Open Access Article
Open Access ArticleTwo-in-one strategy for fluorene-based spirocycles via Pd(0)-catalyzed spiroannulation of o-iodobiaryls with bromonaphthols†
Bojun
Tan‡
,
Long
Liu‡
,
Huayu
Zheng
,
Tianyi
Cheng
,
Dianhu
Zhu
 *,
Xiaofeng
Yang
*,
Xiaofeng
Yang
 and
Xinjun
Luan
and
Xinjun
Luan
 *
*
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, 710127, China. E-mail: zhudianhu@nwu.edu.cn; xluan@nwu.edu.cn
First published on 8th September 2020
Abstract
Rapid assembly of fluorene-based spirocycles represents a highly significant but challenging task in organic synthesis. Reported herein is a novel Pd(0)-catalyzed [4+1] spiroannulation of simple o-iodobiaryls with bromonaphthols for the one-step construction of [4,5]-spirofluorenes in high yields with excellent functional group tolerance. Noteworthily, these valuable fluorene-based coumarin skeletons can enrich the database of C-coumarins and exhibit excellent spectroscopic properties.
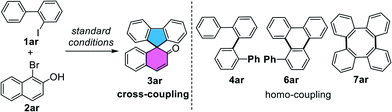
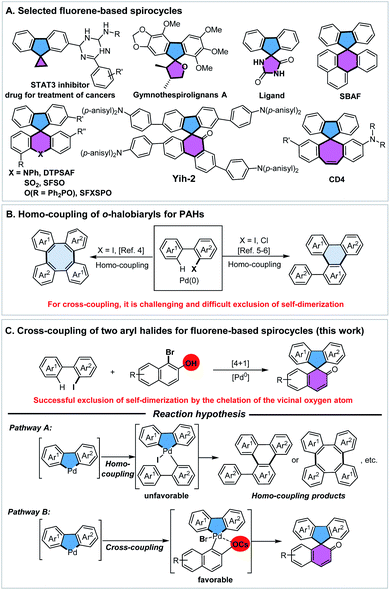
Fluorene-based spirocycles have been employed as a type of privileged structures which constitute the central core of a wide range of bioactive molecules and functional materials in modern organic synthesis.1 Therefore, the pursuit of new methods for the rapid assembly of spirofluorenes and their derivatives is of high interest. So far, most of the established protocols were focused on the preparation of symmetrical spirofluorenes, while unsymmetrical have been rarely investigated. Noteworthily, unsymmetrical spirofluorenes are receiving more and more attention due to the adjustability and modifiability of structures and properties in the design of new pharmaceuticals, ligands and optoelectronic materials (Scheme 1A).2 In this context, we present an unprecedented palladium-catalyzed [4+1] spiroannulation process through cross-coupling of simple o-iodobiaryls and bromonaphthols for the one-step direct access to a variety of unsymmetrical [4,5]-spirofluorenes.
 | ||
| Scheme 1 Selected fluorene-based spirocycles and coupling of o-halobiaryls for PAHs or spirofluorenes. | ||
Bimolecular coupling of aryl halides represents one of the most versatile and powerful tools for the construction of biaryl-derived compounds or polycyclic aromatic hydrocarbons (PAHs).3 However, the overall synthetic efficiency of cross-coupling is often eroded by the accompanying homo-coupling. For example, Zhang4a and Shi4b groups reported reactions of Pd-catalyzed coupling of o-iodobiaryl compounds to prepare long conjugated tetraphenylenes through the double C–H activation/[4+4] annulation (Scheme 1B). Notably, Itami5 and Shi6 made a ground-breaking advancement in the dimerization of o-chloro- or o-iodobiaryls for the synthesis of a library of valuable triphenylenes, which could have potential access to graphene nanoribbon substructures. By contrast, the development of cross-coupling of o-iodobiaryls with other aryl halides is a highly significant but challenging task in organic synthesis. After many efforts, some elegant examples have reported the cross-coupling of two aryl halides by Lautens,7a Zhang,7b Liang,7c Lin,7eetc. However, one of the main obstacles in the reaction process is the occurrence of homo-coupling of aryl iodides.
In connection with our previous work8 and interest in metal-catalyzed dearomatization reactions,9,10 the development of a “two-in-one” strategy for rapid synthesis of a variety of [4,5]-spirofluorenes by Pd(0)-catalyzed spiroannulation through cross-coupling of o-iodobiaryls with bromonaphthols, is highly attractive. In our reaction hypothesis, there will be a competition between the oxidative coupling of C(aryl), C(aryl)-palladacycle with other aryl halides (Scheme 1C). Actually, the activity of aryl iodides is higher than that of aryl bromides. We wonder whether the vicinal oxygen anion of bromonaphthols could be employed to dominate such cross-coupling by suppressing dimerization of o-iodobiaryls through the chelation of aryl/aryl palladacycle, which is beneficial to the oxidative addition of the C–Br bond.
