Methane-trapping metal–organic frameworks with an aliphatic ligand for efficient CH4/N2 separation†
Miao
Chang‡
a,
Yingjie
Zhao‡
a,
Dahuan
Liu
 *a,
Jiangfeng
Yang
*a,
Jiangfeng
Yang
 b,
Jinping
Li
b,
Jinping
Li
 b and
Chongli
Zhong
b and
Chongli
Zhong
 ac
ac
aBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: liudh@mail.buct.edu.cn
bResearch Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
cState Key Laboratory of Separation Membranes and Membrane Processes, Tianjin Polytechnic University, Tianjin 300387, China
First published on 28th October 2019
Abstract
CH4/N2 separation is one of the great challenges in the gas separation field due to their very close physical properties. In this work, a strategy was proposed to construct specific cages using an aliphatic ligand with suitable size to preferentially adsorb CH4 molecules in metal–organic frameworks (MOFs). As a result, a series of MOFs with trans-1,4-cyclohexanedicarboxylic acid (H2CDC) as the ligand and different metal centres (M-CDC, M represents metal ions) exhibit excellent separation performances for CH4/N2 mixture. To the best of our knowledge, the presented MOFs possess higher selectivity (13.1–16.69) and sorbent selection parameter (SSP) in comparison to the reported porous materials. Especially for the integrated descriptor SSP, the value of Al-CDC is up to 82.0, which is at least 2–3 times higher than those in the reported materials. Breakthrough experiments indicate that CH4/N2 can be completely separated using a packed column of Al-CDC. Theoretical calculations confirmed the preferential trap of CH4 in the cages formed by the aliphatic ligand. In addition, the separation performance can be well maintained even after at least 10 cycles and the treatment of the MOF sample with boiling water, acid and base solution. These results not only introduce promising candidates for CH4/N2 separation, but also provide useful information for the separation of other weak adsorbates with similar properties.
Nowadays, the world is facing great challenges related to energy and environment on account of the continuous consumption of fossil fuels, inducing the rapidly increasing demand for clean energy. Methane (CH4), as a kind of clean and economical alternative energy, has been receiving more and more attention. Various sources have been explored to emit a large amount of methane, including conventional natural gas and unconventional natural gas (such as coal bed methane (CBM), landfill gas, and shale gas).1,2 However, in unconventional natural gas, the nitrogen (N2) content is generally very high, limiting its direct utilization. For example, over 30% N2 can be found in CBM, which comprises about 36% of the total natural gas.1,3 Current treatment of these gases, like direct emission into the atmosphere, not only has a negative effect on the utilization of such precious energy, but also extremely arouses environmental issues, since CH4 is also an important greenhouse gas.4 Therefore, it is urgent to efficiently separate CH4/N2 and upgrade these low quality unconventional natural gases. The adsorbent-based separation is one of the promising processes as it is energy efficient and economical.5 In this respect, the key is to develop suitable adsorbents with high separation performance. Unfortunately, even though various kinds of porous materials have been reported for CH4/N2 separation, the selectivities and productivities are still quite low up to now.2,6–25 For example, sOMC exhibits a selectivity of 3.5 at 298 K with a CH4 adsorption capacity of 22 cm3 g−1.8 The selectivity in Na-SAPO-34 is about 2.6.9 For Co-MOF, the uptake of CH4 is very low (9.03 cm3 g−1) and shows a selectivity of 8.5–12.5.2 More recently, ATC-Cu was observed to possess a relatively large uptake of CH4 (64.96 cm3 g−1), while the selectivity was about 9.7.23 The main reason is the similar physical properties of CH4 and N2, including boiling points, polarizabilities, as well as kinetic diameters. Both gases lack a permanent dipole and could be classified as weak adsorbates.26 Thus, these properties present an extremely difficult challenge for the efficient separation of CH4 and N2.
Inspired by the phenomena that weak interactions can induce the cooperative effect to form strong interactions in special spatial arrangements in supramolecular assemblies (such as in cyclodextrin complexes27), it is expected to construct the specific pore structure in adsorbent to preferentially adsorb CH4 using even weak interactions, for example, van der Waals (vdW) interactions. Such non-bonded interactions are highly distance-dependent and can be affected by the presence of other nearby molecules. Towards this target, metal–organic frameworks (MOFs) could represent a suitable platform because of their high designability and tunability of chemical properties and structure.28–31 Herein, a series of MOFs with an aliphatic ligand, H2CDC, were observed to exhibit excellent separation performances for CH4/N2 mixture, especially for Al-CDC with the highest selectivity and sorbent selection parameter (SSP) than all of the reported values in porous materials. Theoretical calculations indicate that CH4 molecules are adsorbed in the low polarity cages surrounded by the aliphatic ligands.
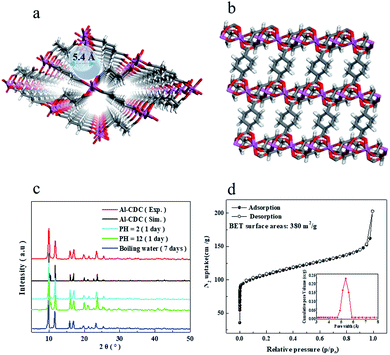
In order to increase the difference in the interaction of CH4 and N2, an aliphatic ligand with low polarity, H2CDC, was selected. It contains saturated C–H bonds and may have relatively strong interaction with the highly polarized CH4 molecule.18,32 For the metal ion, aluminium salt was used in the first step because of its non-toxic and low-cost properties, which are meaningful for applications on a large scale.33 Furthermore, most of the reported Al-MOF materials show remarkable stability.34,35 Thus, Al-CDC (Al2CDC3 in stoichiometry) was synthesized and the structure is given in Fig. 1a and b. In the framework, Al3+ is bonded to six oxygen atoms to form AlO6− octahedra. These connect the zigzag chain of the ligand with the trans corner, resulting in a one-dimensional rhombohedral pore. To increase the synthesis efficiency, we slightly modified the original synthesis method as described in the experimental section. PXRD patterns in Fig. 1c in combination with the TGA curve and N2 adsorption at 77 K as well as the corresponding BET surface area (380 m2 g−1) with the pore size (5.4 Å) in Fig. S11† and 1d indicate the successful preparation of this MOF with good crystallinity and chemical/thermal stability. The sample was further characterized by FESEM and HRTEM. As shown in Fig. S13,† the lattice fringe can be identified to be 0.226 nm.
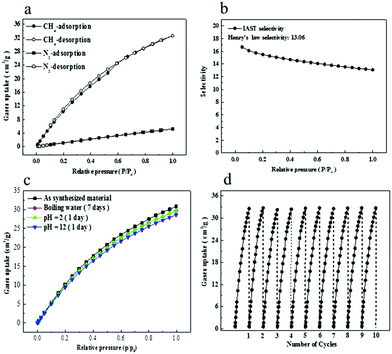
Single gas adsorption isotherms were first measured to examine the separation performance of Al-CDC for CH4/N2 mixture. As shown in Fig. 2a, the adsorption capacity of CH4 is up to 32.06 cm3 g−1 at 298 K and 1.0 bar, while the adsorption capacity of N2 is relatively very low (5.12 cm3 g−1). The rapid increase in CH4 uptakes at low pressures according to the single-component adsorption isotherm indicates that the interaction of CH4 with Al-CDC is stronger than that of N2. In particular, the adsorption isotherms can be maintained even after the treatment of the material using boiling water, acid and base solutions for days (Fig. 2c). Moreover, a regeneration experiment of Al-CDC was performed at 298 K, and the results are shown in Fig. 2d. It is obvious that the adsorption capacity of CH4 remains unchanged even after 10 cycles, indicating the excellent regenerability. According to the ideal adsorbed solution theory (IAST)36 using a dual-site Langmuir model, the selectivity of CH4/N2 is about 13.1–16.69 in the range of the tested pressures (Fig. 2b) and details can be found in the ESI† including the fitting results, which, to the best of our knowledge, are evidently higher than all the reported values in various porous materials (Fig. 3a). In Fig. 3, the selectivities in Cu(INA)2, Co3(HCOO)6, and Ni3(HCOO)6 are obtained from the ratio of Henry's law constants from pure gas adsorption isotherms and IAST-predicted selectivities. For example, IAST-predicted selectivities in Ni-HKUST-1,6 Cu-BTC,11 MOF-889,13 ATC-Cu23 and Co-MOF2 are about 5.1, 3.69, 6.43, 9.7 and 8.5–12.5, respectively, as shown in Table S5 in the ESI.† Moreover, Henry's law selectivity is up to 13.06 (Fig. 2b), which is close to the IAST-predicted values and also higher than those in the reported materials. From a practical point of view, the adsorption capacity of CH4 is another key metric for evaluating the adsorbent performance. From Fig. 3a, the value of Al-CDC is also larger than all the reported ones, except for Ni-HKUST-1 (37.63 cm3 g−1)6 and ATC-Cu,23 which, however, have relatively low selectivities (5.1 and 9.7). For such a MOF with unsaturated metal sites, the water generally existing in the practical CH4/N2 mixture may have a significant negative effect on the separation performance, since the framework is prone to destruction in the presence of water.37 In addition, only one material (Co-MOF2) exhibits a selectivity over 10.0, while the adsorption capacity of CH4 is only about 28% of that in Al-CDC presented in this work.
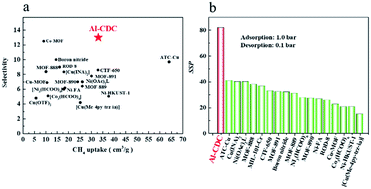 | ||
| Fig. 3 Comparison of the separation performance of CH4/N2: (a) selectivities as a function of the adsorption capacity of CH4 at 298 K and 1.0 bar; (b) SSP at 298 K. | ||
For the most industrialized PSA process, high selectivity may reduce the number of cycles needed for the treatment of input stream to the desired concentration, and good working capacity of the targeted component (described by the difference between adsorbed amounts under adsorption and desorption conditions) can diminish the overall cost. Therefore, it is of great importance to further evaluate the separation performance using an integrated descriptor, which can cover these two factors, like SSP.38 For the convenience of the comparison, 1.0 bar and 0.1 bar are used in this study in consideration of the absence of adsorption data at high pressures in literature. Obviously, the value of SSP of Al-CDC can reach over 82.0, which is at least 2–3 times higher than those in the reported materials (Fig. 3b).
To further confirm the separation of CH4 and N2 in practical applications, a breakthrough experiment was performed using a CH4/N2 (50/50, v/v) binary gas mixture. As shown in Fig. 4, the clear separation curve was realized and the breakthrough of CH4 (occurs at about 15 min) was later than that of N2 (first elutes at about 5 min). The effluent purity for N2 is about 97% during 7–8 minutes and the purity can reach over 99.99%, while CH4 is not detected until 15 minutes, suggesting the preferential adsorption of CH4 over N2 from the gas mixture. An evident roll-up is observed wherein the concentration of N2 in the eluting gas is momentarily higher than that in the feeding gas, revealing the weak interaction with Al-CDC of N2 and the desorption of N2 when CH4 adsorbs in the column. The large gap of residence time (about 10 min) between CH4 and N2 demonstrates the great potential of this MOF for the complete separation of CH4/N2 in industrial processes. Moreover, due to the highest selectivity and CH4 uptake capacity, Al-CDC shows the longest dimensionless time (τ) value (17.00) compared to N2 (5.13), which is longer than that in literature at 298 K and 1.0 bar, for example in Co-MOF(6.02).2 The dynamic selectivity (Sbreakthrough) extracted from breakthrough curves is up to 6.8 at 298 K, which is higher than those reported in literature, for example, in CTF-650 (about 6.0).8 To further confirm the separation performance in practical conditions, the breakthrough experiment was performed in the presence of water (5%), as shown in Fig. S7.† It can be seen that N2 first elutes at the beginning of the experiment while CH4 breaks through later, indicating the ability to separate CH4/N2 in the presence of water. On the basis of these data, Al-CDC exhibits an overall advantage for the separation of CH4 and N2 with respect to selectivity and productivity, superior to other reported adsorbents.2,7,8,11,12,15,18,21
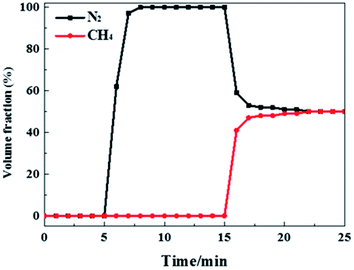 | ||
| Fig. 4 Breakthrough experiment curves of the CH4 and N2 binary mixture component (50/50, v/v) at a constant flow rate of 10 mL min−1 under 298 K and 1.0 bar. | ||
To investigate the mechanism of the excellent separation performance of Al-CDC, a computational study was carried out for CH4 and N2 in the framework. First, 2D potential energy maps for CH4 and N2 in the pore were calculated using the method we proposed previously.38 As shown in Fig. 5a, there are two small regions surrounded by the trans corner of the ligand with the lowest potential energy for CH4, while there is no specific area for N2. Accordingly, DFT calculations were performed to study the interactions between the framework and CH4 or N2. From Table S5,† it can be seen that the binding energy of CH4 is actually larger than that of N2, which is consistent with the isosteric heats of adsorption (Qst) measured in the experiment. The geometric optimization results indicate that the distance between the hydrogen atom of CH4 and the C–H of ligand is about 2.106–2.417 Å in average (Fig. 5b), which is within the range of vdW interaction32 and much shorter than the distances reported in literature (over 3.400 Å).2 The adjacent ligands can form specific cages with low polarity for the CH4 molecule, exhibiting multipoint vdW interactions with cooperative effect leading to strong interaction with CH4. This is similar to the inclusion of guest molecules in cyclodextrins. As weak adsorbates, the polarizability of CH4 is higher than that of N2. In Al-CDC, the aliphatic ligand with low polarity that contains the saturated C–H may have a relatively strong interaction with CH4 since the vdW interaction plays a dominant role in adsorbents with weak polarized surfaces, which is proportional to the polarizability of a gas molecule.32 As a result, Qst of CH4 in Al-CDC is larger than those in other porous materials (Table S5†), which remains unchanged under the whole region of tested pressures, demonstrating the uniformity of the adsorption site in the pore. It should be noted that the size of the cage will be large if the aliphatic ligand is too long, leading to a weak interaction with the CH4 molecule as well as the corresponding low selectivity.
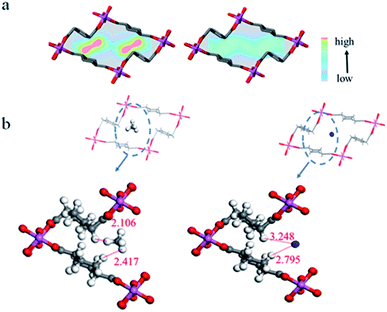 | ||
| Fig. 5 (a) 2D potential energy distributions for CH4 and N2 in the pores of Al-CDC. (b) Optimized adsorption sites for CH4 and N2 within Al-CDC. | ||
Moreover, several control experiments were carried out by synthesizing other kinds of MOFs with Cu and In ions (Fig. S14†). As shown in Fig. S15 and S16,† the selectivity and SSP of Cu-CDC and In-CDC are also high in comparison to other reported porous materials. This confirms the key role of the H2CDC ligand for the excellent separation performance of the CH4/N2 mixture.
In this work, a series of MOFs with H2CDC were prepared with excellent separation performances of CH4/N2 mixture. The values of selectivity and SSP are higher than the reported values. With the help of the saturated C–H as well as the corresponding trans corner in the ligand, specific cages were formed in the framework to preferentially adsorb CH4 molecules, which can be demonstrated by the theoretical calculations. Furthermore, the good stability endows this kind of MOF with a great promise for the separation of CH4/N2 in practical processes, contributing to the upgradation and utilization of unconventional natural gas with high N2 concentrations. These results may also stimulate the applications of such MOFs with aliphatic ligands compared to the well-studied ones with aromatic ligands.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support of the Natural Science Foundation of China (No. 21722602, 21536001, 21978005 and 21576009) is greatly appreciated.Notes and references
- J. Yang, Y. Wang, L. Li, Z. Zhang and J. Li, J. Colloid Interface Sci., 2015, 456, 197–205 CrossRef CAS PubMed.
- L. Li, L. Yang, J. Wang, Z. Zhang, Q. Yang, Y. Yang, Q. Ren and Z. Bao, AIChE J., 2018, 64, 3681–3689 CrossRef CAS.
- M. Kim and J. Kim, Chem. Eng. Res. Des., 2018, 132, 853–864 CrossRef CAS.
- E. Crosson, Appl. Phys. B, 2008, 92, 403–408 CrossRef CAS.
- L. Li, R. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS PubMed.
- X. Jia, N. Yuan, L. Wang, J. Yang and J. Li, Eur. J. Inorg. Chem., 2018, 2018, 1047–1052 CrossRef CAS.
- D. Saha, G. Orkoulas, S. Yohannan, H. Ho, E. Cakmak, J. Chen and S. Ozcan, ACS Appl. Mater. Interfaces, 2017, 9, 14506–14517 CrossRef CAS PubMed.
- K. Yao, Y. Chen, Y. Lu, Y. Zhao and Y. Ding, Carbon, 2017, 122, 258–265 CrossRef CAS.
- M. Rivera-Ramos and A. Hernández-Maldonado, Ind. Eng. Chem. Res., 2007, 46, 4991–5002 CrossRef CAS.
- A. Jayaramana, A. Hernandez-Maldonadoa, R. Yanga, D. Chinnb, C. Munsonb and D. Mohrb, Chem. Eng. Sci., 2004, 59, 2407–2417 CrossRef.
- X. Ren, T. Sun, J. Hu and S. Wang, Microporous Mesoporous Mater., 2014, 186, 137–145 CrossRef CAS.
- J. Möllmer, M. Lange, A. Möller, C. Patzschke, K. Stein, D. Läassig, J. Lincke, R. Gläser, H. Krautscheid and R. Staudt, J. Mater. Chem., 2012, 22, 10274–10286 RSC.
- P. Nguyen, H. Nguyen, H. Pham, J. Kim, K. Cordova and H. Furukawa, Inorg. Chem., 2015, 54, 10065–10072 CrossRef CAS PubMed.
- D. Saha, Z. Bao, F. Jia and S. Deng, Environ. Sci. Technol., 2010, 44, 1820–1826 CrossRef CAS PubMed.
- X. Wang, L. Li, J. Yang and J. Li, Chin. J. Chem. Eng., 2016, 24, 1687–1694 CrossRef CAS.
- B. Liu and B. Smit, J. Phys. Chem. C, 2010, 114, 8515–8522 CrossRef CAS.
- X. Liu, Y. Guo, A. Tao, M. Fischer, T. Sun, P. Moghadam, D. Jimenez and S. Wang, Chem. Commun., 2017, 53, 11437–11440 RSC.
- J. Hu, T. Sun, X. Liu, Y. Guo and S. Wang, RSC Adv., 2016, 6, 64039–64046 RSC.
- J. Prez-Pellitero, H. Amrouche, F. Siperstein, G. Pirngruber, C. Nieto-Draghi, G. Chaplais, A. Simon-Masseron, D. Bazer-Bachi, D. Peralta and N. Bats, Chem.–Eur. J., 2010, 16, 1560–1571 CrossRef PubMed.
- Y. Zhang, W. Su, Y. Sun, J. Liu, X. Liu and X. Wang, J. Chem. Eng. Data, 2015, 60, 2951–2957 CrossRef CAS.
- Y. Guo, J. Hu, X. Liu, T. Sun, S. Zhao and S. Wang, Chem. Eng. J., 2017, 327, 564–572 CrossRef CAS.
- X. Wu, B. Yuan, Z. Bao and S. Deng, J. Colloid Interface Sci., 2014, 430, 78–84 CrossRef CAS PubMed.
- Z. Niu, X. Cui, T. Pham, P. Lan, H. Xing, K. Forrest, L. Wojtas, B. Space and S. Ma, Angew. Chem., Int. Ed., 2019, 58, 10138–10141 CrossRef CAS PubMed.
- R. Li, M. Li, X. Zhou, S. Ng, M. O'Keeffe and D. Li, CrystEngComm, 2014, 16, 6291–6295 RSC.
- C. Kivi, B. Gelfand, H. Dureckova, H. Ho, C. Ma, G. Shimizu, T. Woo and D. Song, Chem. Commun., 2018, 54, 14104–14107 RSC.
- K. Lee, W. Isley, A. Dzubak, P. Verma, S. Stoneburner, L. Lin, J. Howe, E. Bloch, D. Reed, R. Hudson, C. Brown, J. Long, J. Neaton, B. Smit, C. Cramer, D. Truhlar and L. Gagliardi, J. Am. Chem. Soc., 2014, 136, 698–704 CrossRef CAS PubMed.
- K. Connors, Chem. Rev., 1997, 97, 1325–1357 CrossRef CAS PubMed.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- J. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- A. Howarth, M. Katz, T. Wang, A. Platero-Prats, K. Chapman, J. Hupp and O. Farha, J. Am. Chem. Soc., 2015, 137, 7488–7494 CrossRef CAS PubMed.
- H. Noh, C. Kung, T. Islamoglu, A. Peters, Y. Liao, P. Li, S. Garibay, X. Zhang, M. DeStefano, J. Hupp and O. Farha, Chem. Mater., 2018, 30, 2193–2197 CrossRef CAS.
- R. Lin, H. Wu, L. Li, X. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed.
- H. Reinsch and N. Stock, Microporous Mesoporous Mater., 2013, 171, 156–165 CrossRef CAS.
- I. Kang, N. Khan, E. Haque and S. Jhung, Chem.–Eur. J., 2011, 17, 6437–6442 CrossRef CAS PubMed.
- M. Kandiah, M. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. Quadrelli, F. Bonino and K. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- J. Bachman, D. Reed, M. Kapelewski, G. Chachra, D. Jonnavittula, G. Radaelli and J. Long, Energy Environ. Sci., 2018, 11, 2423–2431 RSC.
- P. Maniam and N. Stock, Inorg. Chem., 2011, 50, 5085–5097 CrossRef CAS PubMed.
- M. Tong, Y. Lan, Q. Yang and C. Zhong, Chem. Eng. Sci., 2017, 168, 456–464 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section; adsorption isotherms; calculation of selectivity; characterization of MOFs; comparison with other porous materials. See DOI: 10.1039/c9se00838a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: