (45.7%) and τ2 = 2080 ps (54.3%). Thereby, the average recovery time τA was prolonged to 1.994 ns, which is 1.60 times higher than that of the ZIS, demonstrating that the introduction of N doping in ZIS can effectively separate photo-generated electron–hole pairs. In detail, the slower decay component τ2 should be ascribed to the recombination between electrons from the CB captured by the defect state of N near the VB top and the holes in the VB. Because the defect state of N doping can capture holes, the electrons can transfer from the CB to the surface to eventually decelerate the processes of electron–hole recombination. It is understandable that the carrier lifetime can be extended in the N-doped ZnIn2S4. Meanwhile, the different carrier dynamics of ZIS and NZIS imply that N doping in ZnIn2S4 can alter the electron behavior to promote the separation of photo-generated carriers. Additionally, the appearance of a positive signal demonstrates that the defect state of N in ZnIn2S4 can provide more photoexcited electrons to participate in photocatalytic reactions, which is in good agreement with the theoretical calculations.
In view of the above analysis, N doping in ZnIn2S4 can not only prolong the carrier lifetime which plays an influential role in the photocatalytic activity, but also give rise to more charge carriers in the photocatalytic process, which could boost the photocatalytic performances. Therefore, the charge carrier density was analyzed by Mott–Schottky measurement. As illustrated in Fig. 6c, the slopes of ZIS and NZIS-3 are positive, indicating that they are n-type semiconductors with electrons as the major carriers. The charge carrier density Nd can be calculated from the expression54
| Nd = 2/(eεε0)[d(1/C2)/dV]−1 | (4) |
It is known that the work function is an important parameter to measure the ability of electrons to escape from the Fermi level to a vacuum; therefore, UPS was employed to characterize the work functions of the representative samples ZIS and NZIS-3. Fig. 6d shows that the secondary electron cutoff binding energy Ecutoff of ZIS and NZIS-3 is, respectively, 17.18 and 17.48 eV, which are determined from the intersection of the linear extrapolation and the base line of the UPS spectra.57 And then, the work function (Φ) can be estimated using the formula, Φ = hν − Ecutoff. The Φ of NZIS-3 (3.74 eV) is smaller in contrast to that of ZIS (4.04 eV), indicating that the introduction of N atoms can enable electrons to escape from the surface of ZnIn2S4, resulting in a superior photocatalytic performance. Meanwhile, the zeta potential of NZIS-3 (−33.80 mV) is more negative with respect to that of ZIS (−9.16 mV), which further confirms that the absorption of more protons on the surface by N-doped ZnIn2S4 can result in an enhanced photocatalytic activity.
From the above discussion, the excellent photocatalytic performance of 2D N-doped ZnIn2S4 is related to the enhanced carrier separation, higher electron density and lower work function. Actually, it can be attributed to the essential factor of the regulated band structure due to N doping in ZnIn2S4. As plotted in Fig. 6e, the VB (EVB) potentials of ZIS and NZIS-3 are about 1.50 and 1.11 eV versus the normal hydrogen electrode (NHE), respectively. Therefore, the CB (ECB) potentials of ZIS and NZIS-3 can be calculated to be −0.95 and −1.49 eV according to the values of Eg and EVB. A possible schematic diagram of the photocatalyst is illustrated in Fig. 6f. Clearly, the VB of NZIS-3 upshifts by approximately 0.37 eV compared to that of ZIS, indicating an increase in the VB width due to the N doping.58,59 The increased VB width favors consumption of photo-generated holes owing to the higher mobility of holes, which results in the effective separation of charge carriers. Meanwhile, the elevation of the CB can provide more reductive photoexcited electrons for the photocatalytic reaction.60,61 Moreover, N doping in ZnIn2S4 can not only shift the Fermi energy upward by 0.3 eV, resulting in a higher electron escape ability, but also supply more charge carriers to participate in the photocatalytic process. Consequently, N doping in ZnIn2S4 modulates the electronic structure with favorable features for improving the photocatalytic activity.
Conclusion
2D N-doped ZnIn2S4 was successfully synthesized via a facile solvothermal method. Due to the introduction of the N dopant, the heavily N-doped ZnIn2S4 exhibited a remarkable photocatalytic performance. The modulated electronic structure of ZnIn2S4 not only increases the VB width to promote the mobility of holes for effectively separating the charge carriers, but also elevates the CB to provide more reductive photogenerated electrons and accelerate the transfer of photogenerated electrons for the photocatalytic reaction. In addition, the theoretical calculation further confirms these experimental results. As a result, the photocatalytic H2 generation rate of 2D N-doped ZnIn2S4 reaches 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 μmol g−1 h−1 with visible light illumination, improving by a factor of 13.8 in contrast to that of the pristine ZnIn2S4. These results thus help in boosting the photocatalytic performance of ZnIn2S4 and give an in-depth understanding of the role of the heavily doped photocatalysts.
086 μmol g−1 h−1 with visible light illumination, improving by a factor of 13.8 in contrast to that of the pristine ZnIn2S4. These results thus help in boosting the photocatalytic performance of ZnIn2S4 and give an in-depth understanding of the role of the heavily doped photocatalysts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Basic Research Program of China (2014CB931700) and State Key Laboratory of Optoelectronic Materials and Technologies supported this work.References
- P. W. Du and R. Eisenberg, Energy Environ. Sci., 2012, 5, 6012–6021 RSC.
- S. S. Chen, T. Takata and K. Domen, Nat. Rev. Mater., 2017, 2, 17050 CrossRef CAS.
- W. Wang, M. O. Tade and Z. P. Shao, Prog. Mater. Sci., 2018, 92, 33–63 CrossRef CAS.
- H. X. Zhao, H. T. Yu, X. Quan, S. Chen, H. M. Zhao and H. Wang, RSC Adv., 2014, 4, 624–628 RSC.
- Z. F. Hu, L. Y. Yuan, Z. F. Liu, Z. R. Shen and J. C. Yu, Angew. Chem., Int. Ed., 2016, 55, 9580–9585 CrossRef CAS.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z. X. Guo and J. W. Tang, Energy Environ. Sci., 2015, 8, 731–759 RSC.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS.
- M. S. Akple, J. X. Low, S. Wageh, A. A. Al-Ghamdi, J. G. Yu and J. Zhang, Appl. Surf. Sci., 2015, 358, 196–203 CrossRef CAS.
- Z. Y. Lin, L. H. Li, L. L. Yu, W. J. Li and G. W. Yang, J. Mater. Chem. A, 2017, 5, 5235–5259 RSC.
- C. Y. Yang, Z. Wang, T. Q. Lin, H. Yin, X. J. Lu, D. Y. Wan, T. Xu, C. Zheng, J. H. Lin, F. Q. Huang, X. M. Xie and M. H. Jiangl, J. Am. Chem. Soc., 2013, 135, 17831–17838 CrossRef CAS.
- Z. B. Yu, X. Q. Chen, X. D. Kang, Y. P. Xie, H. Z. Zhu, S. L. Wang, S. Ullah, H. Ma, L. Z. Wang, G. Liu, X. L. Ma and H. M. Cheng, Adv. Mater., 2018, 30, 1706259 CrossRef.
- T. Yang, X. W. Chang, J. H. Chen, K. C. Chou and X. M. Hou, Nanoscale, 2015, 7, 8955–8961 RSC.
- S. C. Sun, P. Gao, Y. R. Yang, P. P. Yang, Y. J. Chen and Y. B. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 18126–18131 CrossRef CAS.
- L. Y. Li, W. Fang, P. Zhang, J. H. Bi, Y. H. He, J. Y. Wang and W. Y. Su, J. Mater. Chem. A, 2016, 4, 12402–12406 RSC.
- J. R. Ran, T. Y. Ma, G. P. Gao, X. W. Du and S. Z. Qiao, Energy Environ. Sci., 2015, 8, 3708–3717 RSC.
- G. J. Lee, S. Anandan, S. J. Masten and J. J. Wu, Renewable Energy, 2016, 89, 18–26 CrossRef CAS.
- A. S. Alshammari, L. N. Chi, X. P. Chen, A. Bagabas, D. Kramer, A. Alromaeh and Z. Jiang, RSC Adv., 2015, 5, 27690–27698 RSC.
- X. H. Wu, W. Qin, X. B. Ding, Y. Wen, H. L. Liu and Z. H. Jiang, J. Phys. Chem. Solids, 2007, 68, 2387–2393 CrossRef CAS.
- J. Thomas, S. Radhika and M. Yoon, J. Mol. Catal. A: Chem., 2016, 411, 146–156 CrossRef CAS.
- Z. B. Lei, W. S. You, M. Y. Liu, G. H. Zhou, T. Takata, M. Hara, K. Domen and C. Li, Chem. Commun., 2003, 2142–2143 RSC.
- Z. Y. Zhang, Y. Z. Huang, K. C. Liu, L. J. Guo, Q. Yuan and B. Dong, Adv. Mater., 2015, 27, 5906–5914 CrossRef CAS.
- Y. J. Chen, S. W. Hu, W. J. Liu, X. Y. Chen, L. Wu, X. X. Wang, P. Liu and Z. H. Li, Dalton Trans., 2011, 40, 2607–2613 RSC.
- J. G. Wang, Y. J. Chen, W. Zhou, G. H. Tian, Y. T. Xiao, H. Y. Fu and H. G. Fu, J. Mater. Chem. A, 2017, 5, 8451–8460 RSC.
- J. S. Chen, F. Xin, X. H. Yin, T. Y. Xiang and Y. W. Wang, RSC Adv., 2015, 5, 3833–3839 RSC.
- S. H. Shen, L. Zhao, Z. H. Zhou and L. J. Guo, J. Phys. Chem. C, 2008, 112, 16148–16155 CrossRef CAS.
- D. W. Jing, M. C. Liu and L. J. Guo, Catal. Lett., 2010, 140, 167–171 CrossRef CAS.
- F. Tian, R. S. Zhu, K. L. Song, F. Ouyang and G. Cao, Int. J. Hydrogen Energy, 2015, 40, 2141–2148 CrossRef CAS.
- Y. Pan, X. Z. Yuan, L. B. Jiang, H. B. Yu, J. Zhang, H. Wang, R. P. Guan and G. M. Zeng, Chem. Eng. J., 2018, 354, 407–431 CrossRef CAS.
- C. Du, Q. Zhang, Z. Y. Lin, B. Yan, C. X. Xia and G. W. Yang, Appl. Catal., B, 2019, 248, 193–201 CrossRef CAS.
- Z. Y. Lin, C. Du, B. Yan, C. X. Wang and G. W. Yang, Nat. Commun., 2018, 9, 4036 CrossRef.
- D. Mocatta, G. Cohen, J. Schattner, O. Millo, E. Rabani and U. Banin, Science, 2011, 332, 77–81 CrossRef CAS.
- M. Zhang, D. D. Lu, Z. H. Zhang and J. J. Yang, J. Electrochem. Soc., 2014, 161, H416–H421 CrossRef CAS.
- P. Barpanda, N. Recham, J. N. Chotard, K. Djellab, W. Walker, M. Armand and J. M. Tarascon, J. Mater. Chem., 2010, 20, 1659–1668 RSC.
- Y. S. Zhou, G. Chen, Y. G. Yu, Y. J. Feng, Y. Zheng, F. He and Z. H. Han, Phys. Chem. Chem. Phys., 2015, 17, 1870–1876 RSC.
- M. Z. Wang, W. J. Xie, H. Hu, Y. Q. Yu, C. Y. Wu, L. Wang and L. B. Luo, Appl. Phys. Lett., 2013, 103, 213111 CrossRef.
- J. M. Bian, X. M. Li, X. D. Gao, W. D. Yu and L. D. Chen, Appl. Phys. Lett., 2004, 84, 541–543 CrossRef CAS.
- T. Nagata, G. Koblmuller, O. Bierwagen, C. S. Gallinat and J. S. Speck, Appl. Phys. Lett., 2009, 95, 132104 CrossRef.
- K. C. Shen, T. Y. Wang, D. S. Wuu and R. H. Horng, Opt. Express, 2012, 20, 15149–15156 CrossRef CAS PubMed.
- B. Yue, Q. Y. Li, H. Iwai, T. Kako and J. H. Ye, Sci. Technol. Adv. Mater., 2011, 12, 034401 CrossRef PubMed.
- Y. P. Yuan, L. W. Ruan, J. Barber, S. C. J. Loo and C. Xue, Energy Environ. Sci., 2014, 7, 3934–3951 RSC.
- T. Jafari, E. Moharreri, A. S. Amin, R. Miao, W. Q. Song and S. L. Suib, Molecules, 2016, 21, 100 CrossRef.
- S. Y. Tee, K. Y. Win, W. S. Teo, L. D. Koh, S. H. Liu, C. P. Teng and M. Y. Han, Adv. Sci., 2017, 4, 1600337 CrossRef PubMed.
- D. Mocatta, G. Cohen, J. Schattner, O. Millo, E. Rabani and U. Banin, Science, 2011, 332, 77–81 CrossRef CAS PubMed.
- C. Du, D. H. Li, Q. Y. He, J. M. Liu, W. Li, G. N. He and Y. Z. Wang, Phys. Chem. Chem. Phys., 2016, 18, 26530–26538 RSC.
- X. L. Tu, J. Lu, M. Li, Y. J. Su, G. L. Yin and D. N. He, Nanoscale, 2018, 10, 4735–4744 RSC.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlop, J. W. J. Hamilton, J. A. Byrne, K. O'Shea, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS.
- C. Du, J. S. Zhou, F. Z. Li, W. Li, Y. Z. Wang and Q. Y. He, Appl. Phys. A: Mater. Sci. Process., 2016, 122, 714 CrossRef.
- J. F. Xie, H. Zhang, S. Li, R. X. Wang, X. Sun, M. Zhou, J. F. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807 CrossRef CAS PubMed.
- M. Dong, P. Zhou, C. J. Jiang, B. Cheng and J. G. Yu, Chem. Phys. Lett., 2017, 668, 1–6 CrossRef CAS.
- Q. Liu, T. X. Chen, Y. R. Guo, Z. G. Zhang and X. M. Fang, Appl. Catal., B, 2016, 193, 248–258 CrossRef CAS.
- Z. L. Xu, C. S. Zhuang, Z. J. Zou, J. Y. Wang, X. C. Xu and T. Y. Peng, Nano Res., 2017, 10, 2193–2209 CrossRef CAS.
- F. C. Lei, L. Zhang, Y. F. Sun, L. Liang, K. T. Liu, J. Q. Xu, Q. Zhang, B. C. Pan, Y. Luo and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 9266–9270 CrossRef CAS PubMed.
- W. L. Yang, L. Zhang, J. F. Xie, X. D. Zhang, Q. H. Liu, T. Yao, S. Q. Wei, Q. Zhang and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 6716–6720 CrossRef CAS PubMed.
- L. D. Li, J. Q. Yan, T. Wang, Z. J. Zhao, J. Zhang, J. L. Gong and N. J. Guan, Nat. Commun., 2015, 6, 5881 CrossRef.
- X. H. Lu, G. M. Wang, S. L. Xie, J. Y. Shi, W. Li, Y. X. Tong and Y. Li, Chem. Commun., 2012, 48, 7717–7719 RSC.
- Z. F. Hu, L. Y. Yuan, Z. F. Liu, Z. R. Shen and J. C. Yu, Angew. Chem., Int. Ed., 2016, 55, 9580–9585 CrossRef CAS.
- Y. L. Gao, Mater. Sci. Eng., R, 2010, 68, 39–87 CrossRef.
- G. Liu, P. Niu, C. H. Sun, S. C. Smith, Z. G. Chen, G. Q. Lu and H. M. Cheng, J. Am. Chem. Soc., 2010, 132, 11642–11648 CrossRef CAS PubMed.
- M. L. Guan, C. Xiao, J. Zhang, S. J. Fan, R. An, Q. M. Cheng, J. F. Xie, M. Zhou, B. J. Ye and Y. Xie, J. Am. Chem. Soc., 2013, 135, 10411–10417 CrossRef CAS PubMed.
- J. Zhang, J. H. Xi and Z. G. Ji, J. Mater. Chem., 2012, 22, 17700–17708 RSC.
- M. Y. Xing, Y. Zhou, C. Y. Dong, L. J. Cai, L. X. Zeng, B. Shen, L. H. Pan, C. C. Dong, Y. Chai, J. L. Zhang and Y. D. Yin, Nano Lett., 2018, 18, 3384–3390 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta11318e |
| This journal is © The Royal Society of Chemistry 2020 |