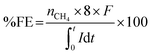Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced methane producing microbial electrolysis cells for wastewater treatment using poly(neutral red) and chitosan modified electrodes†
Hathaichanok
Seelajaroen
 *a,
Sabine
Spiess
*a,
Sabine
Spiess
 *bc,
Marianne
Haberbauer
bc,
Melissa Maki
Hassel
c,
Abdalaziz
Aljabour
a,
Sophie
Thallner
bc,
Georg M.
Guebitz
*bc,
Marianne
Haberbauer
bc,
Melissa Maki
Hassel
c,
Abdalaziz
Aljabour
a,
Sophie
Thallner
bc,
Georg M.
Guebitz
 cd and
Niyazi Serdar
Sariciftci
cd and
Niyazi Serdar
Sariciftci
 a
a
aLinz Institute for Organic Solar Cells (LIOS), Institute of Physical Chemistry, Johannes Kepler University Linz, Altenberger Strasse 69, 4040 Linz, Austria. E-mail: hathaichanok.seelajaroen@jku.at
bK1-MET GmbH, Stahlstrasse 14, 4020 Linz, Austria. E-mail: sabine.spiess@k1-met.com
cacib GmbH (Austrian Centre of Industrial Biotechnology), Krenngasse 37/2, 8010 Graz, Austria
dDepartment of Agrobiotechnology, Institute of Environmental Biotechnology, University of Natural Resources and Life Sciences Vienna, Konrad-Lorenz-Strasse 20, 3430 Tulln an der Donau, Austria
First published on 28th May 2020
Abstract
Microbial electrolysis cells (MECs) consisting of a bioanode and biocathode offer a promising solution for wastewater treatment. These systems can degrade organic substances at the bioanode while converting carbon dioxide (CO2), a major greenhouse gas, to a value-added fuel, methane (CH4) at the biocathode. The bioelectrodes were inoculated with a mixed culture under anaerobic conditions. By applying a constant potential of 0.40 V vs. Ag/AgCl (3 M NaCl), the long-term performance of MECs has been studied by monitoring the removal of chemical oxygen demand (COD) in the anolyte which contained synthetic wastewater and CH4 generation in the cathode chamber. To investigate the effect of electrode modification, poly(neutral red) and chitosan modified carbon felt electrodes were prepared, and applied in MECs. The results revealed that MECs with modified electrodes showed remarkably enhanced overall performance. The average COD removal efficiency, faradaic efficiency towards CO2 reduction to CH4 and CH4 production yield of modified MECs were up to 67%, 55% and 0.14 LCH4/gCOD, respectively.
Introduction
The rise in anthropogenic carbon dioxide (CO2) content, mainly due to fossil fuel burning, leads to an increase in global temperature and further to climate change. This has been first recognized and manually calculated by Svante Arrhenius in his paper back in 1896.1 Not only the environmental effects, but also the depletion of such fuels is critical. Therefore, cleaner and more sustainable sources of energy are urgently needed.2–4 During the last few decades, bio-electrochemical systems (BESs) have gained a lot of interest.3 The BES is, by definition, an electrochemical system where at least one of the redox reactions is microbially assisted and the electrode at which such a reaction occurs is called a bioelectrode (bioanode and biocathode).5 BESs can be implemented in broad applications including electricity generation,5 wastewater treatment,6–8 bioremediation9,10 and production of energy storage chemicals e.g. methane (CH4),2 formate,11 and ethanol.12 Such BESs are operated normally under mild working conditions, show high selectivity towards the desired product, and offer great availability and self-regeneration properties.5 The first evidence of electrical interaction between microorganisms, which were later named electroactive microorganisms, and an electrode was reported by Potter et al. in 1911.13 This extracellular electron transfer3,4,13 includes two mechanisms: direct and indirect/mediated electron transfer.14,15 Direct electron transfer is the inherent interaction between a bacterium, for example Geobacter sulfurreducens, and an electrode through redox proteins such as cytochromes or conductive pili, mentioned as nanowires.14–19 On the other hand, indirect electron transfer describes an interaction between a microorganism and an electrode with the aid of electron shuttles, either endogenous moecules20 (generated by microorganisms themselves as their secondary metabolites, like flavin,20 phenanzine21 and quinones18) or exogenous molecules22,23 (externally added like methylene blue23 and methyl viologen24).Bioanodes have been investigated for their capabilities of oxidizing organic matter, like glucose,25 acetate26 and more complex substrates like landfill leachate27 and wastewater from various sources.6 Such oxidation generates electrons, which are transferred to the anode. For example, 24 electrons could be harvested from the oxidation of glucose, as shown in eqn (1) (E0′ = −0.41 V vs. standard hydrogen electrode (SHE)).28 A system, which contains a bioanode and an abiotic cathode, is called a microbial fuel cell (MFC).4 This technology is considered as a promising wastewater treatment system due to its capability of organic oxidation concomitant with energy harvesting directly in electricity form.6
| C6H12O6 + 6H2O → 6CO2 + 24H+ + 24e− | (1) |
In conventional MFCs, protons are generated from the oxidation of organic matters and coupled with oxygen, an electron acceptor, generating water at the cathode. To improve the efficiency of the system, introduction of prompted catalysts (like platinum) for useful chemical generation in the cathode chamber has been investigated.29 Instead of using expensive materials, microbial biocathodes are an alternative potential option.17,30–33 The direct electron uptake from the cathode through microbes has been adopted for several applications, for example, treatment of heavy-metals (bioremediation),34 reduction of nitrate (denitrification)35 and reduction of CO2 to value-added fuels such as formate11 and CH4 (eqn (2), E0′ = −0.24 V vs. SHE).2 Such BESs, where energy is invested to enhance reaction kinetics or overcome thermodynamic energy barriers, are called microbial electrolysis cells (MECs).5 The electrochemical CO2 reduction to CH4 usually suffers from large overpotential (for example, an overpotential of 1.22 V from using Cu electrodes36). Our group has shown the utilization of such microbial cathodes in a MEC for CO2 reduction to CH4 with a relatively low overpotential of 0.25 V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 and it has been reported that CH4 production was observed in a broad range of production rates.38 The combination of a bioanode and a biocathode now shall accomplish two tasks simultaneously and utilize the advantages of introducing microorganisms to both electrodes.39–42 The coupling of CO2 reduction with the aforementioned microbial oxidation of organic substances is, in principle, feasible by using electrons, protons and CO2 which are generated during oxidation on the bioanode.42 The required voltage of such systems could theoretically be eliminated due to a positive electrochemical cell voltage for CH4 production with glucose oxidation (Ecell = 0.17 V).