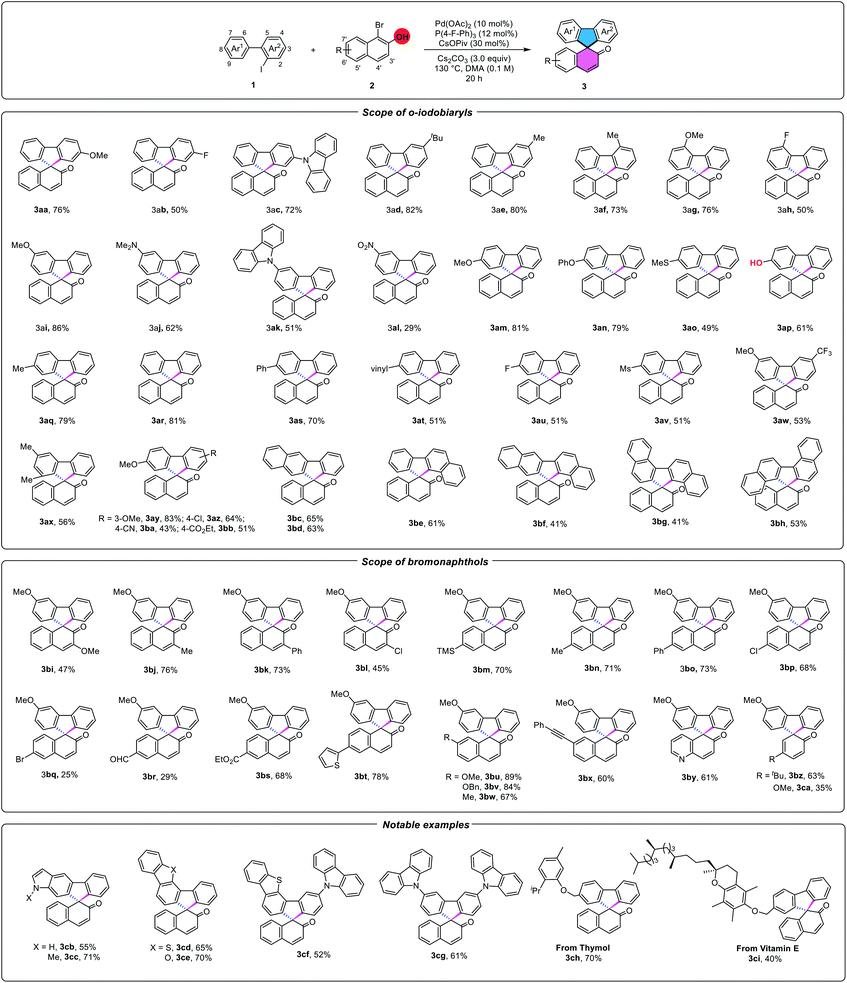
As expected, the reaction indeed led to [4+4] or [4+2] dimerization for producing tetraphenylene 7ar or triphenylene 6ar as major products, according to the known literature,4–6 when we only use 2-iodo-1,1′-biphenyl 1ar as the coupling fragment. To evaluate the feasibility of the proposal outlined in Scheme 1C, we investigated the coupling of 2-iodo-1,1′-biphenyl 1ar with 1-bromo-2-naphthol 2ar by using Pd(0) catalysis. Initial attempts with Pd2(dba)3 barely delivered 29% yield of spiroannulative product 3ar and a lower chemo-selectivity (Table 1, entry 2). Further studies indicated that with the use of monodentated phosphine ligands such as PPh3 or P(p-anisyl)3, and bisdentated phosphine ligands including DPPM (bis(diphenylphosphino)methane), DPPF (1,1′-bis(diphenylphosphino)ferrocene), the reaction proceeded to afford 24–56% yields of 3ar, along with unsatisfactory selectivity (Table 1, entries 3–6). Besides, the nitrogen ligand DIBPY (2,2′-bipyridine) could also deliver 17% yield of the desired product (Table 1, entry 7). Surprisingly, a reaction in the presence of an electron-deficient phosphine ligand P(4-F-Ph)3 using Pd(OAc)2 as the catalyst in DMA at 130 °C for 20 h provided highly chemo-selective [4+1] spiroannulation product 3ar in 83% yield (Table 1, entry 1). High chemo-selectivity of the reaction may be because the electron-deficient P(4-F-Ph)3 makes the palladium center lack electrons and makes it easier to coordinate with the naphthol oxygen anion, which is beneficial to oxidative addition of the C–Br bond of 2ar. In addition to the effect of ligands, we found that bases also play an important role in the chemo-selectivity of the reaction. Homo-coupling product tetraphenylene 7ar (ref. 5) was formed as the major product when we used Li2CO3 or Na2CO3 as the base. However, [4+4] dimerization was inhibited while stronger bases such as K2CO3 or Cs2CO3 were used, especially Cs2CO3 (Table 1, entries 1, 8 and 9). Moreover, the use of CsOPiv is indispensable as the enhancement additive for Pd(II)-catalyzed C–H activation reactions (Table 1, entry 10).11 By contrast, only 30% yield of the desired product was observed when we used 2-bromo-1,1′-biphenyl instead of 1ar under the standard conditions (Table 1, entry 11). Notably, this new reaction was featured by the successful exclusion of self-dimerization of aryl iodides (4ar and 6ar), which assumes that the vicinal oxygen anion might participate in the chelation with aryl/aryl palladacycle in this cross-coupling.
| Entry | Variations from standard conditions | Yieldb (%) | |||
|---|---|---|---|---|---|
| 3ar | 4ar | 6ar | 7ar | ||
| a Standard conditions: 2-iodo-1,1′-biphenyl (1ar, 1.2 equiv), 1-bromo-2-naphthol (2ar, 0.2 mmol), Pd(OAc)2 (10 mol%), P(4-F-Ph)3 (12 mol%), CsOPiv (30 mol%) and Cs2CO3 (3.0 equiv) in DMA (0.1 M) at 130 °C for 20 h. b Yields were determined by 1H NMR analysis. | |||||
| 1 | None | 83 | — | — | — |
| 2 | Pd2(dba)3 instead of Pd(OAc)2 | 29 | <5 | — | 23 |
| 3 | PPh3 instead of P(4-F-Ph)3 | 56 | <5 | <5 | <10 |
| 4 | P(p-anisyl)3 instead of P(4-F-Ph)3 | 37 | <5 | <5 | — |
| 5 | DPPM instead of P(4-F-Ph)3 | 28 | <5 | — | — |
| 6 | DPPF instead of P(4-F-Ph)3 | 24 | <5 | <5 | 15 |
| 7 | DIBPY instead of P(4-F-Ph)3 | 17 | <5 | — | — |
| 8 | Li2CO3 or Na2CO3 instead of Cs2CO3 | <1 | <2 | — | 45 |
| 9 | K2CO3 instead of Cs2CO3 | 20 | <5 | — | <10 |
| 10 | W/o CsOPiv | <5 | <5 | — | — |
| 11 | 2-Bromo-1,1′-biphenyl instead of 1ar | 30 | <5 | — | — |
With the optimized conditions in hand, the reaction scope of a wide range of o-iodobiaryls was investigated in Table 2. Gratifyingly, the envisioned [4+1] spiroannulation proceeded smoothly to generate a set of fascinating spirofluorene molecules (3aa-3bh) in good to excellent yields (41–86%). In general, reactions of 2-iodobiaryls bearing both electron-donating and electron-withdrawing substituted groups at the 3-, 4-, 5-, 6-, 7-, 8- or 9-positions of the phenyl ring occurred smoothly, with the former producing higher yields, implying that the vast change in electronic properties did not shut down C–H activation and the generation of the cyclopalladium intermediate. Notably, methyl substitution at the 9-position of the targeted starting material was also effective (3ax).12 Contrary to classical synthesis of spirofluorenes by using aryl organometallic reagents and ketones, this reaction showed excellent functional group compatibility, as many functional groups such as methoxyl, methylthiol, phenoxyl, dimethylamino, vinyl, trifluoromethyl, fluoro, chloro, cyano and even hydroxyl, ester and carbonyl substituents, were all tolerated to produce the corresponding products in good to high yields (3aa-3bb). When nitro-substituted o-iodobiaryl was applied to the system, the reaction could afford 29% yield of 3al. Besides, the reaction of dinaphthalened iodides also proceeded smoothly in moderate yields (3bc-3bh). For the scope of 1-bromonaphthalen-2-ol derivatives, substituents at the 3, 6, 7-positions of 1-bromo-2-naphthols also could produce moderate to good yields, with good functional group tolerance (such as formyl, