Candle soot nanoparticle-decorated wood for efficient solar vapor generation†
Zhe
Wang
,
Yutao
Yan
,
Xiaoping
Shen
,
Qingfeng
Sun
 * and
Chunde
Jin
*
* and
Chunde
Jin
*
School of Engineering, Zhejiang A & F University, Hangzhou 311300, China. E-mail: qfsun@zafu.edu.cn; jincd@zafu.edu.cn
First published on 21st October 2019
Abstract
Vapor generation by means of harvesting solar energy is an energy-saving, environmentally friendly and efficient method to achieve seawater desalination and water purification. However, solar vapor generation is subjected to poor utilization efficiency of solar energy or it depends on expensive solar absorption materials and complex processes. In this work, inspired by tree transpiration in nature, a novel wood-based vapor generator composed of wood substrates with 3D microchannels and a candle soot decoration surface was designed and developed. Profiting from the simultaneous optimization of the candle soot-decorated wood (CS-wood) properties (hydrophilic wood substrates with microchannels for water transportation and escape, low heat conduction value, and a black CS decoration surface with high solar absorbing abilities), the as-fabricated CS-wood vapor generation material achieved a relatively high net evaporation rate value of 0.950 kg m−2 h−1 when being illuminated at one sun. This bilayer wood-based vapor generator exhibited good capacities of desalination (seawater) and purification (lake water). Such wood-based material as a high-performance, cost-efficient and scalable vapor generator can be a potential candidate for the production of clean water to alleviate the global clean water shortage crisis via the effective utilization of green energy.
1. Introduction
A number of countries in the world such as those in Africa and Western Asia are well aware of the fact that clean fresh water resources are extremely scarce, especially in drought areas with little rainfall and large evaporation.1,2 Furthermore, with the growth of population and aggravation of environmental pollution, the problem of water shortage has become more serious. Seawater desalination and wastewater reuse are considered to be promising methods to address the above-mentioned fresh water scarcity issue because nearly three-fourth of the Earth surface is covered by seawater and a large amount of industrial and domestic wastewater is produced every year.3,4 During the past few years, many methods have been dedicated to improving access to clean water, particularly multistage membrane distillation process and reverse osmosis techniques, which have been successfully commercialized.5–7 However, the reported methods involve high-energy consumption and massive emissions of CO2 greenhouse gases, which has negative effects on the sustainable development of energy and environment.8,9Solar energy, a kind of renewable and green source, has been applied in various fields such as solar water heating systems for buildings,10 electricity generation,11 water purification,12 and so on. Among numerous solar energy utilization technologies, solar vapor generation, as a potential technology for producing clean water by means of the converting light energy to heat energy to facilitate evaporation has recently been the subject of some attention for scientific research and utilization.13–19 The high efficiency achievement of vapor generation via energy conversion depends on the ideal structure composed of light-absorbing materials, low heat conduction and diffusion parts, and water transportation and escape channels.20,21 In terms of the fabrication of the solar vapor generation materials, the common method is that black materials with broadband and high light absorption capacity are coated on various floating supporting substrates, for instance, black TiOx nanoparticle-coated stainless steel meshes,1 polypyrrole-coated polypropylene membranes,22 cotton cloth covered by PEGylated MoS2,23 Au nanoparticle films coated on airlaid papers,24 aluminium nanoparticles deposited on anodic aluminum oxide,25 rGO and MWCNT hybrid coating on PVDF membranes,26 and so on. However, the large-scale utilization of the reported solar vapor generators is still limited as it involves expensive materials and complicated and time-consuming process. Thus, it is necessary to develop a light-harvesting vapor generation material by a low-cost and facile fabrication process.
Based on the above-mentioned situation, in recent years, wood-based solar vapor generation materials have attracted much attention because wood possesses natural hydrophilicity, renewability and 3D multi-scale porous structure and is less expensive, which are conducive to large-scale utilizations.27–29 For instance, Hu's group has prepared several plasmonic metal nanoparticle-decorated wood composites with high efficiency for the conversion of light energy to heat energy under ten sun illumination;30 Liu and co-authors have designed an artificial tree by high-temperature surface carbonization of wood for efficient vapor generation;31 Hu's group has reported a wood-based bilayer structure material with a carbonized top surface for efficient water extraction from groundwater;32 Singamaneni's group has developed a wood-based composite with graphene oxide (GO) coating to achieve a high vapor generation efficiency under twelve sun illumination.33 To date, the fabrication of most of the wood-based solar vapor generation materials is still dependent on relatively expensive coating materials (e.g., Au and GO) or high energy consumption technologies (e.g., high temperature carbonization). Therefore, there is an urgent need to develop wood-based solar vapor generation materials with the potential of scalable applications using low-cost coating materials by an energy saving process.
In this work, inspired by the natural tree transpiration process, wherein water absorbed in the soil by tree roots is transported in a bottom-up way mainly through the tracheid or vessel with microchannels and exhaled into the air through the leaves, we developed a facile and novel decoration method to achieve the low-cost and scalable fabrication of a double-layer wood-based composite for high-efficiency solar vapor generation. This novel wood-based solar absorber was obtained using low-cost household candle soot for solar vapor generation and was applied in seawater desalination and water purification. Furthermore, this bilayer-structured absorber was prepared without using sophisticated chemical reagents or manufacturing equipments, which had greater competing power in practical applications, compared with other wood-based solar absorbers with expensive coating materials and complex preparation processes. The black candle soot coating with the broadband and high light absorption can effectively promote the harvest of solar energy. Furthermore, owing to the low heat conduction property of the natural wood matrix, the heat generated from the conversion of light energy to heat energy can be localized, which can achieve high-efficiency vapor generation. Meanwhile, the hydrophilicity and longitudinal microchannel structure of the natural wood can effectively absorb water and transport it upward, guaranteeing a consecutive water supply for vapor generation. It is worth noting that both the water transport matrix (natural wood slices) and the top solar absorbing layer (candle soot) are easily available, and they are cost-effective materials that can readily achieve scalable applications of wood-based vapor generator for clean water production.
2. Experimental section
2.1 Materials
Candle and balsa wood blocks were provided by Alibaba and were used for structuring the candle soot-decorated wood (CS-wood). Absolute ethanol was supplied by Sinopharm Chemical and was used for cleaning glass slides and dispersing the as-prepared candle soot.2.2 Preparation of candle soot-decorated wood
Candle soot was collected according to the method previously reported in literature.34 Typically, a glass slide was placed in absolute ethanol to perform ultrasonic cleaning for 10 min. After that, the dried glass slide was kept in the middle position of a candle flame for 5 min. The black glass slide with the deposited candle soot was placed in a Petri dish containing absolute ethanol and treated by ultrasound for 20 min to obtain a candle soot suspension. Finally, the CS-wood was obtained by uniformly depositing CS particles on the cross section of wood using a facile drop-casting method.2.3 Characterization
The surface microstructure observations of the raw wood and CS-decorated wood were achieved using a Hitachi S4800 SEM. The surface elemental distribution of the CS-decorated wood were obtained by the corresponding SEM-EDS mapping system. The microstructure observation of the CS particles was implemented using a FEI TECNAI G2 F20 TEM. The surface chemical structures, compositions and bonded valence of the raw wood and CS-decorated wood were analyzed by HORIBA Scientific LabRAM HR Evolution Raman spectra at 633 nm and ThermoFisher ESCALAB 250XI XPS. The sunlight absorption characteristics of the raw wood and CS-decorated wood were analyzed using a PerkinElmer Lambda 750 UV-Vis-NIR spectrometer. The ion concentrations of the seawater and desalted water were evaluated by an Agilent 7500ce inductively coupled plasma mass spectrometer. Conductivity comparisons of seawater, tap water, desalted water, lake water and purified water were carried out using a Mettler Toledo SevenExcellent conductivity tester. The absorption spectra of lake water and purified water were obtained using a Persee TU1901 UV-Vis spectrometer.2.4 Heat conduction property measurements
The laser flash technology has been extensively adopted to investigate the heat conduction properties of many bulk materials.35 Here, the heat conduction property measurements of the raw wood and CS-decorated wood were achieved via a Netzsch LFA 457 laser flash apparatus.28 The heat conduction values of the raw wood and CS-decorated wood were calculated using the equation: k = αρCp, where k (W m−1 K−1), α (mm2 s−1), ρ (g cm−3) and Cp (J g−1 K−1) represent the calculated heat conduction values, measured heat diffusion per unit time along the growth direction of wood, density, and heat capacity of the samples, respectively.2.5 Solar vapor generation experiment
The AuLight CEL-HXF300 simulated solar irradiation system equipped with a standard AM 1.5G spectrum filter serving as an illuminator of one sun intensity (1 kW m−2) was used to conduct the water evaporation experiments of three vapor generation systems (water, wood and CS-wood). The cuboid-shaped wood or CS-wood with a dimension of 10 × 9.5 × 9.5 mm3 (axial × tangential × radial) served as an evaporator and was put on the water surface of a glass container with an inner dimension of 10 mm × 10 mm. The mass changes of three vapor generation systems (water, wood and CS-wood) were monitored using a SOPTOP FA2004 electronic balance (0.0001 g) under irradiation to evaluate the evaporation rate and evaporation efficiency. A FLIR E4 infrared radiation (IR) camera served as the recorder of the evaporator surface temperatures.3. Results and discussion
