Active-powering pressure-sensing fabric devices†
Hongyan
Sun
a,
Ning
Pan
 b,
Xin
Jin
a,
Ka
Deng
a,
Zhiduo
Liu
c,
Cheng-Te
Lin
b,
Xin
Jin
a,
Ka
Deng
a,
Zhiduo
Liu
c,
Cheng-Te
Lin
 d,
Tingrui
Pan
*b and
Yu
Chang
d,
Tingrui
Pan
*b and
Yu
Chang
 *a
*a
aBionic Sensing and Intelligence Center (BSIC), Institute of Biomedical and Health Engineering, Shenzhen Institutes of Advanced Technology, Chinese Academy of Science, 1068 Xueyuan Avenue, Shenzhen 518055, China. E-mail: yu.chang@siat.ac.cn
bMicro-Nano Innovations (MINI) Laboratory, Department of Biomedical Engineering, University of California Davis, One Shields Avenue, Davis, CA 95616, USA. E-mail: trpan@ucdavis.edu
cUniversity of Chinese Academy of Sciences, 19 A Yuquan Rd., Shijingshan District, Beijing, 100049, China
dKey Laboratory of Marine Materials and Related Technologies, Zhejiang Key Laboratory of Marine Materials and Protective Technologies, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, 315201, China
First published on 21st November 2019
Abstract
Smart flexible sensing devices with high-comfort and self-powered characteristics are essential in the future-generation wearable human-sensing interface. However, most of the current sensing devices cannot work independently and require external power. Here, we introduced for the first time an active-powering pressure-sensing fabric (APPS) device, integrating a soft-matter battery unit with a fabric-based sensing substrate into one flexible device architecture, which offered a comfortable and reliable human-sensing interface with continuous system powering capacity for wearable physiological and activity monitoring. Notably, the APPS fabric demonstrated an open voltage of 1 V, short circuit of 35 mA cm−2 and capacity of 2.6 mA h cm−2. The specifically designed solid neutral hydrogel electrolyte showed high safety and high force-resistance, guaranteeing the stability of the power output under pressure. Moreover, it exhibited the highest sensitivity of 19.6 Ω−1 kPa−1 in a test pressure range (of <300 kPa) and a mechanical response time of 9 ms. For wearable applications, we packaged the APPS fabric into an adhesive bandage format, from which a series of pressure detection demonstrations were successfully implemented without using any power source. In particular, the APPS fabric device could power the whole system by itself for signal detection and wireless data transmission via a Bluetooth or LED. Benefitting from the unique properties of the active powering and its remarkable performance, the APPS device holds an enormous potential for emerging wearable applications, including health monitoring, gesture recognition and motion monitoring.
Introduction
As an emerging human–machine interface, flexible electronics hold promise to offer excellent deformability, long-term stability, and extended wearability for a wide range of applications ranging from medical implants to health wearables.1–3 In particular, it has been an active area of research to detect various physiological signals in situ through a flexible and wearable electronic interface based on a variety of chemical, electrical and mechanical measurement principles.4,5 Over the past few years, significant advances in flexible electronic development have been made this noninvasive modality of health monitoring through temporary epidermal attachments, textile implementations, and stretchable designs.6,7 Wearable health monitoring can be of particular interest for real-time, uninterrupted, and comfortable monitoring of vital physiological signals, which can provide previously inaccessible yet clinically critical information for forthcoming preventive healthcare and early disease diagnosis.8 Accordingly, various types of flexible sensors and wearable devices, including elastic biopotential electrodes, wearable pressure sensors, stretchable electrochemical sensors, and flexible temperature/humidity sensors, have been frequently exploited for this purpose.9–11 Among them, wearable pressure sensors, which transform physical touch (in the form of pressure or force) into electrical or optical readouts, have received increasing attention from both industries and academia due to their simple device architecture and extended capacity to obtain an array of physiological signals in an unnoticeable manner, including monitoring arterial pulse waveforms, tracking heart rates and heart rate variabilities, recording respiratory patterns, and analyzing muscle motions and body movements.12–15Despite the rapid progress in the development of wearable devices, several technical hurdles remain in this emerging field.16,17 Remarkably, a reliable yet comfortable human-sensing interface is challenging to maintain for body signal acquisition upon the soft intimate skin contact. For instance, a fabric, as a traditional material throughout civilization, has become a promising platform for flexible electronics, benefiting from its soft yet durable material constructs and breathable and comfortable skin contact.18 A fabric is also known for its capacity of handling a wide range of mechanical loads using its fibrous microstructures and intertwined yarn patterns.19 Recently, Wang's group demonstrated an all-textile pressure-sensing array on regular fabric substrates, and the textile sensor units could recognize real-time pulse waves, acoustic vibrations, hand gestures and finger movements.7 Our group previously introduced a new interfacial sensing modality known as flexible iontronic sensing (FITS) to existing fabric materials and implemented a supercapacitive sensing array on the all-textile substrates. FITS was subsequently applied to continuously track arterial pressure waveforms and to monitor muscular and tendon movements in real time, with extremely low motion artifacts.20 Simultaneously, by naturally offering comfortable and breathable skin contact with mechanical flexibility and adaptability, fabric has become a desirable engineering material platform for wearable pressure sensing and continuous physiological monitoring.
On the other hand, the power source to wearable electronics, as always required, is still difficult to be integrated seamlessly, while staying at the same level of flexibility as the sensing units.21 Accordingly, several research attempts have made to address this issue recently with various forms of self-powering mechanisms to be considered, including active piezoelectric actuations, triboelectric effects, as well as supercapacitive charge storage.22–24 Among them, triboelectricity-derived methods, i.e., harvesting electricity from dynamic mechanical stimuli, have been explored predominantly, meanwhile generated electric signals can also be considered as sensing inputs.25 For example, Wang's groups previously demonstrated a membrane-based pressure sensor powered by an intrinsic triboelectric signal for pneumatic measurements as well as surveillance and health monitoring with high resolution.26 However, although triboelectric sensors can provide appreciably high device sensitivity for body gestures and physiological signals, the related power generation can only produce high output voltages (up to hundreds of voltage) with insufficient electric currents (less than dozens of microamps).27,28 Given the high internal impedance, this unconventional power output from triboelectric devices cannot sustainably fulfill the power demand from modern electronics containing microcontrollers (with power consumption in the order of milliwatts typically).16 Moreover, triboelectric devices can only deliver instantaneous and alternating power/current derived from external stimuli, leading to an even lower level of power on average.25,29 Recent efforts from Wang's groups have been made to address this hurdle by incorporating additional power storage and conversion units, from which the instantaneous peak power has been measured at 2.28 mW and can be used to charge a dedicated power conversion unit as the stored power supply.30 Currently, due to the low-power and time-dependent nature, these triboelectric sensors, without additional power storage or electronic conversion, cannot work reliably and sustainably as sole functional self-powering devices, which largely limits their practical use in a wide range of wearable applications. Thus, on-demand self-powering capacity, i.e., supplying sufficient currents to continuously functional electronics, is still highly sought after for the rapidly growing field of flexible sensors.
