Polypyrrole nanostructures modified with mono- and bimetallic nanoparticles for photocatalytic H2 generation†
Xiaojiao
Yuan
a,
Diana
Dragoe
b,
Patricia
Beaunier
c,
Daniel Bahena
Uribe
d,
Laurence
Ramos
 e,
Maria Guadalupe
Méndez-Medrano
f and
Hynd
Remita
e,
Maria Guadalupe
Méndez-Medrano
f and
Hynd
Remita
 *a
*a
aLaboratoire de Chimie Physique, UMR 8000 CNRS, Université Paris-Sud, Université Paris-Saclay, 91405 Orsay, France. E-mail: hynd.remita@u-psud.fr
bInstitut de Chimie Moléculaire et des Matériaux d'Orsay, UMR 8182 CNRS, Université Paris-Sud, Université Paris-Saclay, 91405 Orsay, France
cSorbonne Université, CNRS UMR-7197, LRS, 4 Place Jussieu, 75005 Paris, France
dLaboratorio Avanzado de Nanoscopía Electrónica, Centro de Investigación y de Estudios Avanzados Del I. P. N, Av. Instituto Politécnico Nacional 2508, San Pedro Zacatenco, Ciudad de México, C. P, 07360, Mexico
eLaboratoire Charles Coulomb (L2C), Université de Montpellier, UMR 5221 CNRS, 34095 Montpellier, France
fLaboratoire de Physique des Interfaces et des Couches Minces, UMR 7647 CNRS, École Polytechnique, 91120 Palaiseau, France
First published on 25th November 2019
Abstract
Green hydrogen production by photocatalytic water splitting offers a promising way to solve environment and energy issues. Conjugated polymer nanostructures (CPNs), with highly π-conjugated structures, have been demonstrated as a new class of photocatalysts. However, pristine CPNs show poor photocatalytic activity for hydrogen generation owing to fast charge carrier recombination and sluggish kinetics. The investigation of application of polypyrrole in photocatalytic hydrogen generation is also rare. Here, we report the surface modification of polypyrrole nanostructures (NSs) with Pt- and Ni-based nanoparticles for photocatalytic hydrogen evolution. PPy nanostructures (NSs) were synthesized using lamellar mesophases as soft templates. The PPy-NSs were modified with mono- and bimetallic (Pt, Ni, Pt–Ni) co-catalyst nanoparticles induced by radiolysis. The prepared Pt-PPy, Ni-PPy and PtNi-PPy exhibit excellent photocatalytic activity for H2 generation and a synergistic effect is obtained with co-modification with Pt and Ni. The effects of the nature of the metal precursors and the loading ratio were studied. We show that the loading rate of co-catalysts is crucial for H2 generation, and an excess of co-catalyst can drastically decrease the activity. The composite photocatalyst Pt-Ni-PPy nanostructures are very active and stable with cycling. Our results indicate that modified CPNs with mono- and bimetallic NPs are promising photocatalysts for hydrogen evolution.
1. Introduction
Hydrogen is a clean, promising and environmentally friendly energy source to solve the energy crisis and environmental pollution. The production of hydrogen through a photocatalytic water-splitting process (WSP) has attracted increasing attention.1–3 Many studies have focused on inorganic metal oxides (e.g., TiO2,4,5 BiVO4,6 and ZnO7), (oxy)sulfides (e.g., CdS8 and SnS2 (ref. 9)), and (oxy)nitrides (such as InN10 and GaN11), which are often based on metal cations possessing d0 and d10 electronic configurations, but generally confined by the relatively limited properties such as large band gap, photocorrosion and self-oxidation.12,13Recently, conjugated polymers (CPs) have been attracting increasing attention for photocatalysis application owing to their unique delocalized conjugated system, high carrier mobility, narrow and tunable band gaps, and efficient absorption of UV-Vis and/or near-infrared light. We have demonstrated that conjugated polymer nanostructures exhibit high photocatalytic activity under ultraviolet and visible light irradiation, and that nanostructuration is a key factor for their photocatalytic application.14–18 These conjugated polymer nanostructures were found to be more active than plasmonic TiO2 under visible light for water treatment and emerge as a new class of photocatalysts.19 However, the photocatalytic hydrogen evolution activity of CPs is weakened by their fast photogenerated electron–hole pair recombination and low catalysis kinetics. On the other hand, CPs are also excellent conductive supports for stabilizing co-catalysts to enhance their properties or extend their functions.20–24 Incorporation of metals into semiconductors has been demonstrated to be a promising way to promote charge carrier separation and increase the active sites as well.25
Noble metals with a low Fermi level can trap electrons from the conduction band (CB) of a semiconductor. Among these noble metals, Pt is a very efficient electron scavenger and is the best candidate as a co-catalyst for hydrogen production due to its largest work function.26 Ni is also a very promising candidate as a co-catalyst for H2 production owing to its low-cost and high activity.27–29 Ni nanoparticles not only provide a large surface area and active sites, but also decrease the electron–hole recombination.27,28
Because of the high cost and scarcity of platinum, its replacement with low cost and abundant catalysts (or co-catalysts) is desirable, but remains a great challenge.30 Many studies focus on decreasing the loading of this precious metal by alloying it with another metal of lower cost to increase the photocatalytic activity of the modified photocatalyst for hydrogen evolution. Indeed formation of alloys can reduce the metal–metal interatomic distance, induce electronic effects, greatly increase the number of active sites and accelerate the mass transfer between the different active sites.31 For example, strategies using Pd–Pt,32,33 Ni–Pt,34,35 Ni–Pd,27 Ni–Au28 or multi-metallic30 heterojunctions have been investigated.
Here we report for the first time that modified conjugated polymer polypyrrole nanostructures with mono (Pt, Ni) and bimetallic (Pt–Ni) nanoparticles (NPs) are very active for hydrogen generation, and that a synergistic effect is obtained by alloying Pt with Ni. Polypyrrole (PPy) nanostructures were synthesized in soft templates formed by lamellar mesophases. Metal nanoparticles (Pt, Ni, and Pt–Ni nanoalloys) of homogeneous size and distribution induced by radiolysis were supported on PPy NPs. Radiolysis is an efficient technique to synthesize metal nanoparticles (NPs) and especially bimetallic NPs of controlled size, distribution and structure in solutions, heterogeneous media or on supports.36,37 The photocatalytic activity of the composite nanomaterials with various metal loadings was investigated for hydrogen generation under UV-visible light irradiation. The obtained composite NSs show excellent photocatalytic performance for hydrogen generation due to the extended absorption region of PPy NSs, efficient electron–hole pair separation due to nanosized Pt, Ni and Pt–Ni particles and a synergistic effect obtained by alloying Pt with Ni.
2. Experimental section
2.1. Chemical reagents
Pyrrole (Py, C4H5N, 98%), as a monomer, iron chloride (FeCl3) as an oxidizing agent, sodium dodecyl sulfate (SDS) as a surfactant, sodium chloride (NaCl), cyclohexane (C6H12, 99.7%), and n-pentanol (C5H12O, ≥99%) as a co-surfactant were used to synthesize PPy NSs. Platinum(II)acetylacetonate (Pt (C5H7O2)2, ≥99.98%) and nickel(II)acetylacetonate (C10H14NiO4, 95%) were used as platinum and nickel precursors, respectively. All the reagents and methanol (CH3OH, 99.9%) were purchased from Sigma-Aldrich, and ethanol (CH3CH3OH, ≥99%) was purchased from VWR International. Deionized water (Milli-Q, 18.6 MΩ) was used throughout all experiments. All the reagents were pure and used without further purification.2.2. Materials preparation
2.3. Characterization
Small Angle X-ray Scattering (SAXS) was used to characterize the mesophases before and after polymerization. The mesophases doped with only Py, only FeCl3 and containing PPy (obtained after oxidation of Py with FeCl3) were put in glass capillaries (diameter = 1.5 mm), and a high brightness X-ray tube with low power and an aspheric multilayer optic (GeniX 3D from Xenocs) were employed to deliver an ultralow divergent beam (0.5 mrad). A two-dimensional Schneider 2D image plate detector prototype was used to collect the scattering intensity.Mott–Schottky (MS) plot measurement was carried out by using a typical three electrode setup (Pt wire and Ag/AgCl as counter and reference electrodes, respectively). A PPy film on FTO was used as the working electrode. The MS spectra were measured in the voltage window of 0.2 V–1.0 V in the dark (increment: 20 mV, frequency: 1 kHz). An aqueous solution of 1 M NaSO4 was used as the electrolyte.
UV-Vis absorption spectra were recorded with an HP 8453 spectrophotometer
The size, dispersion and morphology of the conducting polymer nanostructures and metal nanoparticles were examined by scanning electron microscopy (SEM, ZEISS Supra 55 V P FEG-SEM) and transmission electron microscopy (TEM, JEOL JEM 2010 UHR operating at 200 kV). The chemical analyses were obtained using an Energy-Dispersive X-ray Spectroscopy (EDS) microanalyzer (PGT-IMIX PC) mounted onto the microscope. High angle annular dark field images were obtained using scanning transmission electron microscopy with a Cs corrected JEOL-ARM-200F at 200 kV.