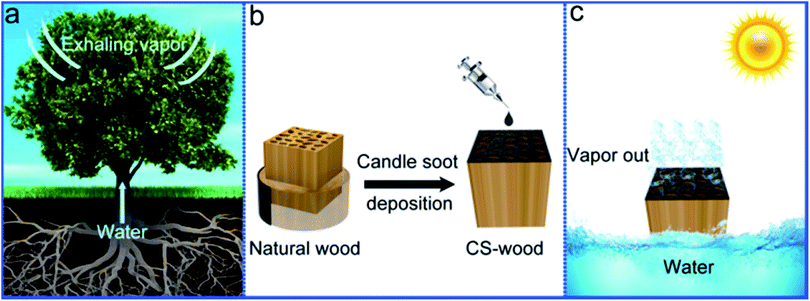
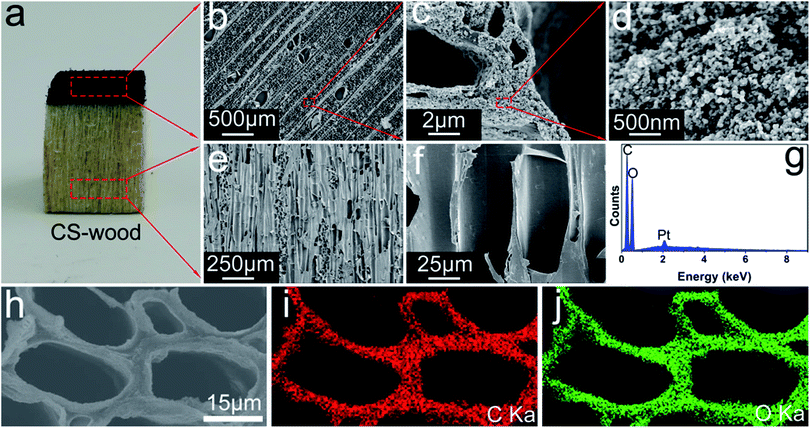
The design concept of the candle soot-decorated wood inspired by the transpiration of natural trees for making a solar vapor generation implement is displayed in Fig. 1. In natural trees, there are a large number of microchannels (vessels or tracheid) along the longitudinal direction of trees for water transportation in a bottom-up way. Part of the transported water finally exhales into the atmosphere through the leaves (Fig. 1a). Inspired by the tree transpiration process, a block of balsa wood with a thickness of 10 mm (in the longitudinal direction) was acquired via cutting trees. Thereafter, the wood block surface was covered by CS particles by the drop-casting process (Fig. 1b). The black CS coating can effectively facilitate solar energy harvesting for enhancing the CS-decorated wood surface temperature. Furthermore, the transformational heat resulting from light absorption can be localized to the evaporation surface owing to the low heat conduction property of wood, and the upward constant supply of water can be implemented for providing an evaporator source due to the hydrophilicity of wood. Based on these performances of the CS-decorated wood, which are conducive to evaporation, a great improvement in the vapor output can be achieved using this system under solar irradiation (Fig. 1c).Fig. 2a displays the appearance photograph of the CS-wood. From this image, the CS-wood exhibited a clear double-layered structure composed of two materials. Of these two materials, the top layer with black color and the subjacent part with light yellow color were the CS particles and wood substance, respectively. The microstructures of the CS particles, the wood substance and the CS-wood were observed in detail by SEM and TEM. The cross-sectional SEM images of the pristine wood exhibited a network-like structure composed of micron-scale channels and cell walls with a thickness of around 1 μm (ESI Fig. S1a–d†). SEM images of the CS-wood top surface demonstrated a microstructure similar to pristine wood with reticular structure (Fig. 2b). However, the cell wall surfaces of the CS-wood were rougher than those of the pristine wood due to the deposition of the CS nanoparticles (Fig. 2c and d). TEM images further indicated that the deposited CS particles possessed diameters of around 30 nm (ESI Fig. S2†). The cross-sectional-view SEM images of the CS-wood displayed a number of long and vertically aligned microchannels with a diameter of dozens of micrometers for water transportation from bottom to top evaporating layer (Fig. 2e and f). The surface elemental composition and distribution of the pristine wood and CS-wood were analyzed by EDS and the corresponding mapping system (Fig. 2g–j). The CS-wood surfaces uniformly distributed two main elements of C and O. The Pt element was observed due to sputtering a conductive layer on the samples for SEM tests.
The surface elemental composition of the pristine wood was similar to that of the CS-wood, which were two main elements of C and O (ESI Fig. S3†). Notably, the atomic ratio of C/O increased from 1.07 (pristine wood) to 1.19 (CS-wood), showing the possible depositions of CS particles on wood surfaces. The Raman spectra of the pristine wood and the CS-wood were characterized to further confirm the successful deposition of the CS particles on wood. As shown in Fig. 3a, there was no absorption peak in the Raman spectra of the pristine wood due to the strong fluorescence effect of lignin in wood. However, the spectrum of CS-wood exhibited two characteristic peaks at around 1350 cm−1 (D band) and 1585 cm−1 (G band) belonging to the sp3 and sp2 hybrid carbons,36 respectively, indicating that the wood surface was successfully covered by CS particles. The XPS spectra served as the method of investigating the surface elemental composition and bonded valence changes of the pristine wood after the coating process was analyzed. There were two main characteristic peaks at around 285 eV and 532 eV assigned to C 1s and O 1s in the XPS survey spectra of both samples (Fig. 3b), respectively. While the relative content of carbon atoms increased from 71.8% (pristine wood) to 93.6% (CS-wood), the relative content of oxygen atoms decreased from 27.3% (pristine wood) to 6.4% (CS-wood). Meanwhile, nitrogen present in wood cannot be observed in the XPS spectra of CS-wood (Fig. 3b and d). In terms of C 1s spectra (Fig. 3c), the pristine wood exhibited four fitting peaks that stemmed from the carbohydrates, lignin and extracts of wood.37 In contrast, the fitting peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O–C–O was absent in the CS-wood C 1s spectra. Furthermore, the relative intensities of the corresponding oxygen-containing peaks of the CS-wood were weaker than those of the pristine wood, indicating that the CS-wood surfaces possessed fewer oxygen-containing groups. The above-mentioned results further confirmed a fact that the CS particles successfully coated the top surface of wood.
O/O–C–O was absent in the CS-wood C 1s spectra. Furthermore, the relative intensities of the corresponding oxygen-containing peaks of the CS-wood were weaker than those of the pristine wood, indicating that the CS-wood surfaces possessed fewer oxygen-containing groups. The above-mentioned results further confirmed a fact that the CS particles successfully coated the top surface of wood.
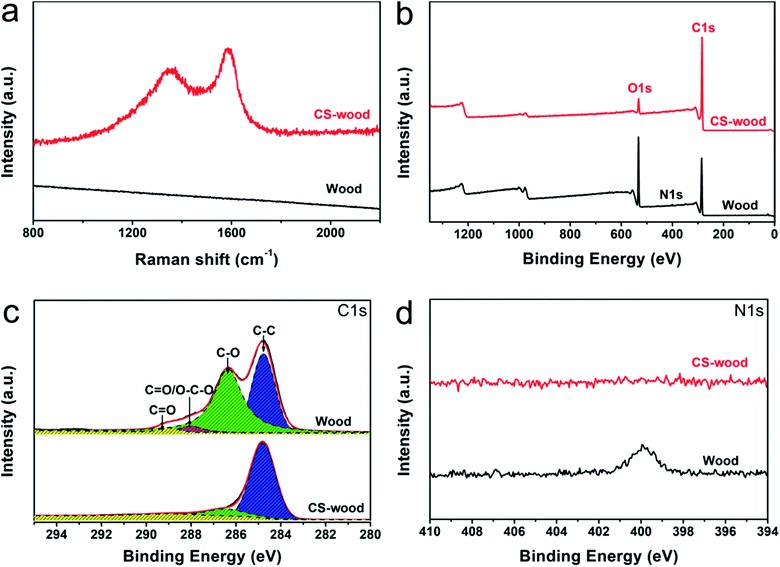 | ||
| Fig. 3 (a) Raman spectroscopy (b) XPS survey spectra (c) C 1s spectra (d) N 1s spectra of the pristine wood and CS-decorated wood. | ||
The hydrophilicity of the matrix is a key factor to achieve a continuous supply of water for vapor generation under solar illumination. The absorption peaks in the FTIR spectrum of the wood substance appeared at around 3360 and 1730 cm−1, demonstrating that –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups existed in wood (ESI Fig. S4†). The existence of the above-mentioned chemical groups with oxygen is conducive to the hydrophilicity of wood. In order to further confirm the hydrophilicity of the wood matrix, the wetting process of CS-wood (30 × 20 × 3 mm3) was recorded by dipping its bottom in methyl blue aqueous solution (ESI Fig. S5†). The methyl blue aqueous solution transportation occurs along the axial direction of the wood. The wetting height of the methyl blue aqueous solution reached 13 mm within 30 s and further rose to 30 mm after 105 s, indicating the rapid water transportation capacity and high hydrophilicity of CS-wood.
O functional groups existed in wood (ESI Fig. S4†). The existence of the above-mentioned chemical groups with oxygen is conducive to the hydrophilicity of wood. In order to further confirm the hydrophilicity of the wood matrix, the wetting process of CS-wood (30 × 20 × 3 mm3) was recorded by dipping its bottom in methyl blue aqueous solution (ESI Fig. S5†). The methyl blue aqueous solution transportation occurs along the axial direction of the wood. The wetting height of the methyl blue aqueous solution reached 13 mm within 30 s and further rose to 30 mm after 105 s, indicating the rapid water transportation capacity and high hydrophilicity of CS-wood.
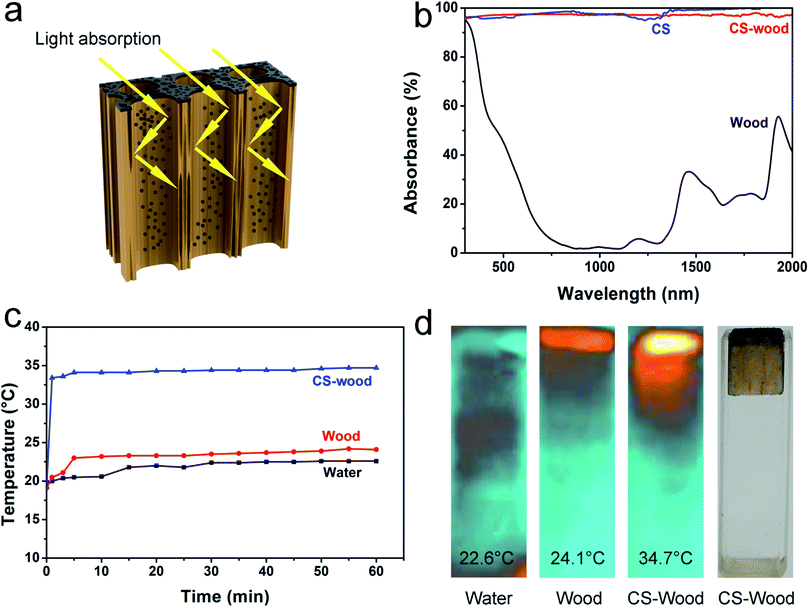
The optical absorptivity comparisons of the pristine wood, the CS and the CS-wood were achieved by using a UV-Vis-NIR spectrometer. The large optical absorptivity differences were observed as shown in Fig. 4b. The CS-wood possesses a relatively high optical absorptivity (>89%) within a broad wavelength range (300–2000 nm), which was similar to the absorbance of CS. By contrast, the natural wood displayed a weaker optical absorptivity (<60%) at the wavenumber region from 500 to 2000 nm As illustrated in Fig. 4a, the stronger optical absorption of CS-wood was profited from the long light absorption path of the longitudinal microchannels of the wood; besides this, it was attributed to the coating of black CS nanoparticles with low reflectivity and the diffusion of CS nanoparticles into the inner wall of wood channels for light absorption.
Another vital factor in the enhancement of water evaporation efficiency is thermal management. The surface temperature values and mapping images of three solar vapor generation systems (pure water, wood and CS-wood) under simulated one sun irradiation were acquired using an IR camera (Fig. 4c and d). After being irradiated for 1 hour at one sun, the recorded temperatures of the three evaporation system surfaces were 22.6 °C (pure water), 24.1 °C (wood) and 34.7 °C (CS-wood). The recorded temperature of the CS-wood evaporation system surface reached 34.1 °C after being illuminated for 5 min, showing the fast response time. After being irradiated for 5 min, the recorded surface temperatures of pure water and floating wood only exhibited a slight increase. However, the recorded surface temperature of the floating CS-wood showed a rise of 14.8 °C after being irradiated for the same time period. These results indicated a fact that CS nanoparticle coverage plays an important role in the transformation of light energy to heat energy. As can be seen from ESI Fig. S6,† CS-wood possesses a relatively low measured thermal conductivity, which did not present a large increase compared with that of the pristine wood. The weak heat conduction property of CS-wood can effectively suppress the loss of converted heat for efficient vapor generation. Furthermore, materials with a high porosity display low bulk density and possess high heat insulation properties. The bulk densities and porosities of the wood and the CS-wood are shown in ESI Section 1.† The wood and the CS-wood showed low bulk densities (0.105 g cm−3 and 0.106 g cm−3, respectively) and high porosities (93.0% and 91.7%, respectively).
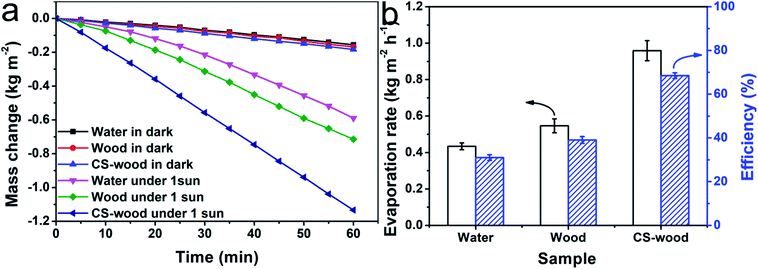
In order to ascertain the photothermal application performance of the CS-wood, the water evaporation measurements of the corresponding three evaporation systems were carried out under dark conditions and a simulated one sun solar illumination intensity. As can be seen from Fig. 5a, without sunlight illumination, the water evaporation capacities of the three systems were similar after 60 minutes. The evaporation capacities of the three systems exhibited large differences after 1 hour of one sun solar illumination. It was apparent that the water evaporation capacity of the CS-wood per unit area was superior to those of the other two evaporation systems after 60 minutes of illumination. The net evaporation rate values (evaporation rate values under illumination subtracted by evaporation rate values under darkness) of the water, wood and CS-wood systems are illustrated in Fig. 5b (black bar). Among the three systems, the pure water system presented the minimum net evaporation rate value of around 0.434 kg m−2 h−1. By contrast, the net evaporation rates were elevated step by step to a maximum value of about 0.959 kg m−2 h−1 (CS-wood system with a 0.001 g cm−2 mass loading of CS nanoparticles). Furthermore, the efficiency value of converting light energy into heat energy is regarded as another important evaluation index of the vapor generation device performance. The evaporation efficiency values of water, wood and CS-wood under the illumination intensity of one sun are defined in terms of the equation: η = ṁhlv/P0Copt, where ṁ, hlv, P0 and Copt represent the net evaporation rate value under the corresponding solar illumination intensity, thermal enthalpy of transformation between liquid and vapor phase and sensible heat, nominal illumination value of 1 kW m−2 and optical concentration.38,39 As illustrated in Fig. 5b (blue bar), the CS-wood evaporation system exhibited an evaporation efficiency value of around 68.5% under one sun illumination, which was greater than those of the wood evaporation system (39.1%) and pure water evaporation system (31.0%). Moreover, it was noteworthy that the evaporation efficiency value of 68.5% for the CS-wood system was comparable with many of the other reported solar vapor generation systems under one sun illumination (ESI Table S1†). The water evaporation tests of CS-wood were performed with different mass loadings of candle soot nanoparticles. As shown in Fig. S7,† the evaporation rate and efficiency of CS-wood showed a slight increase from 0.843 kg m−2 h−1 and 60.2% (with a mass loading of 0.0005 g cm−2) to 0.959 kg m−2 h−1 and 68.5% (with a mass loading of 0.001 g cm−2). When the mass loading further increased, the evaporation rate and efficiency of CS-wood became stable. The energy loss of the CS-wood solar vapor generation system during the evaporation process mainly included radiation loss (4.3%), convection loss (6.7%) and conduction (23.3%) (detailed analysis in ESI Section 2†). Furthermore, water evaporation tests of CS-wood with different mass loadings of candle soot nanoparticles were carried out. As shown in Fig. S7,† the evaporation rate and efficiency of CS-wood showed a slight increase from 0.843 kg m−2 h−1 and 60.2% (with a mass loading of 0.0005 g cm−2) to 0.959 kg m−2 h−1 and 68.5% (with a mass loading of 0.001 g cm−2). When the mass loading further increased, the evaporation rate and efficiency of CS-wood became stable.
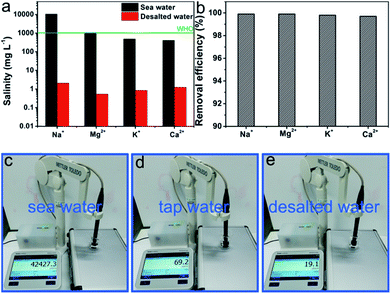
To investigate the solar desalination capability of the CS-wood, the natural seawater from the East China Sea (Zhoushan City, Zhejiang Province) was used to achieve the desalination experiment under natural sunlight by using a homemade system as shown in ESI Fig. S8.† The main cationic concentrations of the natural seawater and the desalinated water obtained from the desalination experiments under the natural sunlight are shown in Fig. 6a and ESI Table S2.† The four main cationic concentrations of the natural seawater presented high values, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 562.85 mg L−1 (Na+), 1086.32 mg L−1 (Mg2+), 492.46 mg L−1 (K+) and 417.40 mg L−1 (Ca2+). However, the corresponding four cationic concentrations of the desalinated water fell to 2.08, 0.54, 0.86 and 1.26 mg L−1 and met the drinking water standards established by the World Health Organization. Meanwhile, the ion removal efficiency of the CS-wood evaporation system was calculated according to the equation R% = [(C0 − Cp)/C0] × 100%, in which R, C0 (mg L−1) and Cp (mg L−1) represent the ion removal efficiency, ions concentrations of natural seawater and desalinated seawater, respectively. As can be seen from Fig. 6b, the four cationic removal efficiencies of the CS-wood evaporation system were higher than 99.7%. Additionally, the desalination level of seawater can also be directly represented by the conductivity value acquired from a digital conductivity instrument (Fig. 6c–e). Three kinds of water, namely, natural seawater, tap water obtained from our lab and desalted water, were used for the conductivity measurement. The seawater, tap water and desalted water displayed conductivity values of 42
562.85 mg L−1 (Na+), 1086.32 mg L−1 (Mg2+), 492.46 mg L−1 (K+) and 417.40 mg L−1 (Ca2+). However, the corresponding four cationic concentrations of the desalinated water fell to 2.08, 0.54, 0.86 and 1.26 mg L−1 and met the drinking water standards established by the World Health Organization. Meanwhile, the ion removal efficiency of the CS-wood evaporation system was calculated according to the equation R% = [(C0 − Cp)/C0] × 100%, in which R, C0 (mg L−1) and Cp (mg L−1) represent the ion removal efficiency, ions concentrations of natural seawater and desalinated seawater, respectively. As can be seen from Fig. 6b, the four cationic removal efficiencies of the CS-wood evaporation system were higher than 99.7%. Additionally, the desalination level of seawater can also be directly represented by the conductivity value acquired from a digital conductivity instrument (Fig. 6c–e). Three kinds of water, namely, natural seawater, tap water obtained from our lab and desalted water, were used for the conductivity measurement. The seawater, tap water and desalted water displayed conductivity values of 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427.3, 69.2 and 19.1 μs cm−1, respectively, indicating the excellent desalination capability of the CS-wood evaporation system.
427.3, 69.2 and 19.1 μs cm−1, respectively, indicating the excellent desalination capability of the CS-wood evaporation system.
Furthermore, it was observed that the CS-wood displayed a self-cleaning ability (Fig. S9†). When the lamp was on, seawater evaporation occurred from the top surface of the CS-wood under irradiation. With the increase of irradiation time, the concentration of seawater on the top surface of the CS-wood increased, which resulted in the formation of salt crystals on the CS-wood. When the lamp was switched off (to simulate night environment), the light-induced seawater evaporation stopped. The deposited salts on the surface of the CS-wood gradually disappeared and dissolved back into the seawater.
As shown in ESI Fig. S10a† inset, the lake water obtained from our university campus was light green in color. By comparison, the purified water was clear and transparent. Moreover, the purification capacity of the CS-wood evaporation system toward lake water was further evaluated by UV-Vis spectroscopy and conductivity measurements. As can be seen from ESI Fig. S10a,† the absorbance of the lake water was higher than that of the purified water from the wavelength of 200 to 900 nm, indicating that the purified water was clearer. The lake water and purified water displayed measured conductivity values of 170.8 and 16.1 μs cm−1 (ESI Fig. S10b†), indicating the existence of fewer ions in the purified water. These results indicate that the CS-wood possesses a potential application value as a solar vapor generation material for desalination and water purification.
4. Conclusions
In summary, inspired by tree transpiration in nature, a novel wood-based material with the function of photothermal conversion was designed and fabricated via uniformly decorating candle soot nanoparticles on the top surfaces of a balsa wood substrate. Benefited from the high optical absorptivity of the candle soot nanoparticles and the long optical absorption path of the wood longitudinal microchannels, the CS-wood possessed a high light absorption capacity (>89%) from the wavelength of 300 to 2500 nm. The wood substrate with a large number of surface hydrophilic chemical groups and longitudinal microchannels can ensure the continuous supply of water for vapor generation owing to the hydrophilicity and capillarity. Furthermore, as wood substrate is a weak heat conductor, the converted heat energy can be localized effectively to the top layers of the CS-wood. Based on these properties, the CS-wood was found to possess a net evaporation rate of 0.950 kg m−2 h−1 and an evaporation efficiency of 67.9% under one sun illumination. The as-fabricated CS-wood also presented excellent capacities of desalination and purification. The CS-wood with high-performance, cost-efficiency and scalable characteristics can open up a new avenue to produce clean water by taking advantage of clean solar energy.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars of China (Grant No. LR19C160001) and Zhejiang Provincial Collaborative Innovation Center for Bamboo Resources and High-efficiency Utilization (Grant No. 2017ZZY2-07).References
- M. Ye, J. Jia, Z. Wu, C. Qian, R. Chen, P. G. O'Brien, W. Sun, Y. Dong and G. A. Ozin, Adv. Energy Mater., 2017, 7, 1601811 CrossRef.
- W. Barnaby, Nature, 2009, 458, 282–283 CrossRef CAS PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- P. Wang and T.-S. Chung, J. Membr. Sci., 2015, 474, 39–56 CrossRef CAS.
- D. Cohen-Tanugi and J. C. Grossman, Desalination, 2015, 366, 59–70 CrossRef CAS.
- A. D. Khawaji, I. K. Kutubkhanah and J.-M. Wie, Desalination, 2008, 221, 47–69 CrossRef CAS.
- Y. Yang, R. Zhao, T. Zhang, K. Zhao, P. Xiao, Y. Ma, P. M. Ajayan, G. Shi and Y. Chen, ACS Nano, 2018, 12, 829–835 CrossRef CAS PubMed.
- R. Semiat, Environ. Sci. Technol., 2014, 42, 8193–8201 CrossRef PubMed.
- Z. Wang, W. Yang, F. Qiu, X. Zhang and X. Zhao, Renewable Sustainable Energy Rev., 2015, 41, 68–84 CrossRef.
- L. Zong, M. Li and C. Li, Nano Energy, 2018, 50, 308–315 CrossRef CAS.
- H. Elasaad, A. Bilton, L. Kelley, O. Duayhe and S. Dubowsky, Desalination, 2015, 375, 71–80 CrossRef CAS.
- F. Zhao, X. Zhou, Y. Shi, X. Qian, M. Alexander, X. Zhao, S. Mendez, R. Yang, L. Qu and G. Yu, Nat. Nanotechnol., 2018, 13, 489–495 CrossRef CAS PubMed.
- P. Zhang, Q. Liao, T. Zhang, H. Cheng, Y. Huang, C. Yang, C. Li, L. Jiang and L. Qu, Nano Energy, 2018, 46, 415–422 CrossRef CAS.
- Y. Li, T. Gao, Z. Yang, C. Chen, W. Luo, J. Song, E. Hitz, C. Jia, Y. Zhou, B. Liu, B. Yang and L. Hu, Adv. Mater., 2017, 29, 1700981 CrossRef PubMed.
- G. Wang, Y. Fu, A. Guo, T. Mei, J. Wang, J. Li and X. Wang, Chem. Mater., 2017, 29, 5629–5635 CrossRef CAS.
