Spatially arranging interfacial droplets at the oil–solid interface†
Ran
Zhang
,
Yao
Wang
and
Zhongqiang
Yang
 *
*
Key Laboratory of Organic Optoelectronics and Molecular Engineering of the Ministry of Education, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: zyang@tsinghua.edu.cn
First published on 16th October 2019
Abstract
The controlling and patterning of small droplets on a solid surface is of significant interest to understand interfacial phenomena and for practical applications. Among interfacial phenomena, the formation of interfacial droplets attracts scientists’ attention, as the mechanism of this phenomenon where water molecules can spontaneously accumulate at the hydrophobic oil/solid interface is still not fully understood. Further investigation is needed to find out specifically where the driving force comes from and how to spatially arrange the interfacial droplets. Herein, self-assembled monolayers are formed on a gold substrate, and it turns out that the hydrophobic surface with a monolayer formed from HS(CH2)11CH3 could inhibit the formation of interfacial droplets; by contrast, the hydrophilic surfaces with monolayers formed from HS(CH2)11COOH, HS(CH2)11NH3·Cl and HS(CH2)11OH, all promote water accumulation. It suggests that the hydrogen bonding between the surface and water proves to be critical in inducing interfacial droplet formation but this has been neglected in past studies. Taking advantage of microcontact printing, the surface chemistry can be controlled at the micron scale and allows spatial arrangement of interfacial droplets at specific regions. This work moves a further step in understanding the mechanism of interfacial droplet formation, and can be potentially exploited for the collection of water and fabrication of microtemplates.
Introduction
There would be an interface for any material with boundaries.1 The study of interfaces not only broadens our knowledge of natural phenomena,2–6 but also directs practical applications.7–11 In reality, natural phenomena and industrial production usually involve multiple phases;12–15 therefore, it is worth studying how multiple interfaces form when two or more phases meet.16–18 For example, surface nanobubbles which are gas filled domains located at a liquid/solid interface are produced by supersaturation of water with gas through a solvent exchange process, resulting in water/gas/solid multiple phases.19–23 When the oversaturated water with oil instead of gas is employed, nanodroplets can be formed in a manner very similar to nanobubbles, ending up with water/oil/solid multiple phases.24–27In analogy to nanobubbles and nanodroplets, another multiple phase system is interfacial droplets. It was reported that water molecules could migrate through a micron-meter thick oil layer and spontaneously form interfacial droplets at the interface between an oil and solid, resulting in oil/water/solid multiple phases.28–30 However, classical thermodynamics cannot explain the reason of spontaneous formation of interfacial droplets, nor where the energy comes from to form a new interface between an oil and solid. Nevertheless, from past study, it is clear that surface chemistry plays an important role. For example, hydrophobic surfaces: gold modified with mixed self-assembled monolayers formed from decanethiol (HS(CH2)9CH3) and hexadecanethiol (HS(CH2)15CH3), and glass treated with octadecyltrichlorosilane (OTS); the wettability of both surfaces was similar but the latter one had higher zeta potential and possessed substantial negative charges. This is mainly contributed by the unreacted residual Si–OH groups, which is known as the driving force for promoting interfacial droplet formation.28 It is learnt from this that, by increasing or decreasing the content of Si–OH groups on the surface, the zeta potential or surface charges can be enhanced or lowered accordingly, which would eventually determine if the formation of interfacial droplets would be promoted or inhibited.29 However, past studies have not explored the surfaces with low zeta potential, which is supposed to have little effect on the induction of interfacial droplet formation, but simultaneously with high wettability. Is the zeta potential or the surface charge the only driving force or should other factors be considered for the interfacial droplet formation? In addition, is it possible to spatially arrange interfacial droplets at a desired location at the micron scale? Both questions have not been answered but would definitely be beneficial for further understanding on the mechanism of interfacial droplet formation and their potential applications such as water collection,31 microtemplate fabrication,32etc.
To obtain new insight into the relation of interfacial droplet formation in dependence of surface wettability and zeta potential, we explored the water/oil/solid sandwich system and investigated how interfacial droplet formation was influenced by surface chemistry which could be easily tuned by forming self-assembled monolayers (SAMs) on a gold surface. For example, thiol molecules with –CH3, –NH3·Cl, –COOH or –OH functional end groups can be anchored on the gold surface via thiol–gold bonds. The –CH3 end group is supposed to provide a hydrophobic modification with low zeta potential, by contrast, the –NH3·Cl, –COOH and –OH end groups would lead to hydrophilic surfaces with positive,33–35 negative and neutral zeta potential, respectively.36,37 We particularly studied how the surface property would promote or inhibit interfacial droplet formation.
Next, inspired by Xuehua Zhang et al.'s work, highly ordered arrays of nanodroplets can be successfully generated on substrates by controlling the surface chemistry at the micron scale.38–40 Herein, we propose that by using microcontact printing, the hydrophilic and hydrophobic micropatterned surfaces can be used selectively to induce or suppress the accumulation of water molecules, leading to a specific arrangement of interfacial droplets at a desired location on the oil–solid interface. The in-depth understanding from this work may facilitate the development of controlling the surface chemistry as a new approach for site selective formation of interfacial droplets and will be potentially useful for various applications, for example, water prefers to set on a hydrophilic area so it would be applied for water collection,41,42 water transportation,43,44 prevention of metal surface corrosion,45 and so on.
Experimental section
1. The preparation of thiol-modified gold substrates
Glass microscope coverslips (22 × 22 mm, Electron Microscopy Sciences, Cat., #72204-1) were immersed in piranha solution, a mixture of H2SO4 and H2O2 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 7
v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and heated to 80 °C for an hour. Then, the coverslips were cooled to room temperature, rinsed with water and then dried in an oven at 100 °C for an hour. Piranha-cleaned glasses were evaporated with 2 nm Cr and 10 nm Au (Tsinghua-Foxconn Nanotechnology Research Center, Tsinghua, Beijing), then gold substrates were obtained. Next, the gold substrate was immersed in 2 mM ethanolic solution containing HS(CH2)11CH3, HS(CH2)15CH3, HS(CH2)17CH3, HS(CH2)11COOH, HS(CH2)11OH and HS(CH2)11NH3·Cl (>99.5%, Sigma-Aldrich) for 2 h, followed by rinsing with ethanol (HPLC grade) and subsequently dried under a stream of nitrogen gas. Thiol–gold substrates with different functional end groups were prepared.
3), and heated to 80 °C for an hour. Then, the coverslips were cooled to room temperature, rinsed with water and then dried in an oven at 100 °C for an hour. Piranha-cleaned glasses were evaporated with 2 nm Cr and 10 nm Au (Tsinghua-Foxconn Nanotechnology Research Center, Tsinghua, Beijing), then gold substrates were obtained. Next, the gold substrate was immersed in 2 mM ethanolic solution containing HS(CH2)11CH3, HS(CH2)15CH3, HS(CH2)17CH3, HS(CH2)11COOH, HS(CH2)11OH and HS(CH2)11NH3·Cl (>99.5%, Sigma-Aldrich) for 2 h, followed by rinsing with ethanol (HPLC grade) and subsequently dried under a stream of nitrogen gas. Thiol–gold substrates with different functional end groups were prepared.
2. X-ray photoelectron spectroscopy (XPS) measurement
XPS experiments were carried out using a PHI Quantera SXM Scanning X-ray Microprobe (INC. ULVAC-PHI. JP). Each data point reported in the paper represents an average of three measurements, which were taken at different fresh portions of the sample.3. Contact angle measurement
Static contact angles of water (3 μL) on substrates were measured at room temperature by using a goniometer (OCA 15 plus, software SCA20, Dataphysics Instruments). The contact angles reported here are the average of at least three measurements. The contact angles of interfacial droplets in oil imaged by a laser scanning confocal microscope (LSCM) were calculated by the image J software from their 3D images.4. Atomic force microscopy (AFM) measurement
The surfaces of bare gold, gold–HS(CH2)11CH3, gold–HS(CH2)11COOH, gold–HS(CH2)11NH3·Cl and gold–HS(CH2)11OH were characterized by AFM (a Brucker, MultiMode 8, RTESP instrument, in tapping mode in air), and the RMS of surfaces was calculated using the nanoscope analysis software.5. The fabrication of thiol-modified gold nanoparticles
For the determination of the zeta potential, we used gold nanoparticles (10 nm, BBI, UK). 200 μL of 2 nM gold nanoparticles in aqueous solution was (i) washed three times by addition of 2 mL water, (ii) centrifuged at 4000 rpm for 15 min, and (iii) the supernatant was removed and resuspended in 200 μL of water. Thiol ethanol solution at a final concentration of 2 mM was added to the solution of gold nanoparticles, sonicated for 15 min, and allowed to incubate for 8 h. The nanoparticles were then washed four times in 400 μL of ethanol by centrifugation at 5000 rpm for 15 min, followed by drying in vacuum and resuspension in 400 μL water by sonication for 15 min. Finally, the thiol-modified gold nanoparticles were obtained.6. Zeta potential measurement
Measurements of zeta-potential were performed using a Zetasizer 3000HS (Malvern Instruments, Worcestershire, UK). The measurements were performed at ambient temperature using an applied voltage of 150 V. The Henry equation was used to calculate zeta-potentials from measurements of electrophoretic mobility.7. The fabrication of polydimethylsiloxane (PDMS) stamps
Prepolymer and crosslinking agent of PDMS (Dow Corning Corporation) were mixed at a weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then casted on the patterned surface of a photolithographic silicon template. After degassing, the sample was cured in an oven at 60 °C for 2 h.46 The cured PDMS was removed and cut into 1 cm × 1 cm size for later use.
1, and then casted on the patterned surface of a photolithographic silicon template. After degassing, the sample was cured in an oven at 60 °C for 2 h.46 The cured PDMS was removed and cut into 1 cm × 1 cm size for later use.
8. The preparation of patterned substrates
A drop of 2 mM HS(CH2)17CH3 ethanol solution was first added onto a PDMS stamp with specific patterns, and dried with nitrogen. Then, the PDMS stamp was put on gold substrates to form a conformal contact for 30 s. Next, the stamp was removed, the gold substrates were washed thoroughly with ethanol and further incubated in 2 mM HS(CH2)11OH ethanol solution for 2 h. Finally, the gold substrates were rinsed with ethanol and dried with nitrogen.9. The formation of interfacial droplets
The protocol from a previous publication30 was adopted. Firstly, thiol–gold substrates were pasted onto culture dishes with a hole (10 mm in diameter). Secondly, gold grids were put on thiol–gold substrates. Thirdly, a drop of oil (n-hexadecane) was dripped onto grids, and the excess was removed by a 10 μL syringe in order to obtain a uniformly filled grid. Lastly, 4 mL of Milli-Q water was poured into the culture dishes. Samples were sealed with parafilm to prevent evaporation of water, and then the samples were incubated in an environmental chamber (temperature: 25 °C, and relative humidity: 50%).10. Optical characterization of interfacial droplets
A Nikon Eclipse Ti microscope with a digital camera (Nikon DS-U3) was used to image the samples. Differential interference contrast (DIC) images were obtained by adding a DIC prism.11. Confocal fluorescence imaging
A Zeiss LSM710 3-channel confocal microscope was used at the Center of Biomedical Analysis, Tsinghua University. 6 μL calcein AM (Sigma) was dissolved into an aqueous phase resulting in a final concentration of 1.5 μM. After incubating for 2 days, the samples were observed using a confocal microscope. A series of bright field, fluorescence and three-dimensional fluorescence images at the same location and selected time intervals were obtained.Results and discussion
1. The properties of substrates
First, we prepared thiol–gold substrates with different functional end groups by reacting thiol with gold to form covalent S–Au bonds; as such a uniform monolayer was obtained.35 In a typical experiment, freshly deposited gold substrates were immersed into 2 mM thiol ethanol solution for 2 h at ambient temperature. Subsequently, they were washed with ethanol and dried with ultrapure nitrogen, resulting in thiol-modified gold substrates. The thiol molecules contain three segments: –SH group on one side, eleven hydrocarbons in the middle, and different functional groups on the other end. Thiols shorter than this length may not guarantee a uniformly packed monolayer.47 Based on the functional end groups, the obtained thiol-modified gold substrates were named as gold–CH3, gold–NH3·Cl, gold–COOH and gold–OH, respectively. In order to fully characterize the surface chemistry, X-ray photoelectron spectroscopy (XPS) was conducted. The results are shown in Table 1 and demonstrated that freshly deposited gold substrates, i.e., bare gold, contained mainly Au(4f). It is known that the gold substrate has high surface energy and it is easy for it to absorb carbon and oxygen from the atmosphere; therefore, the XPS data of the bare gold substrate also showed the presence of C(1s) and O(1s).48 After thiol modification, the content of Au(4f) decreased due to the coverage of thiol molecules. It is noted that from the XPS data, the bare gold surface did not contain sulfur, whereas other thiol–gold substrates all showed the presence of S(2p), which can be attributed to the sulfur in thiols. In addition, XPS of gold–NH3·Cl presented the composition of N(1s) and Cl(2p) due to nitrogen and chloride at the end group of –NH3·Cl. In contrast, both elements were not detected in other substrates in which monolayers contained neither nitrogen nor chloride. The XPS results suggested that thiols with different functional groups were attached to the gold substrates as designed.| End group | C1s | Au4f | O1s | S2p | N1s | Cl2p |
|---|---|---|---|---|---|---|
| –Bare | 36.42 | 56.18 | 7.40 | — | — | — |
| –CH3 | 56.50 | 40.44 | 0.74 | 2.32 | — | — |
| –NH3·Cl | 57.04 | 27.47 | 7.87 | 2.90 | 4.65 | 0.07 |
| –COOH | 58.62 | 30.20 | 8.94 | 2.24 | — | — |
| –OH | 50.31 | 40.85 | 6.57 | 2.27 | — | — |
Next, we further examined the surface wettability and zeta potential of monolayers supported on gold substrates, as shown in Fig. 1. Contact angle measurements indicated that gold–CH3 had the biggest contact angle of about 108.7°, followed by bare gold, gold–NH3·Cl, gold–COOH and gold–OH with the contact angle of about 76.0°, 48.6°, 46.4° and 26.0°, respectively. The gold substrate was known to be hydrophobic,49 while the gold–CH3 substrate presented higher hydrophobicity owing to the hydrophobic functional end group of –CH3. In contrast, –NH3·Cl, –COOH and –OH were hydrophilic functional groups, which drastically changed the hydrophobic bare gold surface into hydrophilic surfaces. The contact angle data had a good agreement with previous reports.35 In order to determine the zeta potential of obtained monolayers, we measured the zeta potential of thiol-modified gold nanoparticles. Fig. 1 shows that the zeta potential of bare gold nanoparticles was about −20.8 mV owing to its high surface charges.50 The zeta potential of gold–NH3·Cl lowered to about −6.4 mV due to the positive charges of the –NH3·Cl group. In contrast, the modification of the –COOH group on the gold surface enhanced the zeta potential to about −58.4 mV, because the negative charges of the −COOH group increased the electronegativity of the gold surface. Besides, –CH3 and –OH were relatively neutral end groups which brought a minor increase on surface charges, and ended up with a zeta potential of about −13.8 mV and −13.0 mV, respectively. Surface roughness of gold–thiol substrates was investigated by AFM measurement, see Table S1 (ESI†). The roughness results were an average of three measurements in an area of 10 × 10 μm2 of substrates. The roughnesses of the five substrates were at a similar range of around 2–5 nm.51 In short, five substrates (gold–CH3, bare gold, gold–NH3·Cl, gold–COOH and gold–OH) were fully characterized by XPS, contact angle, zeta potential and AFM measurement. The substrates with similar roughness but varied wettability and zeta potential were obtained.
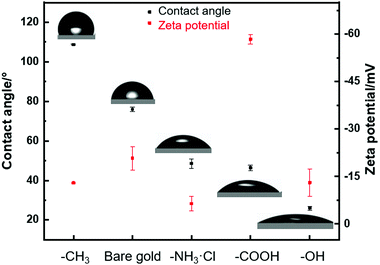 | ||
| Fig. 1 Contact angle (black square) of thiol-modified gold substrates. Zeta potential (red square) of thiol-modified gold nanoparticles. | ||
2. The formation of interfacial droplets on gold–thiol substrates
With the obtained thiol-modified gold substrates containing different functional end groups, we investigated how the surface property, including wettability and zeta potential, influences the formation of interfacial droplets. Following previous experimental procedures,28 a several tens of micrometer thick oil layer, e.g., n-hexadecane, was supported on a solid substrate and then submerged under water, resulting in a water/oil/solid sandwich system. The formation of interfacial droplets was monitored with an optical microscope. The results are shown in Fig. 2; it revealed that for hydrophobic surfaces, including gold–CH3 and bare gold, the formation of interfacial droplets was largely inhibited, and even after 5 days, only a small number of interfacial droplets formed. According to previous study, this phenomenon was due to the low surface charges and little driving force on the substrate to attract water molecules to accumulate.28 In a parallel experiment, at the same time scale, gold–CH3 showed a better suppression on droplet formation than bare gold. It is because the formation of monolayers of dodecanethiol (CH3(CH2)11SH) on the gold substrate lowered the zeta potential of the gold surface from −20.8 mV to −13.8 mV and, as a result, gold–CH3 with lower surface charges could further suppress interfacial droplet formation compared to bare gold. During incubation, the size of interfacial droplets increased, owing to the growth of individual droplets and/or coalescence between droplets. It was noticed that after 5 days, no new interfacial droplets formed and even in the following days till the 28th day (the longest incubation in this study), indicating that solid substrates of gold–CH3 and bare gold were relatively robust for prevention of interfacial droplet formation. We also traced the formation of interfacial droplets and found that the volume of an individual droplet had a linear relationship with time, see Fig. S1 (ESI†). In terms of the morphology of interfacial droplets, it was observed that due to the strong surface tension from the oil and high hydrophobicity from the underlying substrates, the interfacial droplets were squeezed and dewetted to a regular spherical shape.52 The above experimental phenomena related to gold–CH3 and bare gold had a good agreement with a previous study.28 In contrast, hydrophilic surfaces, such as gold–NH3·Cl, gold–COOH and gold–OH, were studied for the first time regarding interfacial droplet formation. It was observed that unlike hydrophobic surfaces, which inhibited droplet formation, the stated three hydrophilic surfaces could significantly promote the growth of a large amount of interfacial droplets within 6 hours of incubation. Inspection of zeta potential data revealed that gold–COOH had the highest zeta potential (−58.4 mV) and corresponded to the highest surface charges among all the substrates. According to a previous study, for example, the higher content of Si–OH groups on substrates usually resulted in higher surface charges and zeta potential. As a consequence, more interfacial droplets formed. However, in the current study, to our surprise, gold–COOH exhibited the highest zeta potential among the three hydrophilic substrates, but formed the fewest interfacial droplets. While the gold–OH substrate, with much lower zeta potential (−13.0 mV) corresponding to lower surface charge than gold–COOH, exhibited much more significant interfacial droplet formation. Furthermore, the zeta potentials of gold–CH3 and gold–OH were very close, −13.8 mV and −13.0 mV, respectively. However, the former surface significantly promoted interfacial droplet formation but the latter surface even inhibited interfacial droplet formation. These results suggested that the zeta potential or the surface charge was not the only factor that triggered the interfacial droplet formation. By carefully examining the above results, we suspected that the hydrogen bonding between the substrate and water molecules might be the origin to induce interfacial droplet formation. Especially, gold–CH3 and gold–OH