XPS measurements were performed on a K alpha spectrometer from ThermoFisher, equipped with a monochromatic X-ray source (Al Kα, 1486.6 eV) with a spot size of 400 μm. The hemispherical analyzer was operated in CAE (Constant Analyser Energy) mode, with a pass energy of 200 eV and a step of 1 eV for the acquisition of survey spectra, while for the acquisition of narrow scans, pass energies of 50 eV and 100 eV and a step of 0.1 eV were used. The charge build-up was neutralized by means of a “dual beam” flood gun. The obtained spectra were treated by means of Avantage software provided by the manufacturer. A Shirley type background subtraction was used and the peak areas were normalized using the Scofield sensitivity factors. The binding energies were calibrated against the C 1s binding energy set at 284.8 eV. The peaks were analyzed using mixed Gaussian–Lorentzian curves (70% Gaussian character).
H2 production from a methanol–water mixture solution was assessed in a closed quartz reactor under a N2 atmosphere with vigorous stirring. For the experiments, 20 mg of the photocatalyst was dispersed in 20 mL of a degassed aqueous solution with 25 vol% methanol, as a hole scavenger. The samples were irradiated using an Oriel 300 W xenon lamp with an infrared water filter for 5 h under stirring. Every 1 h, 0.2 mL of the gas sample was taken using a syringe from the quartz reactor. The amount of H2 was determined by gas chromatography (GC) using a Shimadzu GC-14B instrument.
3. Results and discussion
3.1. Characterization
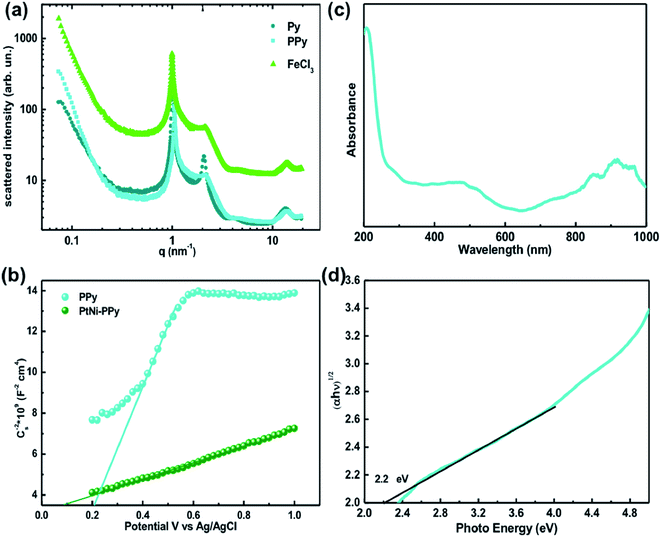
The mesophases doped with Py, with FeCl3 and the mesophase resulting from the mixing of the two previous ones were characterized by small angle X-ray scattering (SAXS) (see Fig. 2a). All the three samples are lamellar phases, characterized by two peaks located at scattering vectors q0 and 2q0; the increase of the scattering intensity at small q is also typical of lamellar phases. The spacing (periodicity of the lamellar phase) d = 2π/q0 = 6.3 nm is the same for the three samples. We note that the lamellar phase containing the monomer is more ordered (sharper second order peak) than the other samples.The electrochemical Mott–Schottky measurement was performed to obtain the flat band potential which can be given by the intercepts of the extrapolated lines (slope) (Fig. 2b). The carrier concentration (NA) is determined using the equation:40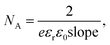 where e is the electron charge, εr is the dielectric constant (∼10 for PPy), and ε0 is the free space (8.85 × 10−14 Fcm−1). The NA of PtNi-PPy (17.7 × 1019 m−3) is five times higher than that of PPy (3.5 × 1019 m−3), indicating enhanced electronic conductivity, which will lead to a decrease of charge carrier recombination and better electron transfer.41–43
where e is the electron charge, εr is the dielectric constant (∼10 for PPy), and ε0 is the free space (8.85 × 10−14 Fcm−1). The NA of PtNi-PPy (17.7 × 1019 m−3) is five times higher than that of PPy (3.5 × 1019 m−3), indicating enhanced electronic conductivity, which will lead to a decrease of charge carrier recombination and better electron transfer.41–43
The UV-Vis spectrum of PPy exhibits an absorption in the visible region (400 nm–700 nm) and near-IR region (700 nm–1000 nm) (Fig. 2c). The peak at 470 nm is due to the π–π* transitions. The corresponding band gap (Eg) of PPy was determined from the Kubelka–Munk function (Fig. 2d): the value of Eg of PPy was found to be 2.2 eV, which is similar to that of previous reports.14
SEM images show uniform PPy NSs with an average size of 40 nm (Fig. S1†). TEM was used to characterize the morphology, size, and distribution of the metal nanoparticles (NPs) synthesized by radiolysis and the composite nanomaterials (Fig. 3). Aggregates of PPy nanoplates were observed (see Fig. 3a, c and e). For platinum modified polymer nanostructures, well dispersed Pt NPs with a diameter of about 2–3 nm were observed on the surface of PPy NSs (Fig. 3a). Fig. 3b shows the (111) facets of Pt with an interplanar spacing of 0.23 nm indicating the formation of crystalline Pt NPs. For nickel-modified polymer nanostructures, Ni-based NPs of 5 nm diameter which were homogeneously dispersed on the surface of PPy-NSs were observed as shown in Fig. 3c and d. Fig. 3d presents the interplanar spacing of the Ni-based nanoparticles. The lattice spacings of 0.20 and 0.24 nm correspond to the (111) plane of Ni and the (111) plane of NiO, respectively, which proves the presence of Ni0–NiO-based nanoparticles on PPy NSs. In the case of co-modification with Pt and Ni, metal NPs of a homogeneous size (2 nm) were observed in Fig. 3e showing a smaller crystallite size than monometallic NPs, which supports the formation of Pt–Ni nanoalloys.44 High-resolution STEM images (Fig. 3f) present the lattice spacings of 0.22 nm and 0.19 nm, which are indexed to the (111) and (200) planes of the fcc PtNi alloy, respectively. EDS analysis of the metal nanoparticles shows that the nanoparticles contain both Pt and Ni (Fig. S2†) indicating the bimetallic nature of the NPs.
 | ||
| Fig. 3 TEM images of 0.2%Pt-PPy-NSs (a and b), 5%Ni-PPy-NSs (c and d), 0.05%Pt0.05%Ni-PPy-NSs (e) and HAADF-STEM image of 0.05%Pt0.05%Ni-PPy (f). | ||
The surface composition and the oxidation states of the metal NPs in the modified PPy NSs were analyzed by X-ray photoelectron spectroscopy (XPS). XPS patterns of Pt-PPy-NSs, Ni-PPy-NSs and PtNi-PPy-NSs are shown in Fig. 4.
The wide region of spectroscopy of PtNi-PPy-NSs is shown in Fig. S3.† The signals at 284.1 eV, 402.5 eV, 533.2 eV and 201.2 eV correspond to C 1s, N 1s, O 1s and Cl 2p, respectively, which are due to PPy.31,45 Other signals at 70.5 eV and 53.3 eV correspond to Pt 4f and Ni 3p, respectively. The narrow range spectra of C 1s and N 1s are depicted in Fig. 4a and b, which also prove the presence of PPy.
The Pt 4f core-level spectrum shows an intense doublet at 71.7 eV and 75.0 eV corresponding to Pt 4f7/2 and Pt 4f5/2 of Pt0 which indicates that Pt2+ was reduced into Pt metal in the Pt-PPy-NS sample (Fig. 4c).
The Ni 2p core-level spectrum shows the presence of metallic nickel, the component Ni 2p3/2 at 852.5 eV, alongside with its oxide and hydroxide, which are expected as nickel is very sensitive to oxygen (Fig. 4d).27,28,46
Given the low loading levels of both Ni and Pt, especially in the case of PtNi-PPy-NSs (Fig. 4e and f), the acquisition conditions were adjusted in order to have the best possible signal/noise ratio in detriment of spectral resolution. Fig. 4e and f show that Pt and Ni nanoparticles are partly oxidized. The Pt 4f signals represent an intense doublet at 71.4 eV and 74.7 eV, corresponding to the core level energies of Pt 4f2/7 and Pt 4f5/2 of the Pt metal. Other doublets (71.4 eV and 75.9 eV) and (75.0 eV and 77.9 eV) can be ascribed to the +2 and +4 oxidation of Pt, respectively. The peaks at 68.0 eV and 72.4 eV correspond to the Ni 3p3/2 and Ni 3p1/2 corresponding to NiO (Fig. 4e).31,47Fig. 4f shows the presence of the Ni metal, NiO and Ni(OH)2. The presence of the oxidized Pt and Ni species is probably caused by the air during the XPS analysis. It is noteworthy that in the bimetallic materials (PtNi), the binding energy of Pt is slightly lower by 0.3 eV compared to that in the monometallic Pt-PPy, which indicates the strong interaction between the metals and PPy supports.48
3.2. Photocatalytic H2 generation
The addition of co-catalyst (such as Pt, Ni–NiO, and PtNi) NPs for photocatalytic hydrogen generation can reduce the activation energy of the reaction, provide active sites for proton reduction and H–H bond formation and decrease the recombination of charge carriers, resulting in an improvement of photocatalytic activity for hydrogen production.27,28 In addition, the amount of loading of the co-catalyst is also a crucial factor for hydrogen generation.49The stability of the photocatalytic activity was investigated with 0.2%Pt-PPy-NSs under UV-visible light (see Fig. 5b). Modified PPy nanostructures with Pt NPs show very good stability with cycling.