37 and it has been reported that CH4 production was observed in a broad range of production rates.38 The combination of a bioanode and a biocathode now shall accomplish two tasks simultaneously and utilize the advantages of introducing microorganisms to both electrodes.39–42 The coupling of CO2 reduction with the aforementioned microbial oxidation of organic substances is, in principle, feasible by using electrons, protons and CO2 which are generated during oxidation on the bioanode.42 The required voltage of such systems could theoretically be eliminated due to a positive electrochemical cell voltage for CH4 production with glucose oxidation (Ecell = 0.17 V).
| CO2 + 8H+ + 8e− → CH4 + 2H2O | (2) |
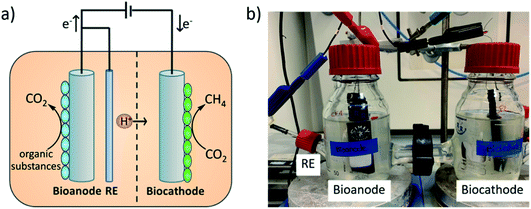
A schematic of an MEC system where a bioanode and a biocathode are combined, is demonstrated in Fig. 1a. Such systems have been investigated for various applications e.g. oxygen reduction to hydrogen peroxide,30 denitrification35 and CO2 reduction to CH4.42,43 The MEC approach for CH4 production offers several advantages, for example the tunability of the degradation/generation rate.41 Therefore, the MEC could be an effective addition to the existing anaerobic digestion systems by improving biogas quality.5,44,45 The performance of BESs largely depends on the quality of electroactive biofilms on electrodes and also on the electron transfer process.46,47 Therefore, several attempts have been made to investigate the effects of different electrode materials and modifications.48 Various electrodes including carbon-based materials (e.g. graphite, carbon felt, carbon cloth and carbon mesh) and other inert materials (e.g. stainless steel) are commonly used in BESs.14,29,46 Further, the surfaces of these materials were modified, aiming to improve microbial growth and electron transfer processes. One example is the modification of carbon-based electrodes with positively charged materials like ammonia,49 chitosan,50 or conductive polymers, for example, polyaniline51 and polypyrrole.52 Chitosan is a bio-polymer and a derivative of chitin, a component of arthropod exoskeletons. It has been applied in many applications due to its low cost, biocompatibility, non-toxicity and high chemical and thermal stability.53 These modifications resulted in improved bacterial colonization and enhanced electron transfer, which further improved the overall performance of BESs.54,55 Another interesting material is poly(neutral red). It is a conductive polymer which could be prepared electrochemically in a slightly acidic to neutral pH solution56 and its monomer, neutral red, is a staining dye which was used in various bio-electrochemical systems as a redox mediator to facilitate electron transfer between the microbe and electrode.57–59 Recently, we reported on the contribution of poly(neutral red) modified carbon felt cathodes, for the enhancement of microbial electrochemical reduction of CO2 to formate by Methylobacterium extorquens. The study showed an improvement in electron transfer processes leading to higher formate production rates, as compared to non-treated electrodes.60 However, to the best of our knowledge, each of the chitosan and poly(neutral red) electrode modifications has not been investigated for the anode and cathode at the same time in a MEC. Therefore, this idea has attracted our interest in order to investigate the effect of modified electrodes not only on one redox reaction but also on both redox processes simultaneously.
In this study, we report a full assembly of an MEC (Fig. 1b), equipped with bioelectrodes, for two applications: wastewater treatment at the bioanode and CO2 reduction to CH4 at the biocathode. The long-term performance of the methane-producing MEC for synthetic wastewater treatment was monitored. The system was evolved by using bioelectrodes (bioanode and biocathode) inoculated with the same mixed culture microorganisms. The experiments were performed at a controlled anode potential of 0.40 V vs. Ag/AgCl (3 M NaCl) and the oxidation of synthetic wastewater was observed by monitoring the change of the anolyte chemical oxygen demand (COD) value, which reflected directly the efficiency of wastewater treatment. Moreover, electromethanogenesis at the biocathode was tracked by CH4 generation in the headspace. Furthermore, carbon felt electrodes were modified with chitosan and poly(neutral red), serving as supports for microorganisms for both the cathode and anode. These two materials were chosen due to the previously reported results, low material costs, good biocompatibility and facile synthesis.52,55 The performance of all three MECs namely MEC 1 (equipped with non-modified carbon felt electrodes), MEC 2 (equipped with poly(neutral red) modified carbon felt electrodes) and MEC 3 (equipped with chitosan modified carbon felt electrodes) were compared, reflecting the effect of electrode modifications.
Results and discussion
Two different carbon felt electrode modifications were investigated by using poly(neutral red) and chitosan. The poly(neutral red) modified carbon felts were prepared by electropolymerization of neutral red, as reported in our previous work.60 The chitosan modified electrodes were prepared chemically through carbodiimide chemistry (Scheme 1). Firstly, the carbon felt surface was oxidized under strong acid conditions of concentrated HNO3. The resulting carboxylic acid groups were later activated through coupling reactions of N-hydroxy-3-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS) prior to reacting with primary amine groups of chitosan.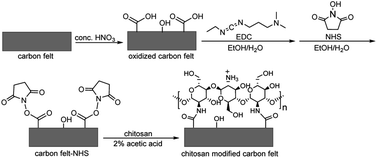 | ||
| Scheme 1 Schematic preparation of chitosan modified carbon felt. EDC: N-hydroxy-3-(3-dimethylaminopropyl)-N′-ethylcarbodiimide; NHS: N-hydroxysuccinimide. | ||
To investigate the long-term performance of MECs, three MECs namely MEC 1, 2 and 3 were built. In MEC 1, non-modified carbon felts were used as the anode and cathode, while MEC 2 and 3 were equipped with poly(neutral red) and chitosan modified electrodes, respectively.
All bioelectrodes in each MEC were inoculated with a mixed culture taken from sewage sludge in a two-compartment electrochemical cell with a controlled potential of 0.40 V vs. Ag/AgCl (3 M NaCl). During the adaptation phase, the bioanode was fed once a week with synthetic wastewater consisting of organic substances like glucose and acetate, while the biocathode was fed with glucose and CO2 to ensure enough biomass formation. After a 4 week adaptation, the faradaic currents were observed at around 2 mA, indicating successfully developed bioelectrodes.61
After the adaptation period, the cathodic solution was replaced with a glucose-free medium. All three developed MECs showed capabilities of organic oxidation in the anodic compartment and simultaneous CH4 generation in the cathodic compartment. To investigate the systems' performance, long-term electrolyses of MEC 1, 2 and 3 were carried out, by applying a constant potential of 0.40 V vs. Ag/AgCl (3 M NaCl) at the anode in a batch operation mode. During the operation of around 90 days, half of the anodic solution was replaced twice a week with a freshly prepared medium, consisting of synthetic wastewater with an average COD concentration of 600 mg L−1, while cathodic chambers were purged with CO2 two times a week. The systems were monitored for 3 running cycles, through analysis of organic degradation by COD determination of anodic solutions and headspace analysis of CH4 production in cathodic chambers.
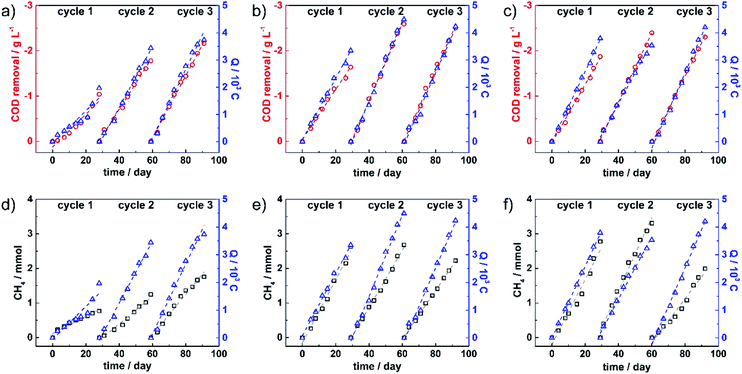
The accumulated COD removal in each running cycle (red circle data point) was plotted together with total electrical charge (Q) (blue triangle data point) over the entire running time, as shown in Fig. 2a–c for MEC 1, 2 and 3, respectively. The data were fitted linearly as presented in dashed lines, showing reaction rates in each cycle. The total COD removal, COD removal rate, average COD removal efficiency, total Q and Q rate are summarized in Table 1.
| MEC 1 | MEC 2 | MEC 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Average ± SD | 1 | 2 | 3 | Average ± SD | 1 | 2 | 3 | Average ± SD | |
| CH4 production/mmol | 0.8 | 1.3 | 1.8 | 1.3 ± 0.5 | 2.6 | 2.7 | 2.2 | 2.5 ± 0.3 | 2.8 | 3.3 | 2.0 | 2.7 ± 0.7 |
| Q/103 C | 2.0 | 3.4 | 3.7 | 3.0 ± 0.9 | 3.3 | 4.5 | 4.2 | 4.0 ± 0.6 | 3.8 | 3.5 | 4.2 | 3.8 ± 0.4 |
| COD removal/g L−1 | 1.0 | 1.8 | 2.2 | 1.7 ± 0.6 | 1.6 | 2.6 | 2.5 | 2.2 ± 0.6 | 1.9 | 2.4 | 2.3 | 2.2 ± 0.3 |
| CH4 production rate/μmol per day | 25 | 40 | 60 | 42 ± 18 | 90 | 80 | 70 | 80 ± 10 | 90 | 110 | 60 | 87 ± 25 |
| Q rate/C per day | 60 | 110 | 130 | 100 ± 36 | 110 | 150 | 140 | 133 ± 21 | 130 | 110 | 140 | 127 ± 15 |
| COD removal rate/mg per L per day | 40 | 60 | 70 | 57 ± 15 | 55 | 85 | 80 | 73 ± 16 | 65 | 80 | 70 | 72 ± 8 |
| Average% FECH4 | 39 | 29 | 38 | 35 ± 6 | 66 | 47 | 44 | 52 ± 12 | 57 | 72 | 36 | 55 ± 18 |
| Average% COD removal efficiency | 25 | 52 | 55 | 44 ± 17 | 52 | 67 | 72 | 64 ± 10 | 56 | 73 | 71 | 67 ± 9 |
| CH4 yield/LCH4/gCOD | 0.09 | 0.08 | 0.09 | 0.09 ± 0.01 | 0.18 | 0.12 | 0.10 | 0.13 ± 0.04 | 0.16 | 0.15 | 0.10 | 0.14 ± 0.04 |
The results revealed that accumulated COD removal in MEC 1 increased from 1.0 g L−1 in the first cycle to 1.8 and 2.2 g L−1 in the second and third cycles, respectively, showing an enhancement of the oxidation process over the running cycles. Significantly higher COD removal values were observed at 1.6 and 1.9 g L−1 in the first cycle of MEC 2 and 3. In the second cycle, the accumulated COD values found in MEC 2 and 3 were enhanced by 1.0 and 0.5 g L−1 from the first cycle, respectively, while a slight drop was detected in the third cycle. The removal rates obtained from the slope of fitting lines, were found to be in the range of 40–85 mg per L per day. The results revealed that the removal rate increased largely from 40 mg per L per day in the first cycle to 70 mg per L per day in the third cycle of MEC 1, while the highest removal rates were observed in MEC 2 and 3 with 85 and 80 mg per L per day in cycle 2, respectively. The large increase in COD removal from the first to the second cycle in all MECs might be related to biofilm growth in the first cycle. Over the whole experiment, average COD removal rates were observed at 57, 73 and 72 mg per L per day in MEC 1, 2 and 3, respectively. Additionally, average COD removal efficiencies were calculated giving the overall efficiencies of 44%, 64% and 67% in MEC 1, 2 and 3, respectively. These results reflected that enhanced organic degradation strongly related to both modifications.
Furthermore, the electrical charge plots revealed that during all three cycles, electrons were consumed continuously in all MECs. In MEC 1, accumulated charges increased from 2.0 × 103 C in the first cycle to 3.4 × 103 and 3.7 × 103 C in the second and third cycles, respectively. The accumulated charges collected in the first cycle of MEC 2 and 3 were found to be almost two times higher than that of MEC 1, suggesting an enhanced electron transfer process at the first cycle in the modified electrode containing systems. This observation might relate to better coverage of biofilms on modified carbon felts. The accumulated charges were further improved in the second cycle and dropped slightly in the third cycle of MEC 2. While those of MEC 3 were of 3.5 × 103 and 4.2 × 103 C in cycle 2 and 3, respectively. Average rates for electron flux were observed for MEC 1, 2 and 3 at 100, 133 and 127 C per day, respectively. The highest electron flux was observed in MEC 2 with 150 C per day in cycle 2, followed by MEC 3 with 140 C per day in cycle 3. Although MEC 1 showed a much lower Q rate in the first cycle relative to MEC 2 and 3, it reached the rate of around 100 C per day which is in the same range as MEC 3 in the second cycle. This observation suggested that biofilm formation on bare carbon felts took longer, as compared to treated carbon felts.
In the cathodic compartment, the CH4 production was quantified twice a week. Fig. 2d–f show the plots of accumulative CH4 concentration (black square data point) and accumulative Q (blue triangle data point) during each running cycle of MEC 1, 2 and 3. The CH4 production rate and the average faradaic efficiencies are summarized in Table 1. The produced CH4 observed in MEC 1 rose continuously from 0.8 mmol in the first cycle to 1.3 mmol in the second cycle to finally 1.8 mmol in the third cycle. In MEC 2, the amount of produced CH4 in cycles 1 and 2 was relatively stable at 2.6 and 2.7 mmol, respectively. However, the production declined to 2.2 mmol in the third cycle, while produced CH4 in MEC 3 increased from 2.8 mmol in cycle 1 to 3.3 mmol in cycle 2 and dropped significantly to 2.0 mmol in cycle 3. The decline observed in MEC 2 and 3 in the third cycle, might result from the instability of the modified electrodes and/or detachment of biofilms. The CH4 production rates in the three MECs were found to be in the range of 25–110 μmol per day and the average production rates over the entire experiments in MEC 1, 2 and 3 were 42, 80 and 87 μmol per day, respectively. Together with the obtained charges, faradaic efficiencies towards the CO2 reduction to CH4 were calculated according to the equation given in the experimental part. The corresponding faradaic efficiencies were averaged within each running cycle. MEC 1 showed efficiencies of 39% in cycle 1, 29% in cycle 2 and 38% in cycle 3, and an overall average efficiency of 35% while the highest faradaic efficiency in MEC 3 was reached at 72% in cycle 2 and then decreased to 36% in cycle 3. The overall average efficiencies of MEC 2 and 3 were reported to be 52 and 55%, respectively.
Considering CH4 production relative to the removed COD (CH4 yield), MEC 1 revealed a relatively stable CH4 yield at 0.09 LCH4/gCOD while the yield observed from MEC 2 and 3 decreased from the first cycle to the third cycle and the average yields were of 0.13 and 0.14 LCH4/gCOD, respectively.
Concerning the performance in both cathodic and anodic chambers, MEC 2 and MEC 3 showed significantly higher organic degradation efficiencies, faradaic efficiencies toward the conversion of CO2 to CH4 and CH4 yield, as compared to those of MEC 1. Moreover, higher numbers of electrons were delivered in the modified electrode containing systems. This might be due to biofilm coverage and/or facilitated electron transfer by the coating. As suggested in the previous report, a positively charged electrode modification with chitosan coating, could enhance the interaction between the electrode and Gram-negative microorganisms like Sporomusa ovata, resulting in improved microbial electrolysis rates.54 However, the instability of the modified systems for CO2 reduction to CH4 was observed during the long-term running.
Compared to the reported studies on methane-producing MECs using non-treated carbon felt electrodes (Table 2), our results (MEC 1) showed lower COD removal efficiency but significantly higher CH4 yield. While, with the electrode modifications (MEC 2 and 3), the higher efficiencies were now comparable to those of the continuous-operating MECs equipped with graphite granules and nickel mesh. Furthermore, using carbon felt offers several advantages as it is highly flexible, has high active surface area, high conductivity and is less corrosive.62 However, it is important to be noted that apart from electrode material, other parameters like cell configuration, inoculation, substrate and operation parameters are of importance.63 Therefore, this comparison was aimed to show the general view of this field and parameters which could be further optimized.
| Anode material | Cathode material | Operation | Cell geometry | E app or Vapp | Substrate | %COD removal | %FECH4 | CH4 yield/LCH4/gCOD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated from the given data; Eapp: applied potential; Vapp: applied voltage; NG: not given, max: maximum value. | |||||||||
| Graphite granules | Graphite granules | Continuous | Two chamber | +0.2 V vs. SHE | Synthetic organic mixture | 75 ± 10% | 61 ± 5% | 0.25a | 65 |
| Graphite granules | Graphite granules | Continuous | Two chamber | +0.2 V vs. SHE | Synthetic organic mixture | 70 ± 2% | 47 ± 2% | 0.17a | 43 |
| Graphite granules | Nickel mesh | Continuous | Upflow | 0.8 V | Beer wastewater | 90% (max) | NG | 0.14 | 66 |
| Graphite granules | Graphite granules | Continuous | Two chamber | +0.2 V vs. SHE | Acetate | 94% | 79 ± 2% | ∼0.25a | 67 |
| Carbon felt | Carbon felt | Continuous | Two chamber | 0.8 V | Synthetic wastewater | ∼80% | NG | 0.04a | 68 |
| Carbon felt | Carbon felt | Batch | Two chamber | +0.4 V vs. Ag/AgCl | Synthetic wastewater | 44 ± 17% | 35 ± 5.5% | 0.09 | This work |
| Poly(neutral red) modified carbon felt | Poly(neutral red) modified carbon felt | Batch | Two chamber | +0.4 V vs. Ag/AgCl | Synthetic wastewater | 64 ± 10% | 52 ± 11.9% | 0.13 | This work |
| Chitosan modified carbon felt | Chitosan modified carbon felt | Batch | Two chamber | +0.4 V vs. Ag/AgCl | Synthetic wastewater | 67 ± 9% | 55 ± 18.1% | 0.14 | This work |
After the long-term operation of around 90 days, samples of bioanodes and biocathodes of all MECs were dried under ambient conditions overnight for SEM measurements. Fig. 3 presents SEM images of bioanodes and biocathodes of MEC 1, 2 and 3. The bioanodes obtained from modified carbon felt electrodes showed better coverage and thicker biofilms (Fig. 3b and c). High magnification images (Fig. 3d–f), from both modified and non-modified carbon felt electrodes are colonized from different cell shape bacteria, revealing mixed microbial strains. In the SEM images of all biocathodes, rod-shaped cells of bacteria were found to be located on carbon fibers (Fig. 3j–l). This characteristic shape refers to some species from phylum Firmicutes like S. ovata64 and the Euryarchaeota Methanobacterium palustre,2 which are capable of growing autotrophically by using a cathode as the sole electron donor and CO2 as the carbon source.
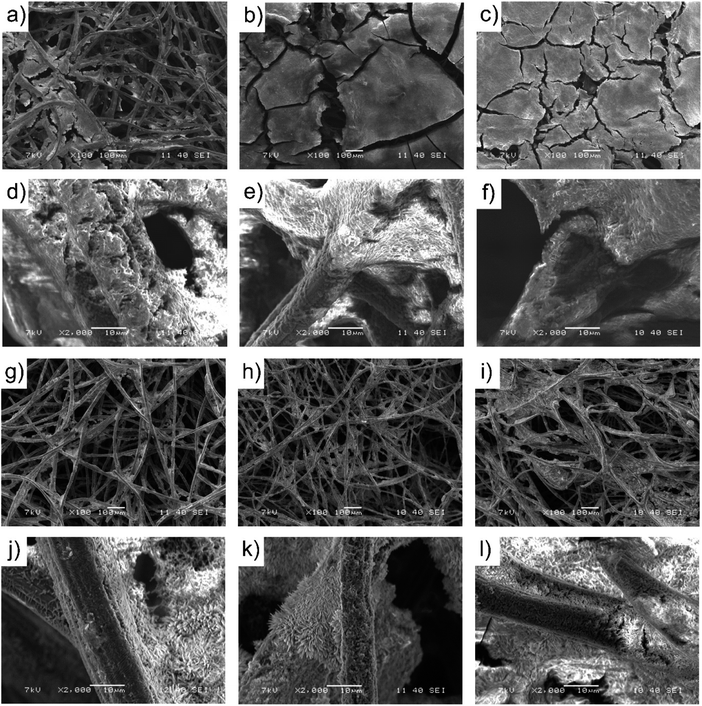 | ||
| Fig. 3 SEM images of bioanodes of MEC 1 (a and d), MEC 2 (b and e) and MEC 3 (c and f) and biocathodes of MEC 1 (g and j), MEC 2 (h and k) and MEC 3 (i and l). | ||
The utilization of electrochemical impedance spectroscopy (EIS) in BESs is highly significant since extensive information of the systems can be extracted, such as the charge transfer resistances and the mechanism of the electron transfer, among many others. In this present study, the electrical loss in the system was evaluated.
