Comparison of different zinc precursors for the construction of zeolitic imidazolate framework-8 artificial shells on living cells†
Wei
Chen
 ,
Shu
Kong
,
Meng
Lu
,
Fangming
Chen
,
Wen
Cai
,
Liping
Du
,
Jian
Wang
and
Chunsheng
Wu
,
Shu
Kong
,
Meng
Lu
,
Fangming
Chen
,
Wen
Cai
,
Liping
Du
,
Jian
Wang
and
Chunsheng
Wu
 *
*
Institute of Medical Engineering, Department of Biophysics, School of Basic Medical Sciences, Health Science Center, Xi’an Jiaotong University, Xi’an 710061, China. E-mail: wuchunsheng@xjtu.edu.cn; Fax: +86-29-82657763; Tel: +86-29-82657763
First published on 21st November 2019
Abstract
The robust cell-in-shell structure is highly desirable for endowing living cells with an artificial exoskeleton to defend them from many environmental factors such as osmotic pressure, shear force, heat, UV radiation, and enzymes. Cell encapsulation has shown potential applications in many fields and attracted increasing interest. However, the influences of the precursors on the cell viability during the shell formation process are not clear and seldom investigated. Here, zinc nitrite, zinc acetate and zinc sulfate were applied individually to synthesize zeolitic imidazolate framework-8 (ZIF-8) shells on living cells. All the zinc salt precursors could convert to a ZIF-8 layer on the living cell surface. The zinc salts and organic ligand did not exhibit obvious toxicity to yeast cells when applied individually. However, dead cells were observed during the living cell encapsulation process using different zinc precursors. Compared with zinc nitrate and zinc acetate, ZIF-8 formed by zinc sulfate led to a higher percentage of cell death, especially under high concentrations of zinc sulfate. Cell division was suppressed by the ZIF-8 shell but restored fully upon shell removal by EDTA solution or pH 4.0 buffer. Escherichia coli (E. coli) cells showed a lower percentage of cell death, indicating excellent tolerance to the ZIF-8 encapsulation process. This work illustrates the cell toxicity during the formation of ZIF-8 cell shells by different zinc salts and engineering of the cell growth by MOF coating, which could provide a foundation for further quantitative analysis and potential applications in biomedicine and bioengineering.
Introduction
Due to the sensitive responses of living organisms to environmental factors, engineering the cell surface is a highly desired approach for cell protection. Nano-coating the cell surface creates a physical barrier between the cell and the external environment without hindering nutrient exchange.1 Besides, these cell-in-shell structures can improve the cellular tolerance against various harsh conditions such as heat,2,3 UV radiation,4,5 toxins,6,7 and mechanical stress.8,9 With incorporation of external molecules to regulate the cell biological behavior or endow cells with new structure and function, cell nanocoating and nanoencapsulation have potential applications in many fields ranging from cell therapy and regenerative medicine, tissue engineering, and sustainable energy to biocatalysis.10–12 Microencapsulation/macroencapsulation cell transplantation processes have been developed for diabetes and other disease treatment and some of them are under preclinical or I/II clinical trials.13The synthetic strategies for cell nanoencapsulation could be classified into three types: the “depositing-to” approach, the “growing-from” approach, and interfacial reactions.10 Specifically, cell surface-induced formation of metal–organic frameworks (MOFs) was reported recently for the formation of cell shells. Viruses, bacteria, and eukaryotic cells could be encapsulated into MOFs.14 For example, S. cerevisiae or Micrococcus luteus were coated with zeolitic imidazole framework-8 (ZIF-8).1,15 The ZIF-8 shell could protect the cells from the damage of lyticase and anti-fungal drugs.15 In addition, the artificial cell shells can be easily removed by EDTA (ethylenediaminetetraacetic acid) treatment if necessary.15 When immobilizing β-galactosidase onto the surface of yeast cells before ZIF-8 formation, the composite shell could enhance the cell viability in the lactose solution since the enzyme can convert the deficient media to nutrients.1 On the other hand, the reaction parameters (e.g. zinc salts, ratio of the two reactants, time, temperature, and surfactants) have direct influences on the crystallinity, size and morphology of ZIF-8.16–18 A low 2-methylimidazole (Hmim)/Zn ratio would lead to the formation of side products in the aqueous solution during the ZIF-8 synthesis.16 Meanwhile, different zinc salts have different dissociation equilibria, inducing differences in the crystal's nucleation and growth.19 Therefore, different zinc salts with different anions, dissociation equilibria and crystal growth behavior could affect the cell viability directly. Different to the cytotoxicity of ZIF-8 nanoparticles to cells, the side effects during the encapsulation process are not clear and it is highly desirable from them to be evaluated.20
In this study, the formation of ZIF-8 on living cells was conducted in a saline solution. Hence, insoluble zinc salts such as zinc chloride, zinc citrate, and zinc bromide cannot be considered. For comparison, three common zinc salt precursors (zinc nitrate, zinc acetate, and zinc sulfate) were used to synthesize the ZIF-8 shell. The cytotoxicity of the zinc salts during the encapsulation process was evaluated on two different types of cells (yeast and bacteria cells). The influence of the artificial shells on cell division was studied on yeast. The results obtained in this study could increase the knowledge on encapsulation of cells by MOFs in general, and specifically on the determination of the concentration of living cells for further applications.
Materials and methods
Materials
Sacharomyces cerevisiae (yeast cells, GMDCC2.167) and Escherichia coli (E. coli, ATCC8739) were supplied by the Guangdong culture collection center. Zinc nitrate (Zn(NO3)2·6H2O), zinc acetate (Zn(Ac)2), zinc sulfate (ZnSO4·7H2O), 2-methylimidazole (Hmim), NaCl, ethylenediaminetetraacetic acid (EDTA) and phosphate buffered saline (PBS) were purchased from Sinopharm Chemical Reagent Co., Ltd. Fluorescein diacetate (FDA) was brought from Sangon Biotech (Shanghai) Co., Ltd.Formation of ZIF-8 shells on yeast cells
One colony of yeast from an agar plate was cultured in yeast culture media (Yeast Mold (YM) medium) with continuous shaking at 30 °C for 18 h. The concentration of the cells was adjusted to about 106 cells per mL with PBS.Due to the degradation of ZIF-8 in PBS, 0.9% NaCl solution was used in the following experiment as the ZIF-8 preparation medium.21 The yeast cells were washed with physiological saline three times. Then, various concentrations of zinc salts and Hmim (dissolved in 0.9% NaCl solution) were added simultaneously under stirring. The reaction was conducted at room temperature for 10 min. The product was collected by centrifugation and washed with saline three times. To distinguish the zinc salt precursors, the ZIF-8 derived from zinc acetate, zinc nitrate and zinc sulfate were named ZIF-8-a, ZIF-8-n and ZIF-s, respectively.
Formation of the ZIF-8 coating on living bacteria
E. coli glycerol stocks were used to inoculate defined overnight cultures in LB medium at 37 °C. 5 mL volumes of cell suspensions were subcultured and harvested at the exponential growth phase. The cells were diluted to an approximate concentration of 106 CFU per mL. The bacteria solution was centrifuged and washed with saline to remove the excess nutrition.The zinc salts and the ligand (dissolved in 0.9% NaCl solution) were added under stirring for 10 min allowing for the formation of crystal ZIF-8 shells. The product was collected by centrifugation and washed with saline several times.
Cell viability test
Cell viability was studied by the FDA method. Metabolically active cells can hydrolyze FDA into fluorescently bright fluorescein by intracellular esterase. An FDA stock solution was prepared by dissolving 10 mg of FDA in 1 mL acetone (a final concentration of 10 mg mL−1). 1 mL of yeast suspension (the shell was removed by EDTA solution, 100 mM in physiological saline) was mixed with 2 μL of the FDA stock solution and incubated for 30 min at 30 °C. The cells were washed with saline three times and tested by fluorescence microscopy.Colony-forming unit (CFU) counting was used to assess the viability of the bacteria.21 EDTA solution (100 mM in 0.9% NaCl solution) was used to dissolve the ZIF-8 shells before the counting. 10 μL of bacteria suspension was taken after the decomposition process. A dilution series was performed to achieve a suitable amount of bacteria on the LB agar plates for counting. The LB agar plates were cultured at 37 °C for 24 h and the results were counted.
Cell growth curves
The untreated cells, encapsulated yeast cells and shell removed yeast cells (two groups: one by EDTA solution (100 mM in 0.9% NaCl solution), another by pH 4.0 buffer (citric acid/sodium citrate in 0.9% NaCl solution)) were cultivated in 50 mL fresh LB medium and the activities of the cells were measured every 2 h at 600 nm by UV-visible spectroscopy.Characterization
The morphology of the structure of the samples was observed using a field emission scanning electron microscope (TESCAN, MALA3 LMH) with an EDS attachment (Aztec X-MaxN). UV-vis absorption spectra were collected using a UNIC UV2600 spectrometer with PBS as the solvent. X-ray diffraction (XRD) patterns of the samples were recorded on an XRD 6100 (SHIMADZU) diffractometer with Cu Kα radiation, 30 kV, 10 mA. Zeta potential data were obtained using a Zetasizer Nano ZSE instrument (Malvern) at 25 °C. Each sample was repeated three times.Results and discussion
Formation of the ZIF-8 coating on yeast cells
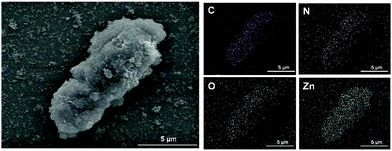
ZIF-8 encapsulated yeast cells were fabricated by an in situ method which dispersed the living cells in the solution containing a desirable precursor for the formation of artificial shells. Three zinc precursors: zinc acetate, zinc nitrate and zinc sulfate, were applied individually due to the different ionization equilibria and coordination ability of the counter anions.22 To characterize the morphology of the artificial cell shells, ZIF-8 encapsulated yeast cells were investigated by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). As shown in Fig. 1, compared with the smooth surface of native yeast (Fig. S1, ESI†), a crystal surface could be found on the encapsulated yeast cells clearly. The hybrid yeast cells derived from the three different zinc salts show similar morphology. However, individual crystals could be found if zinc acetate was used. As is well known, the rate of nucleation is affected by the strength of interactions between the metal ions and counter anions determined by Pearson's acid–base theory.23 A higher rate of nucleation indicates that more nuclei will be formed at the beginning. Besides, it is also found that the zinc nitrate precursor could form a densely-packed continuous layer of nano-sized crystals.22 As shown in Fig. 2, EDS analysis of yeast@ZIF-8-n showed that there are C, N, O, and Zn elements on the surface of the hybrid yeast cells. The existence of the Zn element, which belongs to the ZIF-8, indicates the successful encapsulation of cells with ZIF-8 crystals. | ||
| Fig. 1 SEM images of ZIF-8 coated yeast cells with different zinc salt precursors: (a) yeast@ZIF-8-n, (b) yeast@ZIF-8-a, and (c) yeast@ZIF-8-s. | ||
The formation of artificial shells on the cells was also demonstrated by FTIR (Fig. 3a). The band at 3135 cm−1 was attributed to the aromatic and the aliphatic C–H stretch of imidazole.24 The peak at 424 cm−1 can be assigned as the Zn–N stretch. As shown in Fig. 3b, the XRD patterns of the hybrid yeast cells were all matched to the standard simulation pattern of ZIF-8 data, indicating the existence of ZIF-8 in the hybrid yeast cells. As shown in Fig. 3c, compared with the negative charge of native yeast cells, the zeta potential of the hybrid yeast cells shifted to a positive charge, suggesting the successful ZIF-8 deposition on the surface of the yeast cells.25
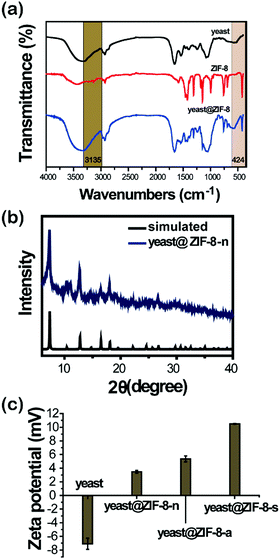 | ||
| Fig. 3 (a) FTIR spectrum of yeast@ZIF-8-n. (b) XRD pattern of yeast@ZIF-8-n. (c) Zeta potential of the yeast and ZIF-8 coated yeast with different zinc salt precursors. | ||
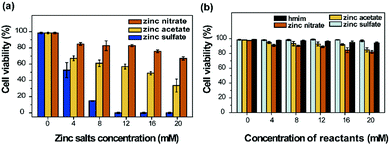
The different types of zinc salts during the cell encapsulation process have shown different toxicity to the yeast cells, as shown in Fig. 4. When the concentration of the zinc salts increased, the cell viability decreased. ZIF-8-s exhibited the highest toxicity to the yeast cells among all the tests. When the concentration of zinc sulfate was 4 mM, nearly half of the cells were dead. All the cells were dead when the concentration was increased to 12 mM. For the zinc acetate, half of the cells were dead when the concentration was 16 mM. The dead cells increased to 60% when the concentration of zinc acetate was increased to 20 mM. The toxicity of the zinc