Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Production of fatty alcohols from non-edible oils by enzymatic cascade reactions†
Yunjian
Ma
a,
Xizhen
Zhang
b,
Yongru
Li
a,
Peilin
Li
 a,
Frank
Hollmann
a,
Frank
Hollmann
 *c and
Yonghua
Wang
*c and
Yonghua
Wang
 *a
*a
aSchool of Food Science and Engineering, Overseas Expertise Introduction Center for Discipline Innovation of Food Nutrition and Human Health (111 Center), South China University of Technology, Guangzhou 510640, China. E-mail: yonghw@scut.edu.cn
bSchool of Biology and Biological Engineering, South China University of Technology, Guangzhou 510640, China
cDepartment of Biotechnology, Delft University of Technology, Van der Maasweg 9, 2629HZ, Delft, The Netherlands. E-mail: F.Hollmann@TUDelft.nl
First published on 15th June 2020
Abstract
A biocatalytic cascade transforming castor oil into (R,Z)-octadec-9-en-7-ol is presented by combining a lipase catalysed hydrolysis of castor oil into ricinoleic acid followed by a photoenzymatic decarboxylation into (R,Z)-octadec-9-en-7-ol. Conversion of up to 41.7% and overall product concentrations of up to 60 mM, this new bienzymatic and photocatalytic cascade exhibits significant potential for the valorisation of non-edible castor oil. The scope and limitations of the current system are described and discussed.
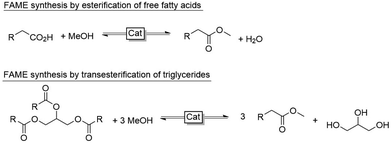
En route to a biobased economy, non-edible plant oils or agricultural waste oils are increasingly getting in focus as renewable feedstock.1 Here, especially castor oil takes a prominent position and is currently evaluated as starting material for ricinoleate-based biodiesels.2,3 To the best of our knowledge, however, the methods of the state-of-the-art almost exclusively centre around the transesterification of castor oil or the esterification of ricinoleic acid. All these methods share the intrinsic disadvantage of being reversible reactions thereby necessitating large molar surpluses of the alcohol to attain significant conversion into the desired biodiesel fatty acid methyl ester (FAME) (Scheme 1).
An alternative approach to valorise (waste) vegetable oils into biofuel is to convert them into the corresponding (C1-shortened) alkanes via decarboxylation (Scheme 1). First, the irreversible nature of the reaction facilitates process design. Second, the alkanes exhibit, compared to the corresponding FAMES and FAEEs, significantly higher caloric values. Today, a focus is put on thermal decarboxylation methods using homogeneous or heterogeneous transition metal catalysts. Generally, these methods require elevated reaction temperatures (typically >200 °C) and therefore are energy-intensive.4
Enzymatic decarboxylation reactions, requiring significantly lower reaction temperatures, today are mostly limited to amino acid decarboxylases5–8 or malonate decarboxylases.9 More recently, oxidative decarboxylation of fatty acids to terminal alkenes10–15 and oxidative decarboxylation of amino acids yielding nitriles16–18 have received some attention.
Recently, an alternative route to valorise long-chain fatty acids into value-added products by using a newly identified photodecarboxylase from the microalga Chlorella variabilis NC64A (CvFAP)19 has come up.20–24CvFAP catalyses the irreversible decarboxylation of a range of fatty acids into the corresponding (C1-shortened) alkanes. Hence, CvFAP not only opens up an irreversible alternative to the established reversible (trans)esterification methods but also yields products exhibition an overall higher degree of reduced carbon atoms and therewith also a higher specific caloric value.