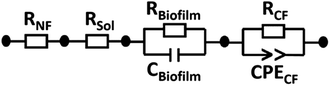
All impedance spectra were recorded in the frequency range of 10−1 to 105 Hz with a perturbation amplitude of 50 mV. Firstly, the resistance of the medium as an electrolyte solution (Rsol) was determined in a one-compartment cell, having platinum electrodes as the working (WE) and counter (CE) electrodes, showing 8.9 Ω. The platinum electrodes were then transferred to a two-compartment electrochemical cell containing a medium to determine the resistance of a Nafion membrane (RNF). The electrical circuit used for data fitting is shown in Fig. 4. In this configuration, RNF was found to be 530 Ω and connected in series with Rsol, the resistance of carbon felt (RCF) and the resistance of biofilm (Rbiofilm). RCF is in parallel with the constant phase element of carbon felt (CPECF) and Rbiofilm is in parallel with the capacitance of the biofilm (Cbiofilm), representing the bioanode.
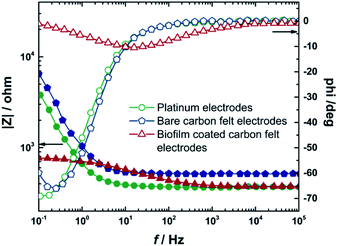
Then, the platinum electrodes were replaced with bare carbon felt electrodes and the impedance spectrum was measured in a similar manner. After that, these carbon felt electrodes were used as electrodes for biofilm formation in MEC 1. With these two bioelectrodes, impedance spectra were recorded to investigate changes of the system in the presence of biofilms. Fig. 5 presents Bode plots for the two-electrode setup of platinum, bare carbon felt and carbon felt with the biofilm, and the data evaluated from the measurements are summarized in Table 3.
| WE | CE | R sol/Ω | R CF/Pt/kΩ | R NF/Ω | R biofilm/kΩ | C biofilm/F | CPE-T (10−4) | CPE-E |
|---|---|---|---|---|---|---|---|---|
| Platinum | Platinum | 8.9 | 8.7 | 530 | — | — | 6.6 | 1.1 |
| Carbon felt | Carbon felt | 70 | 1.1 | 530 | — | — | 2.5 | 1.1 |
| Carbon felt with biofilm | Carbon felt with biofilm | 8 | 0.4 | 530 | 0.4 | 0.1 | 1.6 | 0.7 |
The constant phase element was used for the description of the non-ideal capacity of spongy-like carbon felt electrodes. Thus, the resistances of electrodes (RCF) as bare carbon felt and carbon felt with a biofilm were found to be 1.1 and 0.4 kΩ, respectively. The calculated resistance and capacitance of the biofilm (Rbiofilm, Cbiofilm) were 0.4 kΩ and 0.1 F. Further, the results reveal that, with the biofilm on electrodes, a decrease in capacitive current was observed in a low frequency regime due to enhanced electron transport, indicating the establishment of a conductive biofilm and a larger surface area due to biofilm formation.61 Moreover, EIS results indicated negligible losses of this MEC.
Apart from the observed enhanced performance of the modified electrodes containing MECs, the economic aspects should be addressed as they impact significantly on up-scaling. However, only a few studies have been reported on the cost of electrode modification for this type of MEC. We performed cost studies for such modifications from the price of chemicals which were used in each modification process (see details in the ESI†). The calculation showed that poly(neutral red) modification (∼100 € per m2) was much cheaper, as compared to chitosan modification (∼5400 € per m2) due to the costly coupling reagents (NHS and EDC). Compared to the reported metal based electrodes like Ni mesh66 (∼8600 € per m2), carbon felt (∼170 € per m2) modified with poly(neutral red) (in total ∼270 € per m2) are much cheaper and showed similar results with respect to the CH4 yield (Table 2). Moreover, this poly(neutral red) modified carbon felt is more cost effective than the conventional platinum based electrodes (e.g. Pt (60%) carbon cloth is ∼4100 € per m2,62) which are normally used and, being the main cost of MFC technology made it less feasible for up-scaling reactors.
Experimental
Chemicals
Low molecular weight chitosan and 3-amino-7-dimethylamino-2-methylphenazine hydrochloride (neutral red) were purchased from Sigma-Aldrich. Carbon felt electrodes were received from Alfa Aesar. Other chemicals were of analytical grade and were used as received. Phosphate buffer solutions were prepared from KH2PO4 and K2HPO4 to achieve the desired pH value. A Nafion perfluorinated membrane (Nafion 117) was received from Chemours. With a slight modification to the reported study,69 the Nafion membrane was pre-treated prior to use by boiling in H2O2 (30% v/v), deionized water, 0.5 M H2SO4 and deionized water, sequentially. Each step was carried out for 1 h at 80 °C.Setup
All microbial electrocatalytic experiments were performed in two-compartment electrochemical cells with the same geometrical design (Fig. 1b). Each chamber had a volume of around 250 mL. The cathode and anode chambers were separated by using a pre-treated Nafion 117 membrane allowing proton transport between the two chambers. Carbon felt electrodes (7.0 × 2.5 × 0.6 cm3) connected with Ti wires were used as anodes and cathodes, while a Ag/AgCl (3 M NaCl) electrode (ProSense) was used as a reference electrode. All electrochemical experiments were performed using an IVIUM CompactStat instrument. The potential values reported in this work referred to Ag/AgCl (3 M NaCl) which were calibrated externally with a K3[Fe(III)(CN)6]/K4[Fe(III)(CN)6] redox couple of which the potential value of 0.36 V vs. standard hydrogen electrode (SHE) was used. The calibration showed a Ag/AgCl (3 M NaCl) electrode potential of 0.21 V vs. SHE.Medium
With slight differences from the previous report,2 a medium was prepared in deionized water containing the following ingredients (per L): KH2PO4 (3.0 g), K2HPO4 (2.5 g), NaCl (0.13 g), NH4Cl (0.31 g), NaHCO3 (6.0 g), MgSO4·7H2O (0.040 g), trace element solution (12.5 mL) and vitamin solution (5 mL). The trace element solution consisted of the following ingredients (per L): HCl (25%, 10 mL), FeCl2·4H2O (1.5 g), ZnCl2 (0.070 mg), MnCl2·4H2O (0.10 g), H3BO3 (0.006 g), CoCl2·6H2O (0.19 g), CuCl2·2H2O (0.002 g), NiCl2·6H2O (0.024 g), Na2MoO4·6H2O (0.036 g), Na2WO4·2H2O (0.036 g) and deionized water (990 mL). The vitamin solution contained the following ingredients (per L): biotin (0.002 g), folic acid (0.002 g), pyridoxine hydrochloride (0.010 g), thiamine hydrochloride (0.005 g), riboflavin (0.005 g), nicotinic acid (0.005 g), calcium D-pantothenate (0.005 g), vitamin B12 (0.0001 g), p-aminobenzoic acid (0.005 g), lipoic acid (0.005 g) and deionized water (1 L).Electrode modification
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) containing 50 mM N-hydroxy-3-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and 50 mM of N-hydroxysuccinimide (NHS) at room temperature for 2 h. The electrode was transferred into a 2% acetic acid solution containing chitosan (1% w/v) of which the pH was adjusted to 6.5 with a 1 M NaOH solution. The modification took place at room temperature for 14 h, then the modified carbon felt electrode was carefully washed with ethanol and dried under vacuum.