It is worth mentioning that the nature of the metal precursors is one of the factors that may strongly affect the size, shape and/or the surface state of the metal nanoparticles, and therefore their activity as co-catalysts. Our results show that PPy-NSs modified with metal nanoparticles prepared with platinum (II)acetylacetonate (Pt(C5H7O2)2) and nickel (II)acetylacetonate (Ni(C5H7O2)2), as metallic precursors, exhibit higher photocatalytic properties than modified PPy-NSs prepared with potassium tetrachloroplatinate(II) (K2PtCl4) and nickel formate (C2H2NiO4), respectively (Table S1†). Cui and co-workers investigated the influence of metal precursor ligands on the selectivity of alloy particle shape and structures.52 Therefore, the choice of the metal precursor is very important for the co-catalyst activity for hydrogen production.
The stability of Ni-PPy-NSs with cycling was investigated. As shown in Fig. 5d, after 5 cycles, the amount of H2 dropped by 33%. The small decrease of the photoactivity can be due to the leaching of Ni nanoparticles during the photocatalytic cycles. Indeed, Ni is a very efficient catalyst for many reactions, but it is known to leach in water.53
To investigate the photocatalytic hydrogen production and stability of the bimetallic (PtNi) doping of PPy-NSs, experiments were conducted with the same total metal loading rate (0.1%) under the same conditions (see Fig. 6a–c). Interestingly, we found that a 50/50 mixture of Ni and Pt shows an enhanced photocatalytic activity (664 μmol h−1 g−1) compared with a pure Pt loading (341 μmol h−1 g−1) and a pure Ni loading (52 μmol h−1 g−1) (Fig. 6a and S4†). Some related studies on photocatalytic H2 generation with different photocatalysts have been listed in Table S2† for comparison. The enhanced photocatalytic activity of PtNi-PPy-NSs may be due to the increased photoefficiency by the Schottky barrier leading to a longer lifetime of charge carriers and strong UV-Vis absorption due to the plasmon resonance of Pt in the UV range.55 Synergistic effects are expected (such as electronic and geometry effects) to induce enhancement of photocatalytic activity. Such a synergistic effect for hydrogen production with bimetallic nanoparticles was also recently observed for Ni–Au/TiO2 and Ni–Pd/TiO2: the association of Ni with another metal (such as Pd or Au) induces an increase of the photocatalytic activity of semiconductors for hydrogen production.27,28,56 In the case of Ni–Pd/TiO2, the study of light absorption, charge-carrier dynamics, and photocatalytic activity revealed that the main role of the metal NPs is to act as catalytic sites for recombination of atomic hydrogen. Platinum is very efficient in electron scavenging and proton reduction, while Ni is a very good co-catalyst for H–H bond formation.27,28,35,49
Fig. 6b and S5† show the effect of the total metal loading on photocatalytic hydrogen production for samples with a fixed Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio of 1
Ni ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the catalytic activity strongly depends on the Ni/Pt ratio (Fig. S6†). The 0.1%PtNi-PPy-NSs exhibit the highest photocatalytic activity yield rate of hydrogen compared with the PPy modified with other loading rates. Fig. 6c shows their very high stability with cycling, which indicates that PtNi-PPy-NSs are much more stable than Ni-PPy-NSs during the photocatalytic cycle tests.
1 and the catalytic activity strongly depends on the Ni/Pt ratio (Fig. S6†). The 0.1%PtNi-PPy-NSs exhibit the highest photocatalytic activity yield rate of hydrogen compared with the PPy modified with other loading rates. Fig. 6c shows their very high stability with cycling, which indicates that PtNi-PPy-NSs are much more stable than Ni-PPy-NSs during the photocatalytic cycle tests.
The mechanism of hydrogen formation is similar to that proposed for Pd–Ni/TiO2 and Au–Ni/TiO2.27,28 The proposed photocatalytic mechanism for hydrogen generation is shown in Fig. 6d. The generation of the electron–hole pairs takes place in the organic semiconductor PPy NSs. When the energy of the incident light is higher or equal to the band gap of PPy-NSs, the photogenerated electrons can be promoted from the HOMO to the LUMO of PPy nanostructures, leaving holes in the HOMO of PPy-NSs (PPy-NSs → h+ + e−). Water molecules are reduced by the electrons to produce H2 (H2O + e− → H2 + OH−) in the presence of co-catalysts. Methanol was used as a hole scavenger: CH3OH reacts with the holes to give CO2 and H2. This hole scavenging results in better charge carrier separation and leads to enhancement of the light conversion quantum yield.57 Platinum is known to be an efficient sink for electrons.58 The presence of Pt-based nanoparticles decreases the charge carrier recombination, and therefore induces the increase of the quantum yield. The excited electrons migrate from PPy-NSs to the metal NPs, and then H+ species are reduced on the metal surface to promote proton reduction to generate atomic hydrogen, while these hydrogen radicals recombine to produce molecular hydrogen (Fig. 6d).59 The metal nanoparticles (such as Pt, Ni and Pt–Ni) not only serve as electron sinks, but also provide effective proton reduction sites due to their relatively low over-potential.27 Ni-based nanoparticles are very active co-catalysts in H˙ recombination to form H2.27,28 Finally, H˙ recombination appears on the surface of the metal NPs producing molecular hydrogen H2, which is facilitated by the presence of Ni-based cocatalysts. The enhancement of hydrogen generation compared with that of the monometallic samples is due to a synergistic effect between Ni and Pt: the presence of Pt induces better electron scavenging and higher H˙ production, while the association with Ni promotes H˙ recombination leading to higher H2 production. Furthermore, the modification with bimetallic Pt–Ni nanoparticles leads to photocatalysts with high stability during cycling.
4. Conclusion
In conclusion, polypyrrole nanostructures (NSs) modified with mono- and bimetallic nanoparticles present high photocatalytic activity for hydrogen evolution. PPy NSs were successfully synthesized in soft templates formed by lamellar mesophases. Monometallic Pt, Ni–NiO and bimetallic Pt–Ni nanoparticles of homogeneous size and dispersion on the surface of PPy NSs were obtained by radiolytic reduction. Our work also demonstrates that the photocatalytic activity is very sensitive to the metal loading. 0.2%Pt-PPy-NSs present the best activity compared with the other loading rate percentages. Modification with nickel-based nanoparticles also gives promising results for green hydrogen production. 5%Ni-PPy-NSs show enhanced photocatalytic performance due to the formation of a heterojunction between PPy and NiO–Ni nanoparticles. However, the photocatalytic activity decreases slightly with time, probably because of Ni leaching.Pt-Ni-PPy exhibits much higher activity than its monometallic counterparts, and a synergistic effect is obtained. The presence of Pt leads to better electron scavenging and higher H˙ formation, while the association with Ni promotes H˙ recombination leading to higher H2 generation. These Pt-Ni-PPy nanostructures are also very stable with cycling.
Further studies will focus on the development of composite materials based on conjugated polymer nanostructures modified with co-catalysts made of abundant elements for solar fuel production.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
X. Y. gratefully acknowledges the financial support from the China Scholarship Council (CSC). This work was supported by the University Paris-Saclay and IRS MOMENTOM.References
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- K. Maeda, A. Xiong, T. Yoshinaga, T. Ikeda, N. Sakamoto, T. Hisatomi, M. Takashima, D. Lu, M. Kanehara and T. Setoyama, Angew. Chem., 2010, 122, 4190–4193 CrossRef.
- J. Yu, L. Qi and M. Jaroniec, J. Phys. Chem. C, 2010, 114, 13118–13125 CrossRef CAS.
- A. Tanaka, S. Sakaguchi, K. Hashimoto and H. Kominami, ACS Catal., 2012, 3, 79–85 CrossRef.
- P. Li, X. Chen, H. He, X. Zhou, Y. Zhou and Z. Zou, Adv. Mater., 2018, 30, 1703119 CrossRef PubMed.
- B. E. Sernelius, K.-F. Berggren, Z.-C. Jin, I. Hamberg and C. G. Granqvist, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 10244 CrossRef CAS PubMed.
- S. Chen and L.-W. Wang, Chem. Mater., 2012, 24, 3659–3666 CrossRef CAS.
- T. Di, B. Zhu, B. Cheng, J. Yu and J. Xu, J. Catal., 2017, 352, 532–541 CrossRef CAS.
- Z. Su, H. Li, P. Chen, S. Hu and Y. Yan, Catal. Sci. Technol., 2017, 7, 5105–5112 RSC.
- M. G. Kibria, R. Qiao, W. Yang, I. Boukahil, X. Kong, F. A. Chowdhury, M. L. Trudeau, W. Ji, H. Guo and F. Himpsel, Adv. Mater., 2016, 28, 8388–8397 CrossRef CAS PubMed.
- G. Liao, Y. Gong, L. Zhang, H. Gao, G.-J. Yang and B. Fang, Energy Environ. Sci., 2019, 12, 2080–2147 RSC.
- Y. Inoue, Energy Environ. Sci., 2009, 2, 364–386 RSC.
- X. Yuan, D. Floresyona, P.-H. Aubert, T.-T. Bui, S. Remita, S. Ghosh, F. Brisset, F. Goubard and H. Remita, Appl. Catal., B, 2019, 242, 284–292 CrossRef CAS.
- D. Floresyona, F. Goubard, P.-H. Aubert, I. Lampre, J. Mathurin, A. Dazzi, S. Ghosh, P. Beaunier, F. Brisset and S. Remita, Appl. Catal., B, 2017, 209, 23–32 CrossRef CAS.
- S. Ghosh, N. A. Kouame, S. Remita, L. Ramos, F. Goubard, P.-H. Aubert, A. Dazzi, A. Deniset-Besseau and H. Remita, Sci. Rep., 2015, 5, 18002 CrossRef CAS PubMed.
- S. Ghosh, N. A. Kouamé, L. Ramos, S. Remita, A. Dazzi, A. Deniset-Besseau, P. Beaunier, F. Goubard, P.-H. Aubert and H. Remita, Nat. Mater., 2015, 14, 505 CrossRef CAS PubMed.
- Y. Zou, B. Yang, Y. Liu, Y. Ren, J. Ma, X. Zhou, X. Cheng and Y. Deng, Adv. Funct. Mater., 2018, 28, 1806214 CrossRef.
- S. Ghosh, T. Maiyalagan and R. N. Basu, Nanoscale, 2016, 8, 6921–6947 RSC.
- T. K. Das and S. Prusty, Polym.-Plast. Technol. Eng., 2012, 51, 1487–1500 CrossRef CAS.
- S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324–1338 CrossRef PubMed.
- J. H. Burroughes, D. D. Bradley, A. Brown, R. Marks, K. Mackay, R. H. Friend, P. Burns and A. Holmes, Nature, 1990, 347, 539 CrossRef CAS.
- C. X. Guo, M. Wang, T. Chen, X. W. Lou and C. M. Li, Adv. Energy Mater., 2011, 1, 736–741 CrossRef CAS.
- G. Zhang, Z. A. Lan and X. Wang, Angew. Chem., Int. Ed., 2016, 55, 15712–15727 CrossRef.
- N. Bhandary, A. P. Singh, S. Kumar, P. P. Ingole, G. S. Thakur, A. K. Ganguli and S. Basu, ChemSusChem, 2016, 9, 2816–2823 CrossRef CAS.
- L. Zhang, N. Pan and S. Lin, Int. J. Hydrogen Energy, 2014, 39, 13474–13480 CrossRef CAS.
- A. L. Luna, D. Dragoe, K. Wang, P. Beaunier, E. Kowalska, B. Ohtani, D. Bahena Uribe, M. A. Valenzuela, H. Remita and C. Colbeau-Justin, J. Phys. Chem. C, 2017, 121, 14302–14311 CrossRef CAS.
- A. L. Luna, E. Novoseltceva, E. Louarn, P. Beaunier, E. Kowalska, B. Ohtani, M. A. Valenzuela, H. Remita and C. Colbeau-Justin, Appl. Catal., B, 2016, 191, 18–28 CrossRef CAS.
- L. F. Garay-Rodríguez, S. Murcia-López, T. Andreu, E. Moctezuma, L. M. Torres-Martínez and J. R. Morante, Catalysts, 2019, 9, 285 CrossRef.
- Z. Cai, Z. Lu, Y. Bi, Y. Li, Y. Kuang and X. Sun, Chem. Commun., 2016, 52, 3903–3906 RSC.
- S. Ghosh, S. Bera, S. Bysakh and R. N. Basu, ACS Appl. Mater. Interfaces, 2017, 9, 33775–33790 CrossRef CAS PubMed.
- H. Kobayashi, M. Yamauchi, H. Kitagawa, Y. Kubota, K. Kato and M. Takata, J. Am. Chem. Soc., 2010, 132, 5576–5577 CrossRef CAS PubMed.
- Y. Zhao, F. Pan, H. Li, G. Q. Xu and W. Chen, ChemCatChem, 2014, 6, 454–458 CrossRef CAS.
- N. Cao, J. Su, W. Luo and G. Cheng, Int. J. Hydrogen Energy, 2014, 39, 9726–9734 CrossRef CAS.
- L. Mao, Q. Ba, S. Liu, X. Jia, H. Liu, W. Chen and X. Li, RSC Adv., 2018, 8, 31529–31537 RSC.
- J. Belloni, M. Mostafavi, H. Remita, J.-L. Marignier and M.-O. Delcourt, New J. Chem., 1998, 22, 1239–1255 RSC.
- H. Remita and S. Remita, in Recent Trends in Radiation Chemistry, World Scientific, 2010, pp. 347–383 Search PubMed.
- H. Remita, M. G. Méndez Medrano and C. Colbeau-Justin, Visible Light-Active Photocatalysis: Nanostructured Catalyst Design, Mechanisms, and Applications, 2018, pp. 129–164 Search PubMed.
- A. Galińska and J. Walendziewski, Energy Fuels, 2005, 19, 1143–1147 CrossRef.
- S. Tokgöz, Y. Firat, Z. Safi and A. Peksoz, J. Electrochem. Soc., 2019, 166, G54–G60 CrossRef.
- J. Li, X. Gao, X. Jiang, X.-B. Li, Z. Liu, J. Zhang, C.-H. Tung and L.-Z. Wu, ACS Catal., 2017, 7, 5209–5213 CrossRef CAS.
- S. Wang, P. Chen, J.-H. Yun, Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2017, 56, 8500–8504 CrossRef CAS.
- J. Li, X. Gao, Z. Li, J.-H. Wang, L. Zhu, C. Yin, Y. Wang, X.-B. Li, Z. Liu, J. Zhang, C.-H. Tung and L.-Z. Wu, Adv. Funct. Mater., 2019, 29, 1808079 CrossRef.
- M. Blosi, S. Ortelli, A. Costa, M. Dondi, A. Lolli, S. Andreoli, P. Benito and S. Albonetti, Materials, 2016, 9, 550 CrossRef PubMed.
- M. Šetka, R. Calavia, L. Vojkůvka, E. Llobet, J. Drbohlavová and S. Vallejos, Sci. Rep., 2019, 9, 8465 CrossRef.
- Q. Lu, R. Huang, L. Wang, Z. Wu, C. Li, Q. Luo, S. Zuo, J. Li, D. Peng and G. Han, J. Magn. Magn. Mater., 2015, 394, 253–259 CrossRef CAS.
- K.-W. Park, J.-H. Choi, B.-K. Kwon, S.-A. Lee, Y.-E. Sung, H.-Y. Ha, S.-A. Hong, H. Kim and A. Wieckowski, J. Phys. Chem. B, 2002, 106, 1869–1877 CrossRef CAS.
- F. Wang, Y. Jiang, D. J. Lawes, G. E. Ball, C. Zhou, Z. Liu and R. Amal, ACS Catal., 2015, 5, 3924–3931 CrossRef CAS.
- B. Mei, K. Han and G. Mul, ACS Catal., 2018, 8, 9154–9164 CrossRef CAS PubMed.
- M. Méndez-Medrano, E. Kowalska, A. Lehoux, A. Herissan, B. Ohtani, S. Rau, C. Colbeau-Justin, J. Rodríguez-López and H. Remita, J. Phys. Chem. C, 2016, 120, 25010–25022 CrossRef.
- Y. Xu and R. Xu, Appl. Surf. Sci., 2015, 351, 779–793 CrossRef CAS.
- C. Cui, L. Gan, H.-H. Li, S.-H. Yu, M. Heggen and P. Strasser, Nano Lett., 2012, 12, 5885–5889 CrossRef CAS PubMed.
- E. Y. Kaniukov, A. Shumskaya, M. Kutuzau, V. Bundyukova, D. Yakimchuk, D. Borgekov, M. Ibragimova, I. Korolkov, S. Giniyatova and A. Kozlovskiy, Mater. Chem. Phys., 2019, 223, 88–97 CrossRef CAS.
- T. Deivaraj, W. Chen and J. Y. Lee, J. Mater. Chem., 2003, 13, 2555–2560 RSC.
- S. Shuang, R. Lv, Z. Xie and Z. Zhang, Sci. Rep., 2016, 6, 26670 CrossRef CAS.
- S. Oros-Ruiz, R. Zanella, S. E. Collins, A. Hernández-Gordillo and R. Gómez, Catal. Commun., 2014, 47, 1–6 CrossRef CAS.
- P. V. Kamat and S. Jin, ACS Energy Lett., 2018, 3, 622–623 CrossRef CAS.
- E. Kowalska, H. Remita, C. Colbeau-Justin, J. Hupka and J. Belloni, J. Phys. Chem. C, 2008, 112, 1124–1131 CrossRef CAS.
- J. B. Joo, R. Dillon, I. Lee, Y. Yin, C. J. Bardeen and F. Zaera, Proc. Natl. Acad. Sci., 2014, 111, 7942–7947 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta11088g |
| This journal is © The Royal Society of Chemistry 2020 |