To address the aforementioned challenges, herein, for the first time, we first introduce an active-powering pressure-sensing (APPS) fabric device, integrating a soft-matter battery unit with a fabric-based sensing substrate into one flexible device architecture, which offers a comfortable and reliable human-sensing interface and continuous powering capacity for wearable physiological and activity monitoring. Notably, the APPS fabric with an overall thickness of about 0.7 mm, exhibiting an open-circuit voltage of 1.0 V and a short-circuit current density up to 35 mA cm−2, can fulfill the power requirements from a variety of standard signal processing and transmission units, such as microcontrollers and Bluetooth low-energy modules. In particular, the soft-matter battery, based on the classic zinc–air structure,31 and soft-matter electrolyte matrix exhibit high flexibility and high stability under mechanical loads. The battery component was seamlessly integrated with the sensing fabric in series and directly supplied power to the sensor and external signal acquisition circuitry or device. When loaded with external forces, the resulting electrical changes in the APPS sensor could be detected and converted by the self-powered acquisition unit. Overall, the APPS device possesses a power capacity higher than 2.6 mA h cm−2, which can adequately power the sensor itself and supply the signal acquisition, processing and wireless transmission modules in a continuous fashion. Importantly, the APPS fabric exhibited the highest sensitivity of 19.6 Ω−1 kPa−1 in a test pressure range (of <300 kPa) and a mechanical response time of 9 ms. These specifications certainly allow the APPS device to detect subtle pressure changes generated by vibrations and motions on the human skin. To optimize the desired device performance, a theoretical electromechanical model based on the fibrous micro-structure of the fabric and the contact resistance mechanism was established to evaluate and predict the sensing responses of the APPS device. By connecting the APPS fabric with an appropriate wireless processing circuitry, the measured data, either health-related or activity-related, acquired from the flexible human-sensing interface could be directly transmitted to a modern cloud-based or mobile-based computing platform for further analysis, without involving an active power source. Moreover, no appreciable performance degradation was observed experimentally after extended cyclic testing (>6000 cycles). For wearable applications, we packaged the APPS fabric into an adhesive bandage format with different dimensions, from which a series of practical demonstrations was successfully implemented without using any power source. Furthermore, its capacity of real-time and continuous detecting arterial pulse waveforms from multiple body locations, such as the wrist, neck and instep with excellent signal quality and stability was demonstrated. In addition, complex body motions could also be closely tracked including finger motions and hand gesture recognitions.
Experimental
Preparation of the APPS fabric device
The APPS fabric device was fabricated through a layer-by-layer method. (1) The flexible sandwich structure power unit zinc–air battery was fabricated, which was comprised of a zinc anode, air cathode, and hydrogel electrolyte. Zinc anode: 10 wt% polyvinylidene fluoride (PVDF) was dissolved in N-methyl pyrrolidone (NMP), then zinc powder (1 μm particle size) was added with a weight ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with PVDF, followed by stirring to form a uniform paste with relatively high viscosity. A 50 μm rod coating was used to blade coat the zinc paste on conductive fabric (thickness of 0.08 mm, and the surface has a copper–nickel coating, TOOKUN Company) as the zinc anode, and after coating, the zinc anode was dried in atmosphere at 80 °C for 2 h to evaporate NMP, resulting in the formation of a zinc coating on the conductive fabric. Air cathode: using the same preparation method as the zinc anode, 5 wt% PVDF was dissolved in NMP, then the catalyst (the mixture of MnO2 and carbon black, Changsha Spring New Energy Technology Co. LTD) was added with a weight ratio of 8
1 with PVDF, followed by stirring to form a uniform paste with relatively high viscosity. A 50 μm rod coating was used to blade coat the zinc paste on conductive fabric (thickness of 0.08 mm, and the surface has a copper–nickel coating, TOOKUN Company) as the zinc anode, and after coating, the zinc anode was dried in atmosphere at 80 °C for 2 h to evaporate NMP, resulting in the formation of a zinc coating on the conductive fabric. Air cathode: using the same preparation method as the zinc anode, 5 wt% PVDF was dissolved in NMP, then the catalyst (the mixture of MnO2 and carbon black, Changsha Spring New Energy Technology Co. LTD) was added with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with PVDF, and the catalyst paste was coated on conductive fabric as the air cathode. Hydrogel electrolyte: a solid hydrogel electrolyte was prepared via the UV curing method. 20 wt% poly(ethylene glycol) diacrylate (PEGDA, Aladdin Reagent Company) was dissolved in distilled water under magnetic stirring, and then polyvinyl alcohol (PVA, Aladdin Reagent Company) was added with different weight ratios of PVA (2
2 with PVDF, and the catalyst paste was coated on conductive fabric as the air cathode. Hydrogel electrolyte: a solid hydrogel electrolyte was prepared via the UV curing method. 20 wt% poly(ethylene glycol) diacrylate (PEGDA, Aladdin Reagent Company) was dissolved in distilled water under magnetic stirring, and then polyvinyl alcohol (PVA, Aladdin Reagent Company) was added with different weight ratios of PVA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2
0, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and 2
0.5, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and continuously stirred at 60 °C. After stirring well and cooling to room temperature, NH4Cl was added with a weight ratio of 10–30% with PEGDA solution. Subsequently, diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO, Aladdin Reagent Company) photoinitiator was added with a weight ratio of 2% with PEGDA, until the solution became homogeneous. Finally, the solution was casted onto a homemade plastic tank with the height of 30 μm to form a membrane, which was then treated with UV radiation for 2 min to obtain the final PEGDA/PVA with NH4Cl neutral solid hydrogel electrolyte. (2) To fabricate the flexible sandwich structure sensing unit, which was comprised of sensing materials, both positive and negative. A non-conductive fabric (soft spandex and hard polyester with a thickness of 0.1 mm) was dip-coated with different concentrations (0.5%, 0.75%, and 1.0%) of conductive poly(3,4-ethylenedioxythiophene)/poly (styrenesulfonate) solution (PEDOT:PSS, Nichem-CC018, Taiwan) and dried at 80 °C for 2 h to evaporate water as a sensing material. In addition, the conductivity of the sensing unit was adjusted by diluting the PEDOT:PSS concentration. The sensing unit shared the zinc–air batterie anode as one electrode, and the other electrode was conductive fabric. The final APPS fabric device had a five-layer structure and a thickness of about 0.7 mm.
1), and continuously stirred at 60 °C. After stirring well and cooling to room temperature, NH4Cl was added with a weight ratio of 10–30% with PEGDA solution. Subsequently, diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO, Aladdin Reagent Company) photoinitiator was added with a weight ratio of 2% with PEGDA, until the solution became homogeneous. Finally, the solution was casted onto a homemade plastic tank with the height of 30 μm to form a membrane, which was then treated with UV radiation for 2 min to obtain the final PEGDA/PVA with NH4Cl neutral solid hydrogel electrolyte. (2) To fabricate the flexible sandwich structure sensing unit, which was comprised of sensing materials, both positive and negative. A non-conductive fabric (soft spandex and hard polyester with a thickness of 0.1 mm) was dip-coated with different concentrations (0.5%, 0.75%, and 1.0%) of conductive poly(3,4-ethylenedioxythiophene)/poly (styrenesulfonate) solution (PEDOT:PSS, Nichem-CC018, Taiwan) and dried at 80 °C for 2 h to evaporate water as a sensing material. In addition, the conductivity of the sensing unit was adjusted by diluting the PEDOT:PSS concentration. The sensing unit shared the zinc–air batterie anode as one electrode, and the other electrode was conductive fabric. The final APPS fabric device had a five-layer structure and a thickness of about 0.7 mm.
Characterization
The surface morphology of the sensing materials was characterized using a scanning electron microscope (SEM, Gemini Sigma 300, Germany). The pressure force applied on the device was monitored using a dynamometer (M5-2, Mark-10) mounted on a moving stage (KMTS50E/M, Thorlabs). The electrochemical performance of the batteries was tested using a LAND CT2001A battery testing system, the 1/R curve of the sensor was recorded using a digital multimeter (Tonghui, TH1963), and the real-time voltage curve of the device was recorded by a data acquisition card (DAQ, NI USB-6361, NI Instruments Corporation). The pulse waveform wireless data was transmitted by a custom Bluetooth module (nRF52832, Holyiot) and displayed on a computer via a custom interface.Results and discussion
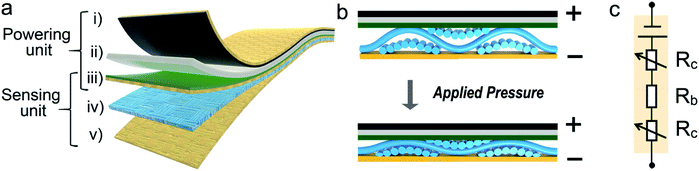
As aforementioned, the APPS fabric had two stacking functional structures, i.e., the soft-matter zinc–air battery sitting on top of the flexible pressure-sensing device. In particular, it consisted of five distinct layers, as illustrated in Fig. 1a, of which four layers were made from commercially available fabric materials, and the other was a hydrogel composite. From the top to the bottom, the powering unit of the flexible zinc–air battery was made up of three flexible layers: (i) a non-reactive cathode, (ii) hydrogel electrolyte; and (iii) zinc powdered-coated anode. Moreover, utilizing the layer-by-layer stacking architecture, the pressure-sensing device and powering unit could share one conductive layer (iii), thus eliminating one functional layer. Consecutively, the sensing component was made up of another three-layer stacking structure with (iii) the top electrode shared with the powering unit, (iv) a piezoresistive sensing fabric, and (v) a bottom electrode layer.The APPS fabric was configured to operate under the piezoresistive sensing principle, which basically measures the resistive changes over the contact area under external loads, as illustrated in Fig. 1b. In particular, the sensing component can be simply modeled as a fibrous elastic contact structure sandwiched in between two flexible fibrous surfaces. When experiencing pressure load, the surface contact area gradually increases due to the bending of the fibers, as predicted by the classic fiber assembly compression theory, and therefore, the contact electrical resistance decreases accordingly.32,33 On the other hand, the powering unit continues converting chemical energy into electronic supply, providing a stable voltage output for external acquisition circuitry despite the mechanical variations. As a result, the external circuitry can be fueled to interrogate the resistive change of the sensing component caused by the mechanical deformation. Fig. 1c exhibits an equivalent electronic circuit model of the APPS fabric device, in which the sensing layers function as a pressure-sensitive component, whereas the powering unit serves as a constant voltage source. By shutting the device to an external load (e.g., a voltage divider), the resistive change of the APPS fabric can be converted into an electrically measurable quantity, while providing adequate electricity to the measurement circuitry itself. Furthermore, Fig. S1 (in ESI†) incorporates a schematic drawing of the functional APPS fabric with appropriate electronics designed for potential wearable health and activity monitoring. The measured sensory signals from the arterial pulse waveforms or muscular activities can be directly processed and transmitted through a BLE-enabled module to a remote terminal or a cloud-based computation platform. Such miniature modern electronics can contain conventional analog-to-digital conversion, signal filtering and conditioning, as well as powerful AI-enabled edge computation in addition to the wireless transmission. From there, the health information and body activities of the subject can be remotely assessed and monitored by health professionals.9,34,35
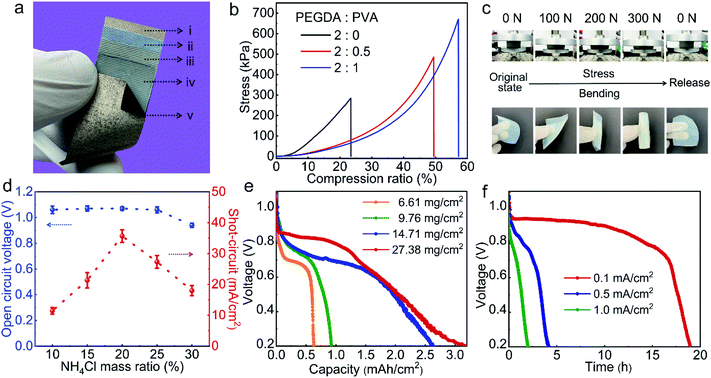
To characterize and optimize the performance of the APPS fabric device, we conducted a series of experimental evaluations on its powering and sensing units. Fig. 2a shows a close-up view of the APPS fabric device showing its layer-by-layer structure and the SEM image shows the cross-section of the APPS fabric device (Fig. S2†). As aforementioned, the powering unit incorporates the conductive fabric coated with an inorganic catalyst powder (i.e., MnO2) as the non-reactive cathode (i); the hydrogel composite of poly(ethylene glycol) diacrylate/polyvinyl alcohol (PEGDA/PVA), containing a neutral electrolyte of ammonium chloride (NH4Cl), as the solid-state electrolyte (ii), and the other conductive fabric coated with zinc paste as the flexible anode (iii). Moreover, applying the near-neutral electrolyte of NH4Cl, the flexible powering unit follows the discharge electrochemical reactions: zinc anode: Zn + H2O → ZnO + 2H+ + 2e−; and air cathode: O2 + 4H+ + 4e− → 2H2O,36 consuming zinc ions at the anode and oxygen from air at the cathode with electrical charges generated under the catalyst of MnO2, while the gel electrolyte provides the buffer for ionic migration.37
As a force sensing component, the powering unit of the APPS device must be stable under pressure, requiring the gel electrolyte, a typically mechanically weak material, to have high compression strength with excellent elasticity.38,39 Therefore, we designed a double network (DN) with PVA and PEGDA to achieve both required mechanical properties. Particularly, PVA hydrogels are considered to possess high compression strength and high flexibility, but relatively low elasticity for recovery.40 In contrast, the PEGDA hydrogel, which can be quickly formed by UV curing with extremely high crosslinking density, possesses a 3D macromolecular structure with high elasticity, but has limited compression strength.41 When combining PVA and PEGDA in the same DN matrix structure, we achieved the desired dual mechanical properties.42 The mechanical properties of the hydrogel were investigated via compression tests, as shown in Fig. 2b, where the compression strength of the hydrogel increased from 280 kPa to 670 kPa, when the weight ratio of PEGDA to PVA changed from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 2
0 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, to 2
0.5, to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (due to the limitation of the solubility of PVA, a gel with a higher PVA concentration was difficult to prepare). These high mechanical properties of the PEGDA/PVA hydrogel originate from the reciprocity of the two polymer networks, which suppress the propagation of cracks. Fig. 2c demonstrates the excellent elasticity of the PEGDA/PVA (2
1 (due to the limitation of the solubility of PVA, a gel with a higher PVA concentration was difficult to prepare). These high mechanical properties of the PEGDA/PVA hydrogel originate from the reciprocity of the two polymer networks, which suppress the propagation of cracks. Fig. 2c demonstrates the excellent elasticity of the PEGDA/PVA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) hydrogel, where the gel could completely recover its original shape after pressing for 300 N (equivalent pressure is 450 kPa) or folding in any arbitrary direction without fracture or deformation, guaranteeing the structural stability of the powering unit as a soft wearable pressure sensing device.
1) hydrogel, where the gel could completely recover its original shape after pressing for 300 N (equivalent pressure is 450 kPa) or folding in any arbitrary direction without fracture or deformation, guaranteeing the structural stability of the powering unit as a soft wearable pressure sensing device.
To continuously power functional electronics, both the output voltage and current of the APPS device have to satisfy the energy requirements. Since the electrochemical voltage typically ranges from 0.7–1.5 V for the zinc–air batteries,43 the current output becomes a dominant parameter to fulfill. In principle, the current density of the powering unit can be regulated by adjusting the concentration of NH4Cl for the APPS. As shown in Fig. 2d, the current density increased with an increase in the NH4Cl concentration from 10 to 30 wt% in the PEGDA/PVA hydrogel, and declined after reaching a peak point (at the concentration of 20%). This is due to the fact that the conductivity of the NH4Cl hydrogel increased with an increase the electrolyte concentration; however, once the NH4Cl solution reached a supersaturation level, NH4Cl crystals may form, which will precipitate on the surfaces of the anode and the cathode, resulting in a declined performance of the battery discharge.36 The results show that at the concentration of 20%, the powering unit exhibited a stable open-circuit voltage of 1.0 V and a maximal short-circuit current density of 35 mA cm−2, leading to an overall power output up to 35 mW cm−2, which can easily power most modern microelectronics, such as microcontrollers, light-emitting devices (LEDs) and Bluetooth communication chips.44 Moreover, the battery capacity determines the operational time of the APPS device. By directly tuning the reactant content (zinc powers) per unit area, the capacity of the powering unit could be efficiently controlled, varying from 0.65 to 3.0 mA h cm−2 with 6.61–27.38 mg cm−2 zinc power (Fig. 2e). As can be seen, a further increase in the content of the zinc powder from 14.71–27.38 mg cm−2 did not cause a significant increase in the battery capacity. This is because the gel electrolyte may not always be in direct contact with the zinc powder due to the limitation of the solid-state format. After the surface layer of zinc paste is fully consumed, the zinc powder stored underneath needs to be reached to react through a gradual diffusion process, the time of which grows parabolically with the distance.40 Consequently, we determined that the thickness of the zinc paste should be limited by ∼0.1 mm (14.7 mg cm−2 zinc paste), of which the overall battery capacity is capped at ∼2.6 mA h cm−2. With this capacity, the flexible zinc–air structure can sufficiently power modern electronics, such as LEDs or digital watch for an extended period of time (as shown in Fig. S3†). In addition, the operation time of the powering unit is also decided by the discharge current, where a lower discharge current leads to a longer operation time. The powering unit exhibited an excellent galvanostatic discharge rate at various current densities (Fig. S4a†) and reasonably high discharge times at 0.1, 0.5, and 1.0 mA cm−2 (Fig. 2f). As a result, for a BLE module with about 1 mW in average power consumption, a 10 cm2 APPS device can theoretically support the system for about 18 h with a discharge current density of about 0.1 mA cm−2. As a flexible powering unit, no obvious change could be found in the output voltage and short of the battery when the powering unit underwent various extreme bending conditions (i.e., 0°, ±90°, and ±180°), as shown in Fig. S4b,† guaranteeing its feasibility as a component of a wearable pressure sensing device.
The sensing unit of the APPS device is composed of (iii) the top conductive fabric layer as the shared electrode and (iv) the resistive sensing layer using a woven fabric dip-coated with a solution of poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT:PSS); and (v) the bottom electrode layer formed by another piece of conductive fabric. The PEDOT:PSS polymer was selected as the filler for the pressure-sensing matrix because of its stable chemical properties, easy processability by dip-coating with the fabric substrate, and high yet tunable conductivity based on its solution concentration.45 As shown in the scanning electron microscopy (SEM) image in Fig. S5,† the PEDOT:PSS-containing fabric maintained the pristine morphology of the interwoven fibers entangled into a 3D network, thus forming a pressure-sensitive interface with the conductive fabric.
To characterize the sensing performance of the APPS device, we investigated an electromechanical model for the multilayer fabric sensing structure by applying the classical compression theory of a fibrous assembly. The whole resistance of the fabric sensor (R) with a resistive sensing fabric (iv) sandwiched between two conductive fabric electrodes (iii) and (v) can be regarded as a connection in series of the bulk resistance (Rb) of the resistive fabric and the contact resistances (Rc) at the two resistive–conductive interfaces (between resistive and conductive fabrics),7,45 as shown in Fig. 1c, and can be defined as R = 2Rc + Rb. It has been previously determined that the variation in the contact resistance plays a more important role than that of the bulk resistance in the fabric sensing structure within the pressure range of up to 760 mmHg.20 The contact resistance is directly related to the contact resistivity (ρc) of the interface divided by the contact area (Ac): Rc = ρc/A. The contact resistivity of the interface is defined as the measured contact resistance at and infinitely small contact area: 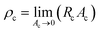 , and mainly dominated by the material category, surface clearness and environmental factors, and therefore can be treated as an intrinsic property.46–48
, and mainly dominated by the material category, surface clearness and environmental factors, and therefore can be treated as an intrinsic property.46–48
Specifically, the pressure-induced bending of the individual fibers can lead to a change in the volume fraction of the fibers (Vf) in the fabric assembly, which successively varies the area fraction of the fibers and the contact area at the interfaces.7 A theoretic equation was derived accordingly to establish the relationship between the resistive change (R) and the pressure load (P) (the detailed derivation of this equation can be found in the ESI†):
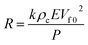 | (1) |
The device sensitivity is classically defined as the slope rate of the 1/R–P curve of the device. As a result, the 1/R value of the APPS devices and the sensitivity of the APPS device can be defined as:
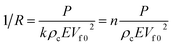 | (2) |
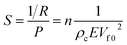 | (3) |
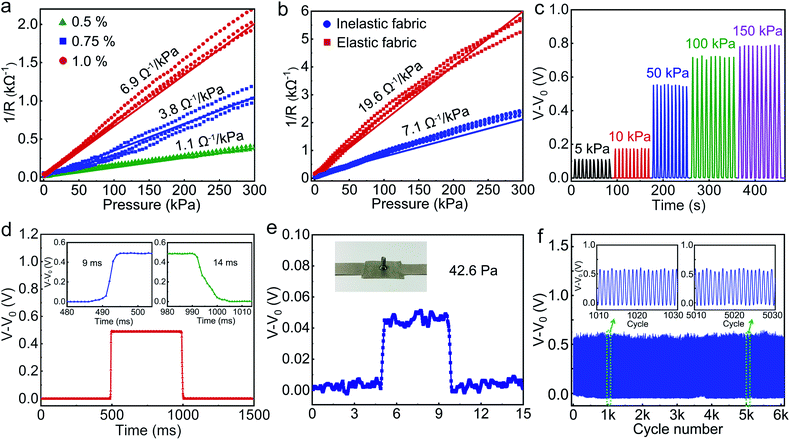
Based on the theoretical model developed above, we further characterized and optimized the pressure-sensing performance of the APPS device incorporated with the highly resistive layer of PEDOT:PSS fabric by modifying the contact resistivity and the Young's modulus, and these parameters can be easily controlled during the preparation of the PEDOT:PSS fabric. The sensitivities were obtained for different APPS devices at various levels of PEDOT:PSS concentration (Fig. 3a). As can be seen, various response curves were obtained by varying the concentration of the PEDOT:PSS solution from 0.5 wt% to 1 wt%. Consequently, the resulting fabrics dip-coated with PEDOT:PSS showed a contact resistivity of 74.3, 26.9 and 14.1 Ω cm−2 (in this case, the contact between unit area PEDOT:PSS/fabric and conductive fabric of the device under 10 MPa, that is, the nominal full contact). Acting as the piezoresistive sensing element, the PEDOT:PSS fabric offered different pressure–resistance response curves based on its interfacial contact with the conductive surface, and thus, led to various device sensitivities. In particular, the pressure response of the device (1 wt% PEDOT:PSS) with a fixed sensing surface area of 1 × 1 cm2 had a pressure sensitivity value of 6.9 Ω−1 kPa−1 below 300 kPa, which has a better linear relationship in the test pressure range. In summary, the PEDOT:PSS concentration in the sensing layer had a significant influence on the contact resistivity sensitivity of the sensing fabric as the higher amount of PEDOT:PSS coated on fiber surface led to a thicker and more completed resistive layer. In conclusion, the lower contact resistivity of the interface results in the higher sensitivity of the APPS device, which is consistent with the theoretic equation.
Moreover, to explore the influence of the hardness of the fabric on the device sensitivity, pressure response characterization was performed on the APPS devices with elastic or the inelastic fabric as the sensing layers. As shown in Fig. 3b, a device sensitivity of 19.6 Ω−1 kPa−1 was obtained for the soft spandex sensing fabric with a Young's modulus of around 0.48 GPa,51 which is 2.7 times higher than that of the hard polyester sensing fabric (7.1 Ω−1 kPa−1) with a Young's modulus of around 1.2 GPa,52 while the ρc and the Vf0 of the two devices remained similar, according with the theoretic prediction. Consequently, to achieve higher sensitivity, a softer sensing fabric pre-treated by a PEDOT:PSS solution with a higher concentration should be chosen, as verified by the theoretical prediction and experiment.
As a whole system with powering unit and sensing unit, the output of the device was measured as the voltage change (V–V0, where V0 is the voltage when no pressure applied) according to the equivalent circuit shown in Fig. S6,† and the voltage change detected also showed a pressure-related variation (Fig. 3c). The stability and repeatability of the pressure sensor were also investigated under different pressures, as shown in Fig. 3c. Response time is another key parameter for the application of the pressure sensor. Using a piezoelectric actuator to apply a periodical load onto the pressure sensor, by evaluating the loading and unloading phases during each duty cycle, the pressure sensor showed a response time of 9 ms and a recovery time of 14 ms (Fig. 3d). In addition, the minimum detection limit and a comparison with other pressure sensors were illustrated successfully, as shown in Fig. 3e and Table S1.† A screw (weight of 0.4349 g) was placed on the APPS fabric device with a 1 × 1 cm2 sensing area, which was around 42.6 Pa. Moreover, to further investigate the stability and reliability of the pressure sensor, a pressure of 50 kPa was repeatedly loaded and unloaded on the pressure sensor for more than 6000 cycles, and the enlarged views of the output waveform insets clearly show that there was no performance degradation during the whole cyclic process, which is greatly important for long-term use (Fig. 3f). All these measurement results indicate that the APPS fabric device exhibits high stability, repeatability, and durability. Fig. S7† shows a prototype of the APPS fabric device with excellent flexibility and foldability. It was then be further cut into different shapes and mounted onto various surface curvatures and topologies.
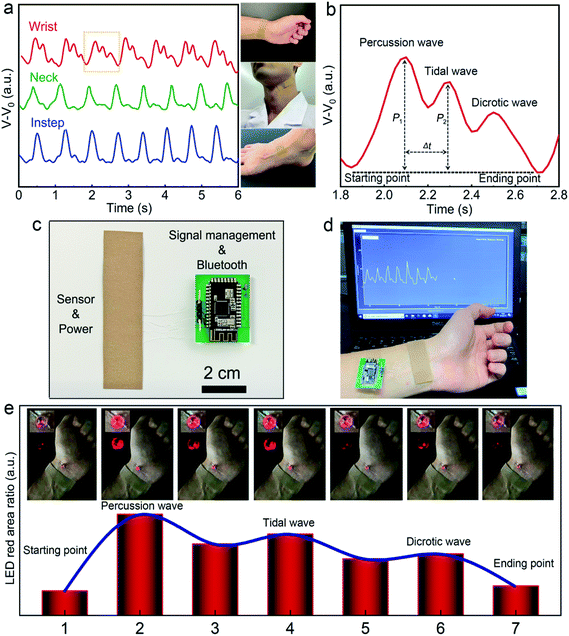
Utilizing the high device sensitivity and fast mechanical response of the APPS architecture, the flexible sensing fabric could be easily mounted on the human body and monitor vital physiological information by capturing the continuous arterial pulse waveforms in a real-time and noninvasive fashion. To provide a simple yet secure attachment to the human body, we configured the APPS fabric device into a bandage format with a medical-grade adhesive layer added to both ends, as shown in Fig. 4a. As a consequence, we could easily fix the APPS device to a palpable location, such as the wrist, neck, or instep, to track the arterial pulse waveforms through the contact with the skin. In this case, the epidermal pressure measurements from the arterial pulses at different body locations can be influenced by many different factors, such as the thickness of the adipose tissue or the shape of the supportive bony structure, leading to variations and distortions in the measured hemodynamic signals over the body surface.53 Historically, the arterial waveforms collected from the wrist have been extensively studied, and from the wrist measurements, we can clearly distinguish the typical hemodynamic features, including the starting point, percussion wave, tidal wave, diastolic wave, and ending point, from a healthy test subject, as noted in Fig. 4b. In particular, due to the high resolution of the APPS pressure sensor, we could clearly observe two distinct pressure peaks from the radial artery pulse measurements, i.e., P1 refers to the reflected wave from percussion wave, while P2 represents the reflected wave from tidal wave.5,8 The corresponding time delay (Δt) between P1 and P2 as well as its ratio, also known as, the radial artery augmentation index (AIr = P2/P1), can be further derived to provide health and potentially pathological information of the cardiovascular circulation, such as risks in arteriosclerosis and hypertension.53
On the other hand, the powering unit offers sufficient electricity to fuel the wireless monitoring circuitry for wearable utilities in a continuous manner. To emphasize the distinct self-powering feature, we implemented two wireless monitoring strategies. The first approach was to connect the wearable sensor to a standard Bluetooth low energy (BLE)-enabled microprocessor via an analog-to-digital (ADC) signal conversion, from which the digital signal could be acquired and transmitted to a mobile device or a cloud-based platform. The required average power to drive a Bluetooth component to continually collect pulse signal and periodically transmit the data wireless was around 1 mW, thus the APPS device can support the external Bluetooth-enabled processor for about 18 h in practice.34 Using the BLE circuitry (Fig. 4c), the pulse waveforms collected from the wireless data transmission sensor system are plotted in Fig. 4d, from which all the important features of the arterial pulse waveforms can be apparently observed and wireless data transmitted to a personal computer or other electronic display equipment. The corresponding video (Video S1, ESI) is provided in the ESI.† Thus, this sensing system holds great potential to be used in on-line monitoring and intelligent remote diagnosis, which has the advantages of being compact, lightweight, and wireless, with no external power supply.
The second strategy was to directly shunt the device to a light-emitting device (LED), where the sensor acted as a variable resistor and a constant power source, as shown in Fig. 4e. Employing the LED approach, the APPS fabric bandage, in a close loop with the external miniature LED, was also attached onto the wrist, and the real-time pressure pulse waveforms were converted into the modulation of the light intensity in LED. This change could be tracked remotely by a video camera, from which the light intensity could be assessed and calculated back into the corresponding pressure values. As shown in Fig. 4e and Video S2 (ESI†), the APPS device-controlled LED periodically blinked, which is consistent with the pulse waveforms on the tested subjects (∼80 times per min). Compared to the BLE-enabled pulse monitoring, the LED method does not require any external hardware component (besides the LED itself) mounted or connected onto the body surface, while still allowing the remote accessibility of vital signals. In particular, this LED configuration offers a significantly lower power consumption of around 0.3 mW, leaving the system with continuous functionality for more than 3 days. However, the acquired signal resolution from the BLE-circuitry was significantly higher than that of the LED method, and it was also compatible with an existing mobile platform without requiring the video-capturing instrument, which can be put into direct use in the immediate future.
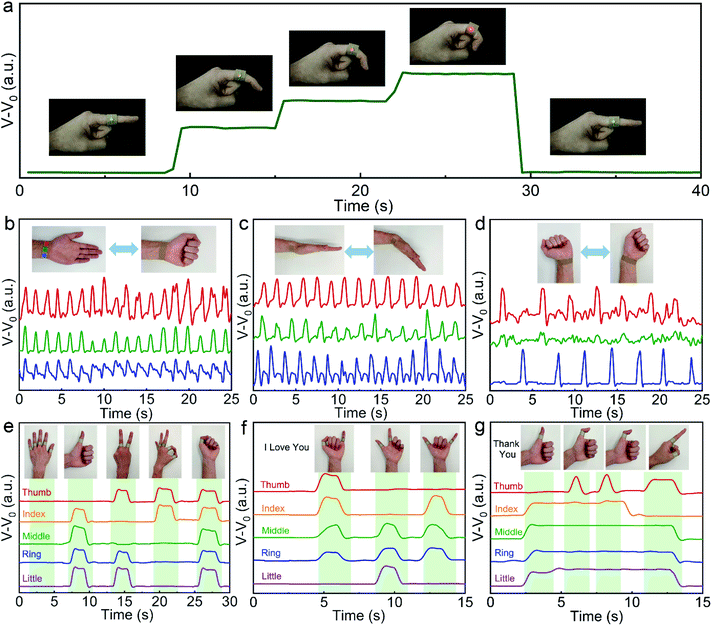
In addition to the small-scale pulse wave monitor, the APPS fabric device can also be used to monitor large-scale joint motion. An APPS fabric device was attached to the curvy skin of an index finger. Once the finger is bent, the contact area between the sensing unit electrodes (iii and v) and PEDOT:PSS/fabric will increase, leading to an increase in the current correspondingly (Fig. 5a). The corresponding active-powering pressure-sensor could independently drive small electronic components, and therefore, a user interface sensing device based on a human readable sign output, which provides real-time visual observation through an LED, was connected in series to the APPS fabric, as demonstrated in the illustrations in Fig. 5a. When the sensing device was attached on the joint of the index finger, the red LED was turned on instantly when the finger was bent, and the intensity of luminescence indicated the degree of finger bending. These demonstrations suggest that our APPS fabric is wearable, power self-sufficient, and easy to integrate more sensing components for pressure sensing application without an external power supply.
Due to the high sensitivity and flexibility of the APPS fabric device, it was employed to detect joint motion with the three-sensor array attached to the wrist, such as monitor clench, wrist down- or up-bending, and swing left or right (Fig. 5b–d). As seen from Fig. 5b–d, the electrical signal variations showed different signal shapes and intensities for the three sensors and three states of motion. This is because the different positions of the joint during the movement of the force were different. Therefore, comprehensively considering the detected signals of both shape and intensity variation, we suppose that the different types of joint movement states can be distinguished, and it is expected to be used as a monitoring device for the rehabilitation training of joint patients.
Moreover, to further explore the application of the APPS fabric in gesture recognition, the integration of five pressure sensors on the five fingers of a man or a glove creates a wearable smart sensor platform to monitor hand movements in real time, as shown in Fig. 5e, where the smart sensor device could detect and distinguish the motion of each finger (the thumb, index, middle, ring, and little finger) simultaneously. Initially, the five fingers were straightened and the output data signals of the five devices were in a smooth initial state. Then, four specific gestures meaning “good”, “yeah”, “OK” and “strong” were performed sequentially, and the recorded data signals well-reflected the gestures with the five fingers. In addition, the continuous gesture language actions of “I Love You” (Fig. 5f) and “Thank You” (Fig. 5g) could be performed to test the monitoring abilities of the smart sensor. The ability of gesture recognition is important part for artificial intelligence technology. Therefore, these promising performance demonstrations indicate that our smart sensor has broad application prospects in gesture language translation.
Conclusions
In conclusion, we developed for the first time an active-powering pressure sensing fabric, which integrates a flexible powering unit with a fabric-based sensing substrate into one intact flexible device architecture, for human physiological and activity monitoring. The powering unit demonstrated an open voltage of 1 V and a short circuit of 35 mA cm−2 and a capacity of 2.6 mA h cm−2 was achieved by controlling the unit area anode weight. Specifically the designed solid neutral hydrogel electrolyte shows high safety and high force-resistance, guaranteeing the stability of the power output under pressure. The resulting APPS fabric device provided the highest sensitivity of 19.6 Ω−1 kPa−1 in the test pressure range (of <300 kPa), a rapid mechanical response time of 9–14 ms, and stable cycling performances (>6000 cycles). Moreover, a theoretical electromechanical model based on the fibrous micro-structure of the fabric and the contact resistance mechanism was established to evaluate and predict the sensing responses of the APPS device. For wearable sensor applications, the APPS fabric device could power the whole system by itself for the real-time detection of pulse waveforms and wireless data transmission via Bluetooth for 18 h or LED for 3 days. Additionally, complex body motions could also be closely tracked including finger motions and hand gesture recognitions. With the desired features of active-power, low cost, and soft human-sensing interface, the APPS fabric exhibits potential and applicability to a wide range of wearable health and activity monitoring applications without involving any active power unit. We believe that this sensing fabric with active-powering will take a step further towards the wide application of wearable electronic devices.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by the National Natural Science Foundation of China (61901460 and 61903355), the Project funded by China Postdoctoral Science Foundation (2019M653125 and 2018M643255), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06D631), the Shenzhen Fundamental Research Program (JCYJ20180305180923182 and JCYJ20170413164102261), and the SIAT Innovation Program for Excellent Young Researchers (201816).References
- S. Park, S. W. Heo, W. Lee, D. Inoue, Z. Jiang, K. Yu, H. Jinno, D. Hashizume, M. Sekino and T. Yokota, Nature, 2018, 561, 516–521 CrossRef CAS PubMed.
- Y. Zang, F. Zhang, C.-a. Di and D. Zhu, Mater. Horiz., 2015, 2, 140–156 RSC.
- Y. Kim, A. Chortos, W. Xu, Y. Liu, J. Y. Oh, D. Son, J. Kang, A. M. Foudeh, C. Zhu and Y. Lee, Science, 2018, 360, 998–1003 CrossRef CAS PubMed.
- B. Nie, R. Li, J. Cao, J. D. Brandt and T. Pan, Adv. Mater., 2015, 27, 6055–6062 CrossRef CAS PubMed.
- C. B. Huang, S. Witomska, A. Aliprandi, M. A. Stoeckel, M. Bonini, A. Ciesielski and P. Samorì, Adv. Mater., 2018, 31, 1804600 CrossRef.
- Z. Zhu, R. Li and T. Pan, Adv. Mater., 2018, 30, 1705122 CrossRef PubMed.
- M. Liu, X. Pu, C. Jiang, T. Liu, X. Huang, L. Chen, C. Du, J. Sun, W. Hu and Z. L. Wang, Adv. Mater., 2017, 29, 1703700 CrossRef PubMed.
- T. Yang, X. Jiang, Y. Zhong, X. Zhao, S. Lin, J. Li, X. Li, J. Xu, Z. Li and H. Zhu, ACS Sens., 2017, 2, 967–974 CrossRef CAS.
- J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim and J. Wang, Lab Chip, 2018, 18, 217–248 RSC.
- W. Weng, P. Chen, S. He, X. Sun and H. Peng, Angew. Chem., Int. Ed., 2016, 55, 6140–6169 CrossRef CAS PubMed.
- J. Wu, Y.-M. Sun, Z. Wu, X. Li, N. Wang, K. Tao and G. P. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 4242–4251 CrossRef CAS PubMed.
- Y. Pang, K. Zhang, Z. Yang, S. Jiang, Z. Ju, Y. Li, X. Wang, D. Wang, M. Jian and Y. Zhang, ACS Nano, 2018, 12, 2346–2354 CrossRef CAS PubMed.
- Z. Zhu, R. Li and T. Pan, Adv. Mater., 2018, 1705122 CrossRef PubMed.
- Y. Han, F. Yi, C. Jiang, K. Dai, Y. Xu, X. Wang and Z. You, Nano Energy, 2019, 56, 516–523 CrossRef CAS.
- S. Han, C. Liu, Z. Huang, J. Zheng, H. Xu, S. Chu, J. Wu and C. Liu, Adv. Mater. Technol., 2019, 4, 1800640 CrossRef.
- H. Chen, Y. Song, X. Cheng and H. Zhang, Nano Energy, 2019, 56, 252–268 CrossRef CAS.
- J. S. Heo, J. Eom, Y. H. Kim and S. K. Park, Small, 2017, 14, 1703034 CrossRef PubMed.
- X. M. T. E. Al, Adv. Mater., 2014, 26, 5310 CrossRef PubMed.
- S. Honda, Q. Zhu, S. Satoh, T. Arie, S. Akita and K. Takei, Adv. Funct. Mater., 2019, 29, 1807957 CrossRef.
- R. Li, Y. Si, Z. Zhu, Y. Guo, Y. Zhang, N. Pan, G. Sun and T. Pan, Adv. Mater., 2017, 29, 1700253 CrossRef PubMed.
- Z. Lou, L. Li, L. Wang and G. Shen, Small, 2017, 13, 1701791 CrossRef PubMed.
- X. Cheng, W. Tang, Y. Song, H. Chen, H. Zhang and Z. L. Wang, Nano Energy, 2019, 61, 517–532 CrossRef CAS.
- J. H. Lee, H. J. Yoon, T. Y. Kim, M. K. Gupta, J. H. Lee, W. Seung, H. Ryu and S. W. Kim, Adv. Funct. Mater., 2015, 25, 3203–3209 CrossRef CAS.
- L. Yu, Y. Yi, T. Yao, Y. Song, Y. Chen, Q. Li, Z. Xia, N. Wei, Z. Tian and B. Nie, Nano Res., 2018, 12, 331–338 CrossRef.
- Z. L. Wang, J. Chen and L. Lin, Energy Environ. Sci., 2015, 8, 2250–2282 RSC.
- P. Bai, G. Zhu, Q. Jing, J. Yang, J. Chen, Y. Su, J. Ma, G. Zhang and Z. L. Wang, Adv. Funct. Mater., 2014, 24, 5807–5813 CrossRef CAS.
- W. Xu, L. B. Huang, M. C. Wong, L. Chen, G. Bai and J. Hao, Adv. Energy Mater., 2017, 7, 1601529 CrossRef.
- F. R. Fan, W. Tang and Z. L. Wang, Adv. Mater., 2016, 28, 4283–4305 CrossRef CAS.
- M. Wu, Y. Wang, S. Gao, R. Wang, C. Ma, Z. Tang, N. Bao, W. Wu, F. Fan and W. Wu, Nano Energy, 2019, 56, 693–699 CrossRef CAS.
- Z. Lin, J. Chen, X. Li, Z. Zhou, K. Meng, W. Wei, J. Yang and Z. L. Wang, ACS Nano, 2017, 11, 8830–8837 CrossRef CAS PubMed.
- C. Y. Su, H. Cheng, W. Li, Z. Q. Liu, N. Li, Z. Hou, F. Q. Bai, H. X. Zhang and T. Y. Ma, Adv. Energy Mater., 2017, 7, 1602420 CrossRef.
- J. Hu, Structure and mechanics of woven fabrics, Elsevier, 2004 Search PubMed.
- N. Pan, J. Compos. Mater., 1994, 28, 1500–1531 CrossRef.
- J. He, P. Xiao, W. Lu, J. Shi, L. Zhang, Y. Liang, C. Pan, S.-W. Kuo and T. Chen, Nano Energy, 2019, 59, 422–433 CrossRef CAS.
- X. Liu, C. Tang, X. Du, S. Xiong, S. Xi, Y. Liu, X. Shen, Q. Zheng, Z. Wang and Y. Wu, Mater. Horiz., 2017, 4, 477–486 RSC.
- F. T. Goh, Z. Liu, T. A. Hor, J. Zhang, X. Ge, Y. Zong, A. Yu and W. Khoo, J. Electrochem. Soc., 2014, 161, A2080–A2086 CrossRef CAS.
- L. An, Z. Zhang, J. Feng, F. Lv, Y. Li, R. Wang, M. Lu, R. B. Gupta, P. Xi and S. Zhang, J. Am. Chem. Soc., 2018, 140, 17624–17631 CrossRef CAS PubMed.
- L. Ma, S. Chen, D. Wang, Q. Yang, F. Mo, G. Liang, N. Li, H. Zhang, J. A. Zapien and C. Zhi, Adv. Energy Mater., 2019, 9, 1803046 CrossRef.
- H. Li, Q. Yang, F. Mo, G. Liang, Z. Liu, Z. Tang, L. Ma, J. Liu, Z. Shi and C. Zhi, Energy Storage Materials, 2019, 19, 94–101 CrossRef.
- Y. J. Li, L. Cui, P. F. Da, K. W. Qiu, W. J. Qin, W. B. Hu, X. W. Du, K. Davey, T. Ling and S. Z. Qiao, Adv. Mater., 2018, 30, 1804653 CrossRef PubMed.
- B. M. Bailey, V. Hui, R. Fei and M. A. Grunlan, J. Mater. Chem., 2011, 21, 18776–18782 RSC.
- J.-Y. Sun, X. Zhao, W. R. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. Suo, Nature, 2012, 489, 133 CrossRef CAS PubMed.
- Y. Li and H. Dai, Chem. Soc. Rev., 2014, 43, 5257–5275 RSC.
- G. Gridling and B. Weiss, Vienna University of Technology Institute of Computer Engineering Embedded Computing Systems Group, 2007 Search PubMed.
- C. L. Choong, M. B. Shim, B. S. Lee, S. Jeon, D. S. Ko, T. H. Kang, J. Bae, S. H. Lee, K. E. Byun and J. Im, Adv. Mater., 2014, 26, 3451–3458 CrossRef CAS PubMed.
- H. Berger, J. Electrochem. Soc., 1972, 119, 507–514 CrossRef CAS.
- L. Kogut and K. Komvopoulos, J. Appl. Phys., 2003, 94, 3153–3162 CrossRef CAS.
- G. S. Marlow and M. B. Das, Solid-State Electron., 1982, 25, 91–94 CrossRef CAS.
- H. Du and J. Zhang, Soft Matter, 2010, 6, 3370–3376 RSC.
- O. Stern, Z. Elektrochem., 1924, 30, 1014–1020 Search PubMed.
- J. L. Yu, Y. M. Liu and B. Z. Jang, Polym. Compos., 1994, 15, 488–495 CrossRef CAS.
- G. Gunduz and, J. Compos. Mater., 2005, 39, 1577–1589 CrossRef.
- K. Meng, J. Chen, X. Li, Y. Wu, W. Fan, Z. Zhou, Q. He, X. Wang, X. Fan and Y. Zhang, Adv. Funct. Mater., 2019, 29, 1806388 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta09395h |
| This journal is © The Royal Society of Chemistry 2020 |