had very similar zeta potential, but the former surface cannot form hydrogen bonds but the latter one can, suggesting that the surface possesses low zeta potential but has strong interaction with water molecules, such as through hydrogen bonds, and thus can still induce and promote interfacial droplet formation. In addition, based on hydrogen bond theory, the strength of hydrogen bonding between the functional end groups on the solid surface and water would follow the sequence of –COOH, –OH and –NH3·Cl.53 However, the gold–COOH substrate did not show the highest promotion of interfacial droplet formation. It might be because the –COOH group could form intermolecular dimers which decreased the probability of forming hydrogen bonds between –COOH and water molecules. As a result, the least amount of interfacial droplets formed on the gold–COOH among the three hydrophilic substrates in this study. Regarding the interfacial droplet size, once interfacial droplets formed, they would grow individually and/or coalesce between droplets, resulting in droplet growth during incubation. The growth mechanism was consistent with previous studies.30 At last, we inspected the morphology of interfacial droplets on these three hydrophilic substrates. Different from hydrophobic surfaces on which droplets appeared to be of spherical shape, interfacial droplets on hydrophilic surfaces tended to be more irregular and even island like.54,55 Moreover, the more hydrophilic the surface was, the more irregular the morphology interfacial droplets exhibited. In particular, gold–OH showed the lowest contact angle and best wetting for water, interfacial droplets appeared to be the most irregular and even formed a relatively continuous water film. We tried to construct models and found that the surface coverage had a linear relationship with time, see Fig. S2 (ESI†). Combining all the above observations, it can be concluded that the formation of interfacial droplets can be determined not only by zeta potential/surface charge but also through the hydrogen bonding between the substrate and water. The morphology of interfacial droplets was mainly controlled by the surface wettability.41,56 These results inspired us that the formation and the morphology of interfacial droplet could be adjusted by controlling the surface wettability and zeta potential.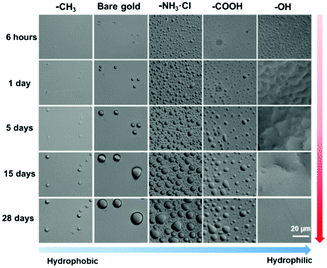 | ||
| Fig. 2 The evolution of interfacial droplets at the interface between different thiol-modified gold substrates and n-hexadecane over incubation in water for 28 days. | ||
3. Spatial arrangement of interfacial droplets
In order to realize the spatial arrangement of interfacial droplets, we chose gold–CH3 and gold–OH, because their surface chemistry has proved to, respectively, inhibit and promote interfacial droplet formation. Here, we took advantage of the microcontact printing technique,46,57,58 so that micron patterns with specific surface chemistry could be achieved. It is expected that interfacial droplets would preferentially form at specific sites where the surface chemistry, such as –OH, shows a strong driving force for water accumulation, whereas at the rest of the sites, the interfacial droplet formation would be significantly hindered by gold–CH3. It is noted that with a longer length of thiol molecules, a better inhibition effect for interfacial droplet formation was achieved and the optimal result in our study was to employ HS(CH2)17CH3 (Fig. S3 and S4, ESI†). The experimental methodology is illustrated in Fig. 3. In a typical experiment, the PDMS stamp (100 μm wide lines separated by 50 μm gaps) inked with HS(CH2)17CH3 was put onto the gold substrate, and the monolayers of HS(CH2)17CH3 with a feature of 100 μm wide lines were obtained, which are supposed to effectively prevent the interfacial droplet formation. Next, by incubation of the post-stamped surface with HS(CH2)11OH ethanol solution, the unstamped bare gold surface (50 μm gaps) was backfilled with HS(CH2)11OH, which would promote the subsequent interfacial droplet formation. In order to characterize the interfacial droplets in three-dimension (3D), upon incubation we added a fluorescent dye, calcein AM, at a final concentration of 1.5 μM into the upper aqueous solution. The fluorescent dye would diffuse into interfacial droplets, allowing 3D visualization of interfacial droplets under a confocal microscope. Fig. 4A showed that after 5 days’ incubation, interfacial droplets with strong fluorescence signals selectively formed at the region of 50 μm wide lines where the surface was modified with HS(CH2)11OH. In contrast, the 100 μm wide lines modified with HS(CH2)17CH3 demonstrated that little interfacial droplets formed. Next, we utlized a stamp with a more complex pattern, that is, “ ”, a Chinese character of water. The line width of the “
”, a Chinese character of water. The line width of the “ ” pattern was 50 μm separated by 100 μm lines. Fig. 4B showed that the feature of “
” pattern was 50 μm separated by 100 μm lines. Fig. 4B showed that the feature of “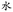 ” with a monolayer formed from HS(CH2)11OH promoted the formation of interfacial droplets. Both experiments suggested that a gold surface micropatterned with a monolayer in a shape of straight lines or a complex character can spatially control the spatial arrangement of interfacial droplet formation.
” with a monolayer formed from HS(CH2)11OH promoted the formation of interfacial droplets. Both experiments suggested that a gold surface micropatterned with a monolayer in a shape of straight lines or a complex character can spatially control the spatial arrangement of interfacial droplet formation.
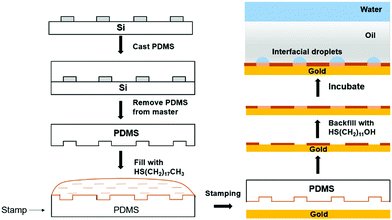 | ||
| Fig. 3 Schematic illustration of the spatial arrangement of interfacial droplets at the oil–solid interface by controlling the surface chemistry at the micron scale via microcontact printing. | ||
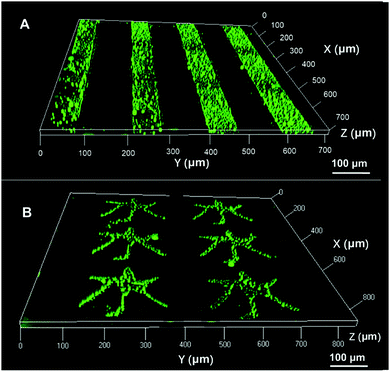 | ||
Fig. 4 The confocal images of interfacial droplets spatially arranged in (A) line or (B) “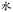 ” (Chinese character of water) micron patterns. ” (Chinese character of water) micron patterns. | ||
Conclusions
In summary, we prepared thiol-modified gold substrates, investigated how surface wettability and zeta potential influenced the behaviors of interfacial droplets formed at oil–solid interfaces and precisely arranged the interfacial droplets to a specific region. Through comprehensive characterizations, we found that for hydrophobic surfaces, gold–CH3 and bare gold, the formation of interfacial droplets was largely inhibited, because both surfaces had low zeta potential/surface charge. The prevention of interfacial droplet formation on the gold–CH3 surface was superior to bare gold, which can be attributed to the lower zeta potential on the former surface. For hydrophilic surfaces, gold–NH3·Cl, gold–COOH and gold–OH, the zeta potential could not fully explain the formation of interfacial droplets.28–30 Instead, it is the first time that hydrogen bonds between surface chemistry and water was proposed to influence the interfacial droplet formation. Among the three surfaces, although –COOH can form the strongest hydrogen bond, it simultaneously formed dimers intermolecularly and decreased the possibility of forming a hydrogen bond with water.53,59 It turned out that the gold–OH formed the most interfacial droplets. Finally, by spatially controlling the monolayers with specific functional end groups, such as modified gold surfaces with HS(CH2)17CH3 and HS(CH2)11OH with a certain pattern, interfacial droplet formation could be specifically inhibited or promoted on the surface, respectively. As a result, interfacial droplets in a micron scale pattern were spatially arranged. Such site-selective formation of interfacial droplets may be potentially utilized for microtemplates60,61 and microreactors.62,63Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21872078).Notes and references
- H. J. Butt, K. Graf and M. Kappl, Physics and Chemistry of Interfaces, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany, 2004 Search PubMed.
- M. H. Wood, M. T. Casford, R. Steitz, A. Zarbakhsh, R. J. L. Welbourn and S. M. Clarke, Langmuir, 2016, 32, 534–540 CrossRef CAS.
- Y. Zheng, H. Bai, Z. Huang, X. Tian, F. Nie, Y. Zhao, J. Zhai and L. Jiang, Nature, 2010, 463, 640–643 CrossRef CAS.
- T. L. Liu and C. J. Kim, Science, 2014, 346, 1096–1100 CrossRef CAS.
- J. M. Campbell, F. C. Meldrum and H. K. Christenson, J. Phys. Chem. C, 2015, 119, 1164–1169 CrossRef CAS.
- L. O. Figura and A. A. Teixeira, Food Physics:Physical Properties–Measurement and Applications, Springer-Verlag, Berlin Heidelberg, 2007 Search PubMed.
- B. Su, Y. Tian and L. Jiang, J. Am. Chem. Soc., 2016, 138, 1727–1748 CrossRef CAS PubMed.
- B. P. Binks and R. Murakami, Nat. Mater., 2006, 5, 865–869 CrossRef CAS PubMed.
- D. Daniel, J. V. I. Timonen, R. Li, S. J. Velling and J. Aizenberg, Nat. Phys., 2017, 13, 1020–1025 Search PubMed.
- J. Lv, Y. Song, L. Jiang and J. Wang, ACS Nano, 2014, 8, 3152–3169 CrossRef CAS.
- S. Liao, Y. Tao, W. Du and Y. Wang, Langmuir, 2018, 34, 11655–11666 CrossRef CAS.
- P. H. Dixneuf and V. Cadierno, Metal-Catalyzed Reactions in Water, Wiley-VCH Verlag GmbH & Co. KGaA, 2013 Search PubMed.
- D. J. am Ende, Chemical Engineering in the Pharmaceutical Industry: R&D to Manufacturing, John Wiley & Sons, Inc., 2010 Search PubMed.
- S. A. Vitale and J. L. Katz, Langmuir, 2003, 19, 4105–4110 CrossRef CAS.
- A. Méndez-Vilas, A. B. Jódar-Reyes and M. L. González-Martín, Small, 2009, 5, 1366–1390 CrossRef.
- T. Kajiya, F. Schellenberger, P. Papadopoulos, D. Vollmer and H. J. Butt, Sci. Rep., 2016, 6, 23687 CrossRef CAS.
- X. Huang, H. Dong, Z. Liu and Y. Zhao, Langmuir, 2019, 35, 5442–5447 CrossRef CAS PubMed.
- J. S. Turner and T. G. L. Silirtcliefe, Nature, 1970, 228, 1083–1084 CrossRef CAS.
- J. L. Parker, P. M. Claesson and P. Attard, J. Phys. Chem., 1994, 98, 8468–8480 CrossRef CAS.
- D. Lohse and X. Zhang, Rev. Mod. Phys., 2015, 87, 981–1035 CrossRef CAS.
- S. Lou, Z. Ouyang, Y. Zhang, X. Li, J. Hu, M. Li and F. Yang, J. Vac. Sci. Technol., B, 2000, 18, 2573–2575 CrossRef CAS.
- N. Ishida, T. Inoue, M. Miyahara and K. Higashitani, Langmuir, 2000, 16, 6377–6380 CrossRef CAS.
- R. P. Berkelaar, E. Dietrich, G. A. M. Kip, E. S. Kooij, H. J. W. Zandvliet and D. Lohse, Soft Matter, 2014, 10, 4947–4955 RSC.
- X. Zhang and W. Ducker, Langmuir, 2007, 23, 12478–12480 CrossRef CAS.
- X. Zhang, Z. Lu, H. Tan, L. Bao, Y. He, C. Sun and D. Lohse, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 9253–9257 CrossRef CAS.
- M. Li, L. Bao, H. Yu and X. Zhang, J. Phys. Chem. C, 2018, 122, 8647–8654 CrossRef CAS.
- C. Xu, H. Yu, S. Peng, Z. Lu, L. Lei, D. Lohse and X. Zhang, Soft Matter, 2017, 13, 937–944 RSC.
- Z. Yang and N. L. Abbott, Langmuir, 2010, 26, 13797–13804 CrossRef CAS PubMed.
- Y. Li, Q. Yang, M. R. Andy, M. Cai, J. Y. Y. Heng and Z. Yang, J. Phys. Chem. B, 2017, 121, 6766–6772 CrossRef CAS PubMed.
- R. Zhang, W. Liao, Y. Sun, J. Y. Y. Heng and Z. Yang, J. Phys. Chem. C, 2019, 123, 1151–1159 CrossRef CAS.
- M. Ma, G. Tocci, A. Michaelides and G. Aeppli, Nat. Mater., 2016, 15, 66–72 CrossRef CAS.
- Y. He, X. Xiao, Y. Wu and J. Fu, Sci. Rep., 2015, 5, 13522 CrossRef CAS PubMed.
- C. Xu, S. Peng, G. Qiao and X. Zhang, Langmuir, 2016, 32, 11197–11202 CrossRef CAS PubMed.
- M. Vinoba, K. S. Lim, S. H. Lee, S. K. Jeong and M. Alagar, Langmuir, 2011, 27, 6227–6234 CrossRef CAS PubMed.
- V. Chechik and C. J. M. Stirling, Gold-thiol self-assembled monolayers, John Wiley & Sons, Ltd, 2009 Search PubMed.
- V. Chegel, O. Rachkov, A. Lopatynskyi, S. Ishihara, I. Yanchuk, Y. Nemoto, J. P. Hill and K. Ariga, J. Phys. Chem. C, 2012, 116, 2683–2690 CrossRef CAS.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Chem. Soc. Rev., 2010, 39, 1805–1834 RSC.
- L. Bao, A. R. Rezk, L. Y. Yeo and X. Zhang, Small, 2015, 11, 4850–4855 CrossRef CAS PubMed.
- L. Bao, Z. Werbiuk, D. Lohse and X. Zhang, J. Phys. Chem. Lett., 2016, 7, 1055–1059 CrossRef CAS.
- S. Peng, B. Pinchasik, H. Hao, H. Möhwald and X. Zhang, J. Phys. Chem. Lett., 2017, 8, 584–590 CrossRef CAS.
- X. Dai, N. Sun, S. O. Nielsen, B. B. Stogin and J. Wang, Sci. Adv., 2018, 4, 919 Search PubMed.
- H. Bai, L. Wang, J. Ju, R. Sun, Y. Zheng and L. Jiang, Adv. Mater., 2014, 26, 5025–5030 CrossRef CAS.
- M. Ma, F. Grey, L. Shen, M. Urbakh, S. Wu, J. Liu and Q. Zheng, Nat. Nanotechnol., 2015, 10, 692–695 CrossRef CAS.
- H. Chen, P. Zhang, L. Zhang, H. Liu, Y. Jiang, D. Zhang, Z. Han and L. Jiang, Nature, 2016, 532, 85–89 CrossRef CAS.
- O. Lebedeva, G. Jungurova, A. Zakharov, D. Kultin, E. Chernikova and L. Kustov, J. Phys. Chem. C, 2012, 116, 22526–22531 CrossRef CAS.
- J. L. Wilbur, A. Kumar, H. A. Biebuyck, E. Kim and G. M. Whitesides, Nanotechnology, 1996, 7, 452–457 CrossRef CAS.
- C. D. Bain, E. B. Troughton, Y. Tao, J. Evall, G. M. Whitesides, M. Hill and G. M. Whitesides, J. Am. Chem. Soc., 1990, 28, 263–328 Search PubMed.
- T. Ishida, S. Tsuneda, N. Nishida, M. Hara, H. Sasabe and W. Knoll, Langmuir, 1997, 13, 4638–4643 CrossRef CAS.
- C. D. Bain, E. B. Troughton, Y. T. Tao, J. Evall, G. M. Whitesides and R. G. Nuzzo, J. Am. Chem. Soc., 1989, 111, 321–335 CrossRef CAS.
- M. Barquins and J. Cognard, Gold Bull., 1986, 19, 82–86 CrossRef CAS.
- H. Nakae, R. Inui, Y. Hirata and H. Saito, J. Biomater. Appl., 1998, 46, 2313–2318 CAS.
- Y. Wang, Y. Wang, M. Cai, H. Li and Z. Yang, Chem. Res. Chin. Univ., 2018, 39, 2003–2009 CAS.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Elsevier Butterworth-Heinemann, 1997 Search PubMed.
- H. Zhao and D. Beysens, Langmuir, 1995, 11, 627–634 CrossRef CAS.
- S. Karpitschka, A. Pandey, L. A. Lubbers, J. H. Weijs, L. Botto and S. Das, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7403–7407 CrossRef CAS.
- A. Faghihnejad and H. Zeng, Langmuir, 2013, 29, 12443–12445 CrossRef CAS.
- S. Alom and C. S. Chen, Soft Matter, 2007, 3, 168–177 RSC.
- L. Yan, X. Zhao and G. M. Whitesides, J. Am. Chem. Soc., 1998, 7863, 6179–6180 CrossRef.
- J. Ran and M. Wah Wong, Aust. J. Chem., 2009, 62, 1062–1067 CrossRef CAS.
- H. Tara and P. Janda, Langmuir, 2016, 32, 11221–11229 CrossRef.
- A. Hozumi, S. Kojima, S. Nagano, T. Seki, N. Shirahata and T. Kameyama, Langmuir, 2007, 23, 3265–3272 CrossRef CAS.
- X. Yao, Y. Zhang, L. Du, J. Liu and J. Yao, Renewable Sustainable Energy Rev., 2015, 47, 519–539 CrossRef CAS.
- B. Ahmed-omer, C. Brandt and T. Wirth, Org. Biomol. Chem., 2007, 5, 733–740 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sm01720h |
| This journal is © The Royal Society of Chemistry 2020 |