salts and the ligand was investigated separately as a comparison. When the concentration of the zinc salts was 16 mM, no obvious cytotoxicity was observed. When the concentration increased, the ratio of cell death gradually increased. The three zinc salts and the ligand had tiny differences in the toxicity to the yeast cells. Thus, there is an obvious difference in the cytotoxicity between the metal salt precursors and the encapsulation process. The toxicity of ZIF-8 to the yeast cells was related to the precursors, the reaction conditions, the side products and the synergistic effect of the precursors. According to previous reports, the oral LD50 of Zn metal is 0.35 g kg−1, and the rat oral dose of 1-methylimidazole is 8.4 g kg−1.26,27 For human cells (e.g. MCF-7 and HepG2 cells) and zebrafish, ZIF-8 showed high cytotoxicity at a high concentration.28 When the concentration of ZIF-8 was up to 200 μM, all the cells and zebrafish embryos were dead. The significant cytotoxicity of ZIF-8 could be caused by the release of Zn2+ and ligands to the intracellular solution, the generated reactive oxygen species (ROS), and size effects.29–31 Moreover, the nanoparticle-to-cell contact plays an important role in the cytotoxicity evaluation. The modification of the nanoparticles’ uptake and their intracellular solubility are the key components in modulating the bioactivity of ZnO nanoparticles (NPs).32 Upon indirect exposure to ZnO NPs (via dialysis), they showed no cytotoxic effect even at extremely high concentrations, highlighting a requirement for NP-to-cell contact. In this study, the yeast cell wall is composed primarily of β(1,3)-glucan, manoproteins, β(1,6)-glucan and chitin.33 The carbohydrate fraction may represent as much as 90% (w/w) of mannoproteins. Another important feature is that phosphodiester bridges in mannosyl side chains contribute numerous negative charges on the cell surface. Available experimental support suggests that peptidoglycan and polysaccharides play important roles in toxin binding.34 It was found that carbohydrates could be encapsulated into ZIF-8 with 100% efficiency.35,36 Carbohydrates decorated with COO− functional groups could be employed reproducibly for the encapsulation of carbohydrates in MOFs due to the coulombic attraction between the functional groups and Zn2+ cations. Due to the similar structure and surface charge of the cell surface and carbohydrates, the shell was formed on the surface of the cells. Therefore, the existence of the cell wall provides metal ion binding sites (for cell encapsulation) and a biochemical and physical barrier. Although the individual precursors exhibited tiny toxicity, a NP-to-cell contact effect and synergistic effect occurred in the encapsulation process. Besides, the side products, the change of pH, and physical damage should also be considered.
Artificial shells underpin the advancement of engineering strategies toward governing cell division, proliferation, and differentiation. Herein, the time-dependent cellular responses of ZIF-8 coated yeast cells were investigated. OD600 experiments quantitatively measure cell proliferation by determining turbidity and are widely used in the study of the stage of cultured cells.37 For the native yeast, the OD600 remained stable for 4 h before exponential growth was observed, indicating that the yeast cells were initially in a dormant/hibernation state before entering a rapid cell proliferation state. In contrast, the OD600 measurements of the ZIF-8 encapsulated yeast cells showed no obvious growth even after 20 h. It is postulated that the ZIF-8 exoskeleton acted as a physical restriction suppressing cell budding. Fig. 5 shows the OD600 measurements after the removal of ZIF-8 by EDTA solution and pH 4.0 buffer.15,38 Compared to the incubation period of the naked cells (4 h), the lag phase for the removed sample was longer (18 h). The OD600 data showed that the cell growth rate and final cell number of the yeast after the removal of the ZIF-8 shell reached a similar level to the native yeast. Furthermore, there was no difference between the growth curves of the yeast after removal of the MOF shell by EDTA solution and pH 4.0 buffer. Thus, it can be concluded that the ZIF-8 coatings have no measurably negative impact on the yeast cells.
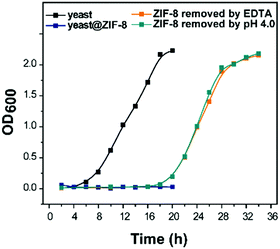 | ||
| Fig. 5 Yeast growth measurement (OD600) for native and ZIF-8 coated yeast before and after the removal of MOF coatings by EDTA solution and pH 4.0 buffer. | ||
Formation of ZIF-8 artificial shells on bacteria cells
The ZIF-8 exoskeleton can also been applied in coating bacteria cells (e.g. E. coli cells). Due to the high toxicity of ZIF-8-s, ZIF-8-n and ZIF-8-a were applied in the treatment of bacteria cells. The ZIF-8 crystal layer could be clearly found from the SEM image as shown in Fig. 6. The compact crystal layer could be observed distinctly for both precursors. Moreover, the preparation process did not affect the cell viability of E. coli. Different to yeast cells, bacteria cells have a smaller size, which benefits the nucleation and growth. Thus, the bacteria hybrid cells have higher crystallinity, in accordance with the simulated pattern. The FTIR spectrum of the hybrid bacteria cells was similar to the hybrid yeast cells. The peaks at 3135 and 2927 cm−1 were assigned to the aromatic and aliphatic C–H stretch of imidazole, respectively. The peak at 424 cm−1 was attributed to the Zn–N stretch.24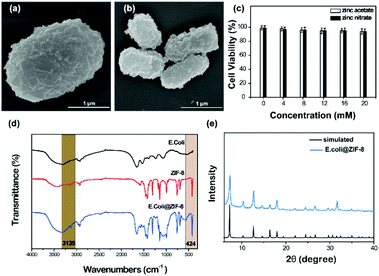 | ||
| Fig. 6 (a) and (b) SEM image of E. coli@ZIF-8-n and E. coli@ZIF-8-a. (c) Cell viability of the coating process. (d) FTIR spectrum of E. coli@ZIF-8-n. (e) XRD pattern of E. coli@ZIF-8-n. | ||
Different to yeast cells, the polymeric structure of the bacterial cell wall comprises glycan strands covalently linked to each other by an interconnecting peptide stem attached to an alternating saccharide of the glycan strands.39 The cell wall of Gram-negative bacteria can be divided into two distinct membranes, inner and outer. The outer leaflet of the cell outer membrane is composed primarily of lipopolysaccharide (LPS), a repeating polysaccharide chain termed “O-antigen” that extends into the extracellular space.40 LPS plays important roles in many fields such as nutrient transport, absorbing metal ions, and mediating the physiological and pathophysiological interaction of bacteria with host organisms.41 Due to the existence of LPS, Gram-negative bacteria exhibit higher tolerance to toxins and antibiotics.42 LPS could absorb zinc ions and benefit nuclei formation and particle growth. On the other hand, LPS could hinder ions entering the cell wall and protect the cells from metal ions. The cell wall of Gram-negative bacteria is stress-stiffening, which could maintain the bacterial cell elasticity by preventing abrupt cell shape changes during changes in external pressure or osmolarity.41 Therefore, the biochemical protection of LPS and the physical structure of the cell wall could hinder the damage from a high osmotic pressure solution.39,43
Conclusions
In conclusion, a cell-in-shell structure was successfully achieved through ZIF-8 coated living cells. Zinc nitrite, zinc acetate and zinc sulfate lead to similar hybrid yeast morphology and structure. Although the three zinc salts and the organic ligand exhibited tiny toxicity to the yeast cells, the in situ encapsulation process involved cell death, especially at the higher concentration of zinc sulfate. The ZIF-8 coating could engineer the cell division, suppressing cell budding and growth. After removal of the MOF shell, the cell growth could recover. This coating method could also be applied in treatment of bacteria cells. In contrast to yeast cells, much less cell death occurred in the encapsulation of E. coli cells. The construction of the MOF coating represents a new and promising approach for the next-generation of cell-based research and applications, endowing living cells with new properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported in part by grants from National Natural Science Foundation of China (Grant No. 51861145307, 31661143030, 31700859, and 31470956), China Postdoctoral Science Foundation (Grant No. 2018M633524), and Fundamental Research Funds for the Central Universities.References
- L. Kang, R. J. Richardson, C. J. Doonan, X. Mulet, Y. Ju, J. Cui, F. Caruso and P. Falcaro, Angew. Chem., Int. Ed., 2017, 56, 8510 CrossRef.
- G. Wang, L. Wang, P. Liu, Y. Yan, X. Xu and R. Tang, ChemBioChem, 2010, 11, 2368 CrossRef CAS.
- E. H. Ko, Y. Yoon, J. H. Park, S. H. Yang, D. Hong, K.-B. Lee, H. K. Shon, T. G. Lee and I. S. Choi, Angew. Chem., Int. Ed., 2013, 125, 12505 CrossRef.
- J. H. Park, K. Kim, J. Lee, J. Y. Choi, D. Hong, S. H. Yang, F. Caruso, Y. Lee and I. S. Choi, Angew. Chem., Int. Ed., 2014, 53, 12420 CAS.
- N. Jiang, X.-Y. Yang, G.-L. Ying, L. Shen, J. Liu, W. Geng, L.-J. Dai, S.-Y. Liu, J. Cao, G. Tian, T.-L. Sun, S.-P. Li and B.-L. Su, Chem. Sci., 2015, 6, 486 RSC.
- D. Hong, H. Lee, E. H. Ko, J. Lee, H. Cho, M. Park, S. H. Yang and I. S. Choi, Chem. Sci., 2015, 6, 203 RSC.
- B. Wang, P. Liu, W. Jiang, H. Pan, X. Xu and R. Tang, Angew. Chem., Int. Ed., 2008, 47, 3560–3564 CrossRef CAS.
- A. Matsuzawa, M. Matsusaki and M. Akashi, Langmuir, 2013, 29, 7362 CrossRef CAS.
- C. Ye, Z. A. Combs, R. Calabrese, H. Dai, D. L. Kaplan and V. V. Tsukruk, Small, 2014, 10, 5087 CAS.
- B. J. Kim, H. Cho, J. H. Park, J. F. Mano and I. S. Choi, Adv. Mater., 2018, 30, 1706063 CrossRef.
- T.-F. Liu, Y. Wang, W. Zhong, B. Li, K. Mequanint, G.-X. Luo and M. Xing, Adv. Healthcare Mater., 2019, 8, 1800939 CrossRef.
- W. Geng, L. Wang, N. Jiang, J. Cao, Y.-X. Xiao, H. Wei, A. K. Yetisen, X.-Y. Yang and B.-L. Su, Nanoscale, 2018, 10, 3112 RSC.
- M. Farina, J. F. Alexander, U. Thekkedath, M. Ferrari and A. Grattoni, Adv. Drug Delivery Rev., 2019, 139, 92 CrossRef CAS PubMed.
- R. Ricco, W. Liang, S. Li, J. J. Gassensmith, F. Caruso, C. Doonan and P. Falcaro, ACS Nano, 2018, 12, 13 CrossRef CAS.
- K. Liang, J. J. Richardson, J. Cui, F. Caruso, C. J. Doonan and P. Falcaro, Adv. Mater., 2016, 28, 7910 CrossRef CAS.
- K. Kida, M. Okita, K. Fujita, S. Tanaka and Y. Miyake, CrystEngComm, 2013, 15, 1794 RSC.
- Y. Pan, D. Heryadi, F. Zhou, L. Zhao, G. Lestari, H. Su and Z. Lai, CrystEngComm, 2011, 13, 6937–6940 RSC.
- K. Kida, K. Fujita, T. Shimada, S. Tanaka and Y. Miyake, Dalton Trans., 2013, 42, 11128 RSC.
- A. Schejn, L. Balan, V. Falk, L. Aranda, G. Medjahdi and R. Schneider, CrystEngComm, 2014, 16, 4493 RSC.
- M. Hoop, C. F. Walde, R. Riccò, F. Mushtaq, A. Terzopoulou, X.-Z. Chen, A. J. deMello, C. J. Doonan, P. Falcaro, B. J. Nelson, J. Puigmartí-Luis and S. Pané, Appl. Mater. Today, 2018, 11, 13 CrossRef.
- M. J. Velasquez-Hernandez, R. Ricco, F. Carraro, F. T. Limpoco, M. L. Moreau, E. Leitner, H. Wiltsche, J. Rattenberger, H. Schrottner, P. Fruhwirt, E. M. Stadler, G. Gescheidt, H. Amenitsch, C. J. Doonan and P. Falcaro, CrystEngComm, 2019, 21, 4538 RSC.
- Y. Cai, M. Strømme and K. Welch, 3 Biotech., 2014, 4, 149 CrossRef.
- G. Ramu, M. Lee and H.-K. Jeong, Microporous Mesoporous Mater., 2018, 259, 155 CrossRef CAS.
- F. Hillman, J. M. Zimmerman, S.-M. Paek, M. R. A. Hamid, W. T. Lim and H.-K. Jeong, J. Mater. Chem. A, 2017, 5, 6090 RSC.
- Y. Hu, H. Kazemian, S. Rohani, Y. Huang and Y. Song, Chem. Commun., 2011, 47, 12694 RSC.
- A. Chakraborty, S. Laha, K. Kamali, C. Narayana, M. Eswaramoorthy and T. K. Maji, Inorg. Chem., 2017, 56, 9426 CrossRef CAS.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232 CrossRef CAS PubMed.
- R. C. Huxford, J. Della Rocca and W. Lin, Curr. Opin. Chem. Biol., 2010, 14, 262 CrossRef CAS.
- À. Ruyra, A. Yazdi, J. Espín, A. Carné-Sánchez, N. Roher, J. Lorenzo, I. Imaz and D. Maspoch, Chem. – Eur. J., 2015, 21, 2508 CrossRef.
- F. Ren, B. Yang, J. Cai, Y. Jiang, J. Xu and S. Wang, J. Hazard. Mater., 2014, 271, 283 CrossRef CAS.
- V. Valdiglesias, C. Costa, G. Kiliç, S. Costa, E. Pásaro, B. Laffon and J. P. Teixeira, Environ. Int., 2013, 55, 92 CrossRef CAS.
- A. Nel, T. Xia, L. Mädler and N. Li, Science, 2006, 311, 622 CrossRef CAS.
- C. Shen, S. A. James, M. D. de Jonge, T. W. Turney, P. F. A. Wright and B. N. Feltis, Toxicol. Sci, 2013, 136, 120 CrossRef CAS.
- L. Popolo, T. Gualtieri and E. Ragni, Med. Mycol., 2001, 39, 111 CrossRef CAS.
- M. A. Lazuriaga, C. E. Benjamin, M. W. Gaertner, H. Lee, F. C. Herbert, S. Mallick and J. J. Gassensmith, Supramol. Chem., 2019, 31, 485 CrossRef.
- E. Astria, M. Thonhofer, R. Ricco, W. Liang, A. Chemelli, A. Tarzia, K. Alt, C. E. Hagemeyer, J. Rattenberger, H. Schroettner, T. Wrodnigg, H. Amenitsch, D. M. Huang, C. J. Doonan and P. Falcaro, Mater. Horiz., 2019, 6, 969 RSC.
- P. H. Shetty and L. Jespersen, Trends Food Sci. Technol., 2006, 17, 48 CrossRef CAS.
- A. L. Koch, Biochim. Biophys. Acta, 1961, 51, 429 CrossRef CAS.
- L. Yan, X. Chen, Z. Wang, X. Zhang, X. Zhu, M. Zhou, W. Chen, L. Huang, V. A. L. Roy, P. K. N. Yu, G. Zhu and W. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 32990 CrossRef CAS.
- D. A. Dik, J. F. Fisher and S. Mobashery, Chem. Rev., 2018, 118, 5952 CrossRef CAS.
- H. Hwang, N. Paracini, J. M. Parks, J. H. Lakey and J. C. Gumbart, Biochim. Biophys. Acta, Biomembr., 2018, 1860, 2566 CrossRef CAS.
- E. T. Rietschel, T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zähringer, U. Seydel and F. D. Padova, FASEB J., 1994, 8, 217 CrossRef CAS.
- H. Dong, Q. Xiang, Y. Gu, Z. Wang, N. G. Paterson, P. J. Stansfeld, C. He, Y. Zhang, W. Wang and C. Dong, Nature, 2014, 511, 52 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sm01907c |
| This journal is © The Royal Society of Chemistry 2020 |