1 v/v) containing 50 mM N-hydroxy-3-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and 50 mM of N-hydroxysuccinimide (NHS) at room temperature for 2 h. The electrode was transferred into a 2% acetic acid solution containing chitosan (1% w/v) of which the pH was adjusted to 6.5 with a 1 M NaOH solution. The modification took place at room temperature for 14 h, then the modified carbon felt electrode was carefully washed with ethanol and dried under vacuum.
Adaptation phase
For all MECs, anodes and cathodes were inoculated with the same inoculum prepared by centrifugation (4000 rpm for 10 min) of sewage sludge, collected from a communal wastewater treatment plant (Austria). The bioelectrodes were developed in the aforementioned two-compartment cell in which each chamber contained 20 mL of prepared inoculum. In cathodic chambers, 200 mL of the previously described medium and glucose (0.22 g) were added. While anodic chambers were filled with 200 mL of medium, containing additional carbon sources, to simulate municipal wastewater which included the following composition (per L): peptone (0.138 g), yeast extract (0.075 g), sodium acetate (0.088 g) and glucose (0.336 g).43 The adaption phase was carried out at room temperature by applying a constant potential of 0.40 V vs. Ag/AgCl (3 M NaCl) at the anode for 4 weeks. In this phase, carbon sources were supplied once a week. For the anode compartment, half of the solution was replaced with a freshly prepared medium containing synthetic wastewater, while glucose (0.22 g) was added into the cathodic compartment without replacing the solution. Both chambers were kept saturated with CO2 to ensure anaerobic conditions. After 4 weeks, 200 mL of the cathodic solution was replaced with a freshly prepared medium without glucose addition.Long-term performance studies
After the 4 week adaptation, all three MECs were studied continuously by applying a constant potential of 0.40 V vs. Ag/AgCl (3 M NaCl) at the anode in a batch operation mode. Headspace samples from cathode chambers and liquid samples from the anolyte were taken twice a week for headspace product analysis and COD measurements, respectively. After sampling, the cathode chambers were purged with CO2 to keep the chamber saturated, while half of the anodic solution was replaced with fresh medium containing synthetic wastewater with an average COD concentration of 600 mg L−1 and the chamber was flushed with N2 to achieve anaerobic conditions. Every 4 weeks, 200 mL of cathodic solution was replaced with fresh medium to provide microorganisms with sufficient trace elements and vitamins, labelled as running cycles 1–3.Determination of COD
The COD of liquid samples taken from the anodic solution were analyzed photometrically. Firstly, 2 mL of the liquid sample was mixed with the reagent in a COD test tube (Nanocolor COD 160 and 1500, Macherey-Nagel). Then, the mixture was heated at 148 °C for 2 h in a thermostat (Nanocolor Vario Mini, Macherey-Nagel). Afterwards, the tubes were allowed to cool down to room temperature. Then COD was determined by using a LED photometer (Compact Photometer PF-3, Machery-Nagel) at wavelength 620 nm. COD removal efficiency can be calculated using the following calculation:in which ΔCOD is calculated from the change of COD1 (COD concentration of anodic solution before reaction) to COD2 (COD concentration of anodic solution after reaction).
Headspace product analysis
Headspace samples from cathode chambers were taken by using a gas-tight syringe for CH4 analysis. The analysis was done by using a gas chromatograph (Thermo Scientific GC ultra) equipped with a thermal conductivity detector (TCD). The faradaic efficiency (FE) can be calculated using the following equation:where nCH4 is the number of moles of CH4 calculated from the amount of CH4 produced in the system, 8 is the number of electrons needed for the reduction of CO2 to CH4, F is the faradaic constant (96
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.33 C mol−1), I is current recorded during the reaction and t is time of the reaction.
485.33 C mol−1), I is current recorded during the reaction and t is time of the reaction.
Scanning electron microscopy measurements
The samples were taken by cutting the bottom edge of each bioelectrode (size of 1.5 × 0.5 cm2) and allowing them to dry under ambient conditions overnight. Scanning electron microscope (SEM) images of dried bioelectrodes were taken using the JEOL JSM-6360 LV scanning electron microscope at the accelerating voltage of an electron beam of 7.0 kV with a working distance of 15 mm.Electrochemical impedance
The impedance experiments were carried out in a two-compartment electrochemical cell separated with a pre-treated Nafion 117 membrane (as mentioned above for microbial electrocatalytic experiments) by using an IVIUM CompactStat instrument. The impedance spectra were recorded within the frequency range of 105 to 10−1 Hz at the perturbation amplitude of 50 mV.Conclusion
In this study, we investigated the long-term performance of CH4 producing MECs equipped with bioanodes and biocathodes for wastewater treatment, in terms of COD removal and CH4 production. By applying a constant potential of 0.40 V vs. Ag/AgCl (3 M NaCl) at the bioanode, organic degradation and CH4 generation were monitored through COD measurement of the anodic electrolyte solution and cathodic headspace analysis, respectively. In addition, carbon felt electrodes were modified with poly(neutral red) and chitosan, serving as electrodes and supports for microbial colonization in MECs. The results of all three MECs revealed the possibility of oxidation of organic substances at bioanodes and simultaneous CH4 generation at biocathodes. Compared to non-modified MECs, higher COD removal rates and CH4 production rates of modified electrode containing systems were observed, especially in the first running cycle. These revealed that modified electrodes with poly(neutral red) and chitosan could enhance biofilm formation and activity. Furthermore, the corresponding efficiencies from those were improved, suggesting efficient electron transfer processes between electrodes and microorganisms. Compared to the previously reported MECs, our MECs showed comparable results even though they were batch operated which limited the substrate input. Further, the cost studies on the electrode material and modification prices suggested that the poly(neutral red) modified electrode is more cost-effective, as compared to other metal based materials, which provides economic feasibility for large-scale operation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Arne Ragoβnig and Vanessa Zawodnik from RM Umweltkonsulenten ZT GmbH for valuable discussion. Financial support by the Austrian Climate and Energy Fund within the MELOS project (861392) is gratefully acknowledged. Further financial support of the Austrian Science Foundation (FWF) with the Wittgenstein Prize for Prof. Sariciftci is gratefully acknowledged (Z222-N19). The authors gratefully acknowledge the funding support of K1-MET GmbH, the metallurgical competence centre. The research program of the K1-MET competence centre is supported by COMET (Competence Centre for Excellent Technologies), the Austrian program for competence centres. COMET is funded by the Federal Ministry for Transport, Innovation, and Technology; the Federal Ministry for Digital and Economic Affairs, the provinces of Upper Austria, Tyrol, and Styria, as well as the Styrian Business Promotion Agency (SFG).Notes and references
- S. Arrhenius, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1896, 41, 237–276 CrossRef CAS.
- S. Cheng, D. Xing, D. F. Call and B. E. Logan, Environ. Sci. Technol., 2009, 43, 3953–3958 CrossRef CAS PubMed.
- S. Bajracharya, M. Sharma, G. Mohanakrishna, X. Dominguez Benneton, D. P. B. T. B. Strik, P. M. Sarma and D. Pant, Renewable Energy, 2016, 98, 153–170 CrossRef CAS.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS PubMed.
- K. Rabaey and R. A. Rozendal, Nat. Rev. Microbiol., 2010, 8, 706–716 CrossRef CAS PubMed.
- H. Hiegemann, D. Herzer, E. Nettmann, M. Lübken, P. Schulte, K. G. Schmelz, S. Gredigk-Hoffmann and M. Wichern, Bioresour. Technol., 2016, 218, 115–122 CrossRef CAS PubMed.
- D. Pant, G. Van Bogaert, L. Diels and K. Vanbroekhoven, Bioresour. Technol., 2010, 101, 1533–1543 CrossRef CAS PubMed.
- D. Pant, A. Singh, G. Van Bogaert, S. Irving Olsen, P. Singh Nigam, L. Diels and K. Vanbroekhoven, RSC Adv., 2012, 2, 1248–1263 RSC.
- Y. Mu, K. Rabaey, R. A. Rozendal, Z. Yuan and J. Keller, Environ. Sci. Technol., 2009, 43, 5137–5143 CrossRef CAS PubMed.
- S. M. Strycharz, T. L. Woodard, J. P. Johnson, K. P. Nevin, R. A. Sanford, F. E. Löffler and D. R. Lovley, Appl. Environ. Microbiol., 2008, 74, 5943–5947 CrossRef CAS PubMed.
- H. Z. Zhao, Y. Zhang, Y. Y. Chang and Z. S. Li, J. Power Sources, 2012, 217, 59–64 CrossRef CAS.
- K. J. J. Steinbusch, H. V. M. Hamelers, J. D. Schaap, C. Kampman and C. J. N. Buisman, Environ. Sci. Technol., 2010, 44, 513–517 CrossRef CAS PubMed.
- M. C. Potter, Proc. R. Soc. B, 1911, 84, 260–276 Search PubMed.
- A. J. Slate, K. A. Whitehead, D. A. C. Brownson and C. E. Banks, Renewable Sustainable Energy Rev., 2019, 101, 60–81 CrossRef CAS.
- C. Koch and F. Harnisch, ChemElectroChem, 2016, 3, 1282–1295 CrossRef CAS.
- H. J. Kim, H. S. Park, M. S. Hyun, I. S. Chang, M. Kim and B. H. Kim, Enzyme Microb. Technol., 2002, 30, 145–152 CrossRef CAS.
- M. Rosenbaum, F. Aulenta, M. Villano and L. T. Angenent, Bioresour. Technol., 2011, 102, 324–333 CrossRef CAS PubMed.
- R. Kumar, L. Singh and A. W. Zularisam, Renewable Sustainable Energy Rev., 2016, 56, 1322–1336 CrossRef CAS.
- N. S. Malvankar, M. T. Tuominen and D. R. Lovley, Energy Environ. Sci., 2012, 5, 5790–5797 RSC.
- E. Marsili, D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick and D. R. Bond, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3968–3973 CrossRef CAS PubMed.
- K. Rabaey, N. Boon, M. Höfte and W. Verstraete, Environ. Sci. Technol., 2005, 39, 3401–3408 CrossRef CAS PubMed.
- S. B. Velasquez-Orta, I. M. Head, T. P. Curtis, K. Scott, J. R. Lloyd and H. Von Canstein, Appl. Microbiol. Biotechnol., 2010, 85, 1373–1381 CrossRef CAS PubMed.
- S. Babanova, Y. Hubenova and M. Mitov, J. Biosci. Bioeng., 2011, 112, 379–387 CrossRef CAS PubMed.
- F. Aulenta, A. Catervi, M. Majone, S. Panero, P. Reale and S. Rossetti, Environ. Sci. Technol., 2007, 41, 2554–2559 CrossRef CAS PubMed.
- T. Catal, K. Li, H. Bermek and H. Liu, J. Power Sources, 2008, 175, 196–200 CrossRef CAS.
- B. Logan, S. Cheng, V. Watson and G. Estadt, Environ. Sci. Technol., 2007, 41, 3341–3346 CrossRef CAS PubMed.
- A. Gálvez, J. Greenman and I. Ieropoulos, Bioresour. Technol., 2009, 100, 5085–5091 CrossRef PubMed.
- S. K. Chaudhuri and D. R. Lovley, Nat. Biotechnol., 2003, 21, 1229–1232 CrossRef CAS PubMed.
- C. Santoro, C. Arbizzani, B. Erable and I. Ieropoulos, J. Power Sources, 2017, 356, 225–244 CrossRef CAS PubMed.
- P. Clauwaert, D. Van Der Ha, N. Boon, K. Verbeken, M. Verhaege, K. Rabaey and W. Verstraete, Environ. Sci. Technol., 2007, 41, 7564–7569 CrossRef CAS PubMed.
- R. A. Rozendal, A. W. Jeremiasse, H. V. M. Hamelers and C. J. N. Buisman, Environ. Sci. Technol., 2008, 42, 629–634 CrossRef CAS PubMed.
- K. P. Nevin, S. A. Hensley, A. E. Franks, Z. M. Summers, J. Ou, T. L. Woodard, O. L. Snoeyenbos-West and D. R. Lovley, Appl. Environ. Microbiol., 2011, 77, 2882–2886 CrossRef CAS PubMed.
- D. R. Lovley, Environ. Microbiol. Rep., 2011, 3, 27–35 CrossRef CAS PubMed.
- L. Huang, J. Chen, X. Quan and F. Yang, Bioprocess Biosyst. Eng., 2010, 33, 937–945 CrossRef CAS PubMed.
- P. Clauwaert, K. Rabaey, P. Aelterman, L. De Schamphelaire, T. H. Pham, P. Boeckx, N. Boon and W. Verstraete, Environ. Sci. Technol., 2007, 41, 3354–3360 CrossRef CAS PubMed.
- K. P. Kuhl, T. Hatsukade, E. R. Cave, D. N. Abram, J. Kibsgaard and T. F. Jaramillo, J. Am. Chem. Soc., 2014, 136, 14107–14113 CrossRef CAS PubMed.
- S. Schlager, M. Haberbauer, A. Fuchsbauer, C. Hemmelmair, L. M. Dumitru, G. Hinterberger, H. Neugebauer and N. S. Sariciftci, ChemSusChem, 2017, 10, 226–233 CrossRef CAS PubMed.
- F. Geppert, D. Liu, M. van Eerten-Jansen, E. Weidner, C. Buisman and A. ter Heijne, Trends Biotechnol., 2016, 34, 879–894 CrossRef CAS PubMed.
- Y. Jiang, M. Su and D. Li, Appl. Biochem. Biotechnol., 2014, 172, 2720–2731 CrossRef CAS PubMed.
- M. Villano, G. Monaco, F. Aulenta and M. Majone, J. Power Sources, 2011, 196, 9467–9472 CrossRef CAS.
- M. Villano, C. Ralo, M. Zeppilli, F. Aulenta and M. Majone, Bioelectrochemistry, 2016, 107, 1–6 CrossRef CAS PubMed.
- M. Villano, F. Aulenta, C. Ciucci, T. Ferri, A. Giuliano and M. Majone, Bioresour. Technol., 2010, 101, 3085–3090 CrossRef CAS PubMed.
- M. Zeppilli, M. Villano, F. Aulenta, S. Lampis, G. Vallini and M. Majone, Environ. Sci. Pollut. Res., 2015, 22, 7349–7360 CrossRef CAS PubMed.
- P. Clauwaert and W. Verstraete, Appl. Microbiol. Biotechnol., 2009, 82, 829–836 CrossRef CAS PubMed.
- N. Aryal, T. Kvist, F. Ammam, D. Pant and L. D. M. Ottosen, Bioresour. Technol., 2018, 264, 359–369 CrossRef CAS PubMed.
- S. Kalathil, S. A. Patil and D. Pant, in Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, Elsevier Inc., Amsterdam, 2017, pp. 309–318 Search PubMed.
- L. Huang, L. Jiang, Q. Wang, X. Quan, J. Yang and L. Chen, Chem. Eng. J., 2014, 253, 281–290 CrossRef CAS.
- Y. Hindatu, M. S. M. Annuar and A. M. Gumel, Renewable Sustainable Energy Rev., 2017, 73, 236–248 CrossRef CAS.
- S. Cheng and B. E. Logan, Electrochem. Commun., 2007, 9, 492–496 CrossRef.
- S. R. Higgins, D. Foerster, A. Cheung, C. Lau, O. Bretschger, S. D. Minteer, K. Nealson, P. Atanassov and M. J. Cooney, Enzyme Microb. Technol., 2011, 48, 458–465 CrossRef CAS PubMed.
- B. Lai, X. Tang, H. Li, Z. Du, X. Liu and Q. Zhang, Biosens. Bioelectron., 2011, 28, 373–377 CrossRef CAS PubMed.
- N. Zhao, Z. Ma, H. Song, Y. Xie and M. Zhang, Electrochim. Acta, 2019, 296, 69–74 CrossRef CAS.
- P. Srinophakun, A. Thanapimmetha, S. Plangsri, S. Vetchayakunchai and M. Saisriyoot, J. Cleaner Prod., 2017, 142, 1274–1282 CrossRef CAS.
- T. Zhang, H. Nie, T. S. Bain, H. Lu, M. Cui, O. L. Snoeyenbos-West, A. E. Franks, K. P. Nevin, T. P. Russell and D. R. Lovley, Energy Environ. Sci., 2013, 6, 217–224 RSC.
- T. Cai, L. Meng, G. Chen, Y. Xi, N. Jiang, J. Song, S. Zheng, Y. Liu, G. Zhen and M. Huang, Chemosphere, 2020, 248, 125985 CrossRef CAS PubMed.
- R. Pauliukaite, M. E. Ghica, M. Barsan and C. M. A. Brett, J. Solid State Electrochem., 2007, 11, 899–908 CrossRef CAS.
- T. D. Harrington, V. N. Tran, A. Mohamed, R. Renslow, S. Biria, L. Orfe, D. R. Call and H. Beyenal, Bioresour. Technol., 2015, 192, 689–695 CrossRef CAS PubMed.
- D. H. Park and J. G. Zeikus, J. Bacteriol., 1999, 181, 2403–2410 CrossRef CAS PubMed.
- D. H. Park and J. G. Zeikus, Appl. Environ. Microbiol., 2000, 66, 1292–1297 CrossRef CAS PubMed.
- H. Seelajaroen, M. Haberbauer, C. Hemmelmair, A. Aljabour, L. M. Dumitru, A. W. Hassel and N. S. Sariciftci, ChemBioChem, 2019, 20, 1196–1205 CrossRef CAS PubMed.
- B. Wang, W. Liu, Y. Zhang and A. Wang, Water Res., 2020, 175, 115696 CrossRef CAS PubMed.
- M. T. Noori, M. T. Vu, R. B. Ali and B. Min, Chem. Eng. J., 2020, 392, 123689 CrossRef CAS.
- Y. Zhang, J. Sun, Y. Hu, S. Li and Q. Xu, Int. J. Hydrogen Energy, 2012, 37, 16935–16942 CrossRef CAS.
- K. P. Nevin, T. L. Woodard, A. E. Franks, Z. M. Summers, D. R. Lovley, K. P. Citation Nevin, T. L. Woodard, A. E. Franks, Z. M. Summers and D. R. Lovley, mBio, 2010, 1, e00103–10 CrossRef PubMed.
- M. Zeppilli, P. Paiano, M. Villano and M. Majone, Biochem. Eng. J., 2019, 152, 107393 CrossRef CAS.
- T. Sangeetha, Z. Guo, W. Liu, M. Cui, C. Yang, L. Wang and A. Wang, Int. J. Hydrogen Energy, 2016, 41, 2189–2196 CrossRef CAS.
- M. Villano, S. Scardala, F. Aulenta and M. Majone, Bioresour. Technol., 2013, 130, 366–371 CrossRef CAS PubMed.
- A. Ding, Y. Yang, G. Sun and D. Wu, Chem. Eng. J., 2015, 283, 260–265 CrossRef.
- S. Kondaveeti and B. Min, Bioprocess Biosyst. Eng., 2013, 36, 231–238 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0se00770f |
| This journal is © The Royal Society of Chemistry 2020 |