- L. Zhu, M. Gao, C. K. N. Peh and G. W. Ho, Nano Energy, 2019, 57, 507 CrossRef CAS.
- L. Zhu, T. Ding, M. Gao, C. K. N. Peh and G. W. Ho, Adv. Energy Mater., 2019, 9, 1900250 CrossRef.
- M. Gao, C. K. Peh, H. T. Phan, L. Zhu and G. W. Ho, Adv. Energy Mater., 2018, 8, 1800711 CrossRef.
- G. Ni, G. Li, S. V. Boriskina, H. Li, W. Yang, T. Zhang and G. Chen, Nat. Energy, 2016, 1, 16126 CrossRef CAS.
- Q. Jiang, H. Gholami Derami, D. Ghim, S. Cao, Y.-S. Jun and S. Singamaneni, J. Mater. Chem. A, 2017, 5, 18397–18402 RSC.
- X. Huang, Y.-H. Yu, O. L. de Llergo, S. M. Marquez and Z. Cheng, RSC Adv., 2017, 7, 9495–9499 RSC.
- Z. Guo, G. Wang, X. Ming, T. Mei, J. Wang, J. Li, J. Qian and X. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 24583–24589 CrossRef CAS PubMed.
- Y. Liu, S. Yu, R. Feng, A. Bernard, Y. Liu, Y. Zhang, H. Duan, W. Shang, P. Tao, C. Song and T. Deng, Adv. Mater., 2015, 27, 2768–2774 CrossRef CAS PubMed.
- L. Zhou, Y. Tan, J. Wang, W. Xu, Y. Yuan, W. Cai, S. Zhu and J. Zhu, Nat. Photonics, 2016, 10, 393–398 CrossRef CAS.
- Y. Wang, C. Wang, X. Song, S. K. Megarajan and H. Jiang, J. Mater. Chem. A, 2018, 6, 963–971 RSC.
- M. Wang, P. Wang, J. Zhang, C. Li and Y. Jin, ChemSusChem, 2019, 12, 467–472 CrossRef CAS PubMed.
- Y. Kuang, C. Chen, S. He, E. M. Hitz, Y. Wang, W. Gan, R. Mi and L. Hu, Adv. Mater., 2019, 31, e1900498 CrossRef PubMed.
- S. He, C. Chen, Y. Kuang, R. Mi, Y. Liu, Y. Pei, W. Kong, W. Gan, H. Xie, E. Hitz, C. Jia, X. Chen, A. Gong, J. Liao, J. Li, Z. J. Ren, B. Yang, S. Das and L. Hu, Energy Environ. Sci., 2019, 12, 1558–1567 RSC.
- M. Zhu, Y. Li, F. Chen, X. Zhu, J. Dai, Y. Li, Z. Yang, X. Yan, J. Song, Y. Wang, E. Hitz, W. Luo, M. Lu, B. Yang and L. Hu, Adv. Energy Mater., 2018, 8, 1701028 CrossRef.
- H. Liu, C. Chen, G. Chen, Y. Kuang, X. Zhao, J. Song, C. Jia, X. Xu, E. Hitz, H. Xie, S. Wang, F. Jiang, T. Li, Y. Li, A. Gong, R. Yang, S. Das and L. Hu, Adv. Energy Mater., 2018, 8, 1701616 CrossRef.
- M. Zhu, Y. Li, G. Chen, F. Jiang, Z. Yang, X. Luo, Y. Wang, S. D. Lacey, J. Dai, C. Wang, C. Jia, J. Wan, Y. Yao, A. Gong, B. Yang, Z. Yu, S. Das and L. Hu, Adv. Mater., 2017, 29, 1704107 CrossRef PubMed.
- K. K. Liu, Q. Jiang, S. Tadepalli, R. Raliya, P. Biswas, R. R. Naik and S. Singamaneni, ACS Appl. Mater. Interfaces, 2017, 9, 7675–7681 CrossRef CAS PubMed.
- X. Deng, L. Mammen, H. J. Butt and D. Vollmer, Science, 2012, 335, 67–70 CrossRef CAS PubMed.
- D. Zhao, X. Qian, X. Gu, S. A. Jajja and R. Yang, J. Electron. Packag., 2016, 138, 040802 CrossRef.
- Y. Hu, J. Yang, J. Tian, L. Jia and J.-S. Yu, Carbon, 2014, 77, 775–782 CrossRef CAS.
- S. Migneault, A. Koubaa, P. Perré and B. Riedl, Appl. Surf. Sci., 2015, 343, 11–18 CrossRef CAS.
- F. Liu, B. Zhao, W. Wu, H. Yang, Y. Ning, Y. Lai and R. Bradley, Adv. Funct. Mater., 2018, 28, 1803266 CrossRef.
- P. Zhang, J. Li, L. Lv, Y. Zhao and L. Qu, ACS Nano, 2017, 11, 5087–5093 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9se00617f |
| This journal is © The Royal Society of Chemistry 2020 |