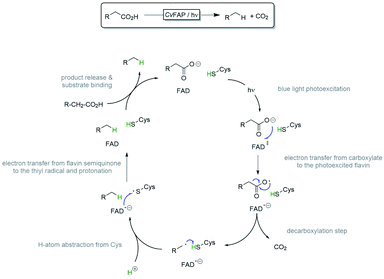
For catalytic activity this enzyme requires activation with blue (λ = 450 nm) light. Though the catalytic mechanism has not been elucidated fully yet, it is clear that a photoactivated flavin prosthetic group catalyses a single electron transfer from the enzyme-bound, deprotonated carboxylate yielding a carboxyl radical and a flavin semiquinone intermediate. The latter rapidly decarboxylates, assisted by a nearby cysteine serving as H-atom donor for the transient carbon radical species. Electron transfer from the flavin to the thiyl radical and reprotonation closes the catalytic cycle (Scheme 2).25
Very recently, we have reported the preparative application of CvFAP for the conversion of some (short and medium chain) carboxylic acids as well as for some natural fatty acids.20–24 However, only edible oils and fatty acids have been considered so far, which we believe have only a limited potential for the preparation of biofuels. Therefore, we became interested in evaluating non-edible castor oil as possible source for ‘next generation’ biofuels.
Castor oil, containing approximately 90% of ricinoleic acid as fatty acid component, is produced at some 600–800 million pounds every year.26 Its main applications so far are as lubricant, polymer precursor as well for the synthesis of surfactants.27–29
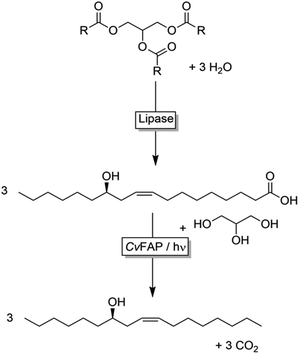
Here, we propose using castor oil as starting material for the synthesis of ((R,Z)-heptadec-9-en-7-ol) by a bienzymatic cascade reaction. Castor oil is first hydrolysed using the commercially available (immobilised) lipase from Rhizopus oryzae (ROL); the free ricinoleic acid then is decarboxylated to (R,Z)-octadec-9-en-7-ol using the aforementioned CvFAP (Scheme 3). Factors influencing the efficiency of the single steps and the combined system have been investigated.
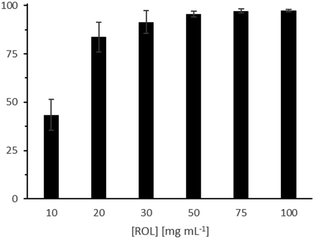
In a first set of experiments, we investigated the first step of the cascade, i.e. the ROL-catalysed hydrolysis of castor oil. A ROL concentration of 100 mg mL−1 was sufficient to reach >95% hydrolysis within 24 h (Fig. 1; Table S2,† entries 1–6).
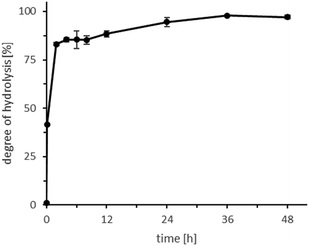
This preliminary evaluation was confirmed by a more detailed time-course of the reaction (Fig. 2; Table S2,† entries 7–15). Already after 2 h, more than 80% of the initial castor oil was hydrolysed; the reaction was essentially complete (98%) after 36 h.
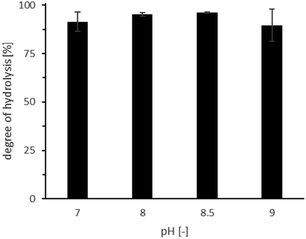
Having the preference of CvFAP for alkaline media in mind, we also evaluated the effect of pH on the ROL-catalysed hydrolysis (Fig. 3; Table S2,† entries 16–19). A very minor reduction at pH 9 may be suspected from these experiments, however, we did not consider this as a major obstacle for the envisioned cascade.
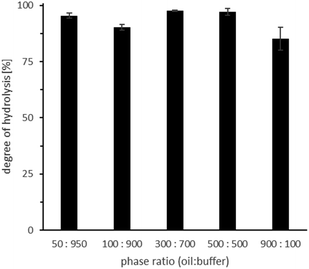
The catalytic potential of ROL is even higher than estimated for these experiments because increasing the substrate amount hardly influenced the overall conversion after 24 h reaction time (Fig. 4; Table S2,† entries 20–24).
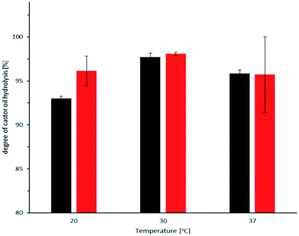
Again, having the bienzymatic cascade in mind, we also investigated the influence of blue-light irradiation on the activity of ROL. As shown in Fig. 5 (and in Table S2,† entries 25–27) no significant influence of blue-light illumination was observed. In these experiments we also varied the reaction temperature and found only a minor influence on the overall reaction rate.
Therefore, we concluded that ROL is highly compatible with the reaction conditions required for the CvFAP-catalysed photodecarboxylation reaction and drew our attention to the photoenzyme.
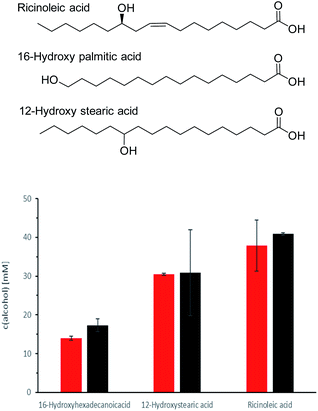
So far, CvFAP had been applied mainly for non-functionalised fatty acids. We therefore also investigated whether CvFAP also displays catalytic activity on some hydroxylated fatty acids (such as ricinoleic acid) (Fig. 6, Table S3†).
Ricinoleic acid (C18) was converted to more than 80% into the corresponding (C1-shortened product), also 12-hydroxy stearic acid (C18) gave very good conversions while 16-hydroxy palmitic acid (C16) was converted to only about 30%. In these experiments we also compared the efficiency of crude cell extracts and whole cells (from which the crude extracts had been prepared) for the photoenzymatic decarboxylation reactions and found no significant difference.
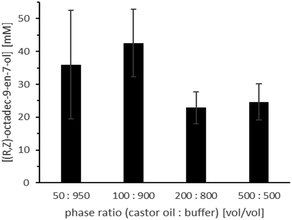
Next, we used both catalysts in a one-pot-one-step reaction cascade and characterised the factors influencing the overall reaction. In contrast to the similar experiment with ROL alone (Fig. 4) we found a significant influence of the substrate (castor oil) loading (Fig. 7; Table S4,† entries 9–12).
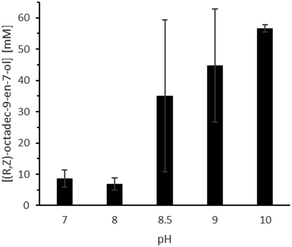
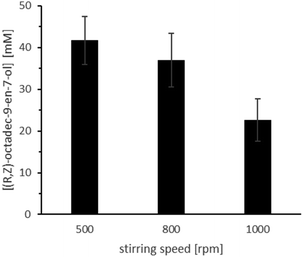
The overall conversion caved in significantly in the presence of higher substrate amounts. A possible explanation for this observation is that a higher castor oil amount also provides higher amounts of ricinoleic acid, which can acidify the aqueous reaction medium. Also, increasing the castor oil amount results in a lower absolute amount of buffer and further favours acidification. This hypothesis is supported by the clear preference of the overall reaction system for more alkaline reaction conditions (Fig. 8; Table S4,† entries 4–8) and the peculiar influence of the stirring rate (Fig. 9, Table S4,† entries 1–3). In the latter case, reduced phase transfer limitations probably accelerated hydrolysis rate and thereby the acidification of the aqueous reaction medium.
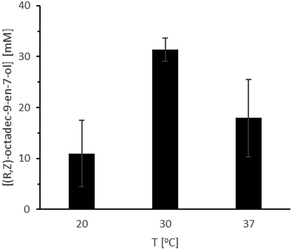
The temperature dependence of the bienzymatic system differed somewhat on the dependency of the ROL-catalysed hydrolysis (Fig. 5) as a clear optimum at 30 °C was observed (Fig. 10, Table S4,† entries 13–15), which can be rationalised by the comparably poor thermal stability of CvFAP.23
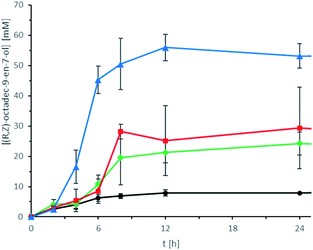
This assumption is also supported by the influence of the photodecarboxylase concentration on the overall product concentration. As shown in Fig. 11, a very clear dependency of the overall product concentration on the amount of CvFAP (as whole cells) added to the reaction mixture was observed.
Even though decently high concentrations of the desired (R,Z)-octadec-9-en-7-ol have been obtained, one striking observation was that in almost all cases product formation ceased after approx. 8–12 h. This is a clear indication that the robustness of CvFAP soon becomes the overall limitation for the envisaged transformation of castor oil into (R,Z)-octadec-9-en-7-ol.30
Conclusions
In the current contribution we have demonstrated the proof-of-concept, scope and limitation of a bienzymatic cascade to transform non-edible castor oil into value added (R,Z)-octadec-9-en-7-ol.Final product concentrations as high as 60 mM have been achieved, which may already be sufficient for an economically feasible synthesis of high-value added products. To the best of our knowledge, this contribution represents the first synthesis of (R,Z)-octadec-9-en-7-ol, which as chiral, unsaturated alcohol may find various applications ranging from bioactive compound to new lubricant. However, envisioning an application for the synthesis of biofuels, the current system probably fails in efficiency. Further optimisation of the activity ratios of ROL and CvFAP will be needed to alleviate the acidification issue of the bienzymatic reaction and to eventually increase the product titres. Also the sometimes rather high standard deviations observed in our triplicate experiments necessitate further improvements in the standardisation of the experimental procedure to improve reproducibility. Also the robustness of the photodecarboxylase needs to be improved. Probably, protein engineering represents the most promising approach here.
Overall, provided the aforementioned current limitations have been addressed, we are convinced that the here-proposed valorisation of agricultural by-products may significantly contribute to the development of a more sustainable, bio-based economy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Outstanding Youth Science Foundation of China (31725022), National Key R&D Program of China (2018YFC0311104), International Collaboration Base for Molecular Enzymology and Enzyme Engineering (2017A050503001), and also supported by the 111 Project (B17018). The Netherlands Organization for Scientific Research is gratefully acknowledged for financial support through a VICI grant (no. 724.014.003).Notes and references
- M. M. Gui, K. T. Lee and S. Bhatia, Energy, 2008, 33, 1646–1653 CrossRef CAS.
- P. Berman, S. Nizri and Z. Wiesman, Biomass Bioenergy, 2011, 35, 2861–2866 CrossRef CAS.
- N. Sánchez, J. M. Encinar, S. Nogales and J. F. González, Catalysts, 2019, 9, 864 CrossRef.
- G. J. S. Dawes, E. L. Scott, J. Le Notre, J. P. M. Sanders and J. H. Bitter, Green Chem., 2015, 17, 3231–3250 RSC.
- P. M. Könst, M. C. R. Franssen, E. L. Scott and J. P. M. Sanders, Green Chem., 2011, 13, 1167–1174 RSC.
- P. M. Könst, P. M. C. C. D. Turras, M. C. R. Franssen, E. L. Scott and J. P. M. Sanders, Adv. Synth. Catal., 2010, 352, 1493–1502 CrossRef.
- P. M. Könst, M. C. R. Franssen, E. L. Scott and J. P. M. Sanders, Green Chem., 2009, 11, 1646–1652 RSC.
- Y. Teng, E. L. Scott, A. N. T. van Zeeland and J. P. M. Sanders, Green Chem., 2011, 13, 624–630 RSC.
- R. Kourist, P. Dominguez de Maria and K. Miyamoto, Green Chem., 2011, 13, 2607–2618 RSC.
- C. E. Wise, C. H. Hsieh, N. L. Poplin and T. M. Makris, ACS Catal., 2018, 8, 9342–9352 CrossRef CAS.
- C. Lu, F. Shen, S. Wang, Y. Wang, J. Liu, W.-J. Bai and X. Wang, ACS Catal., 2018, 8, 5794–5798 CrossRef CAS.
- J. Wang, R. Lonsdale and M. T. Reetz, Chem. Commun., 2016, 52, 8131–8133 RSC.
- I. Zachos, S. Gassmeyer, D. Bauer, V. Sieber, F. Hollmann and R. Kourist, Chem. Commun., 2015, 51, 1918–1921 RSC.
- M. Pickl, S. Kurakin, F. G. Cantú Reinhard, P. Schmid, A. Pöcheim, C. Winkler, W. Kroutil, S. P. de Visser and K. Faber, ACS Catal., 2018, 9, 565–577 CrossRef PubMed.
- A. Dennig, S. Kurakin, M. Kuhn, A. Dordic, M. Hall and K. Faber, Eur. J. Org. Chem., 2016, 3473–3477, DOI:10.1002/ejoc.201600358.
- X. M. Xu, A. But, R. Wever and F. Hollmann, ChemCatChem, 2020, 12, 2180–2183 CrossRef CAS.
- A. But, A. van Noord, F. Poletto, J. P. M. Sanders, M. C. R. Franssen and E. L. Scott, Mol. Catal., 2017, 443, 92–100 CrossRef CAS.
- A. But, J. Le Nôtre, E. L. Scott, R. Wever and J. P. M. Sanders, ChemSusChem, 2012, 5, 1199–1202 CrossRef CAS PubMed.
- D. Sorigué, B. Légeret, S. Cuiné, S. Blangy, S. Moulin, E. Billon, P. Richaud, S. Brugière, Y. Couté, D. Nurizzo, P. Müller, K. Brettel, D. Pignol, P. Arnoux, Y. Li-Beisson, G. Peltier and F. Beisson, Science, 2017, 357, 903–907 CrossRef PubMed.
- W. Zhang, J.-H. Lee, S. H. H. Younes, F. Tonin, P.-L. Hagedoorn, H. Pichler, Y. Baeg, J.-B. Park, R. Kourist and F. Hollmann, Nat. Commun., 2020, 11, 2258 CrossRef CAS PubMed.
- H. J. Cha, S. Y. Hwang, D. S. Lee, A. R. Kumar, Y. U. Kwon, M. Voss, E. Schuiten, U. T. Bornscheuer, F. Hollmann, D. K. Oh and J. B. Park, Angew. Chem., Int. Ed., 2020, 59, 7024–7028 CrossRef CAS PubMed.
- W. Zhang, M. Ma, M. Huijbers, G. A. Filonenko, E. A. Pidko, M. van Schie, S. de Boer, B. O. Burek, J. Bloh, W. J. H. van Berkel, P. W. A. Smith and F. Hollmann, J. Am. Chem. Soc., 2019, 141, 3116–3120 CrossRef CAS PubMed.
- Y. Ma, X. Zhang, W. Zhang, P. Li, Y. Li, F. Hollmann and Y. Wang, ChemPhotoChem, 2020, 4, 39–44 CrossRef CAS.
- M. Huijbers, W. Zhang and F. Hollmann, Angew. Chem., Int. Ed., 2018, 57, 13648–13651 CrossRef CAS PubMed.
- D. J. Heyes, B. Lakavath, S. J. O. Hardman, M. Sakuma, T. M. Hedison and N. S. Scrutton, ACS Catal., 2020, 6691–6696, DOI:10.1021/acscatal.0c01684.
- H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2010, 112, 10–30 CrossRef CAS.
- S. Allauddin, R. Narayan and K. V. S. N. Raju, ACS Sustainable Chem. Eng., 2013, 1, 910–918 CrossRef CAS.
- J. Fiorelli, D. D. Curtolo, N. G. Barrero, H. Savastano, E. M. de Jesus Agnolon Pallone and R. Johnson, Ind. Crops Prod., 2012, 40, 69–75 CrossRef CAS.
- L. Zhang, M. Zhang, L. Hu and Y. Zhou, Ind. Crops Prod., 2014, 52, 380–388 CrossRef CAS.
- B. Lakavath, T. M. Hedison, D. J. Heyes, M. Shanmugam, M. Sakuma, R. Hoeven, V. Tilakaratna and N. S. Scrutton, Anal. Biochem., 2020, 600, 113749 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0se00848f |
| This journal is © The Royal Society of Chemistry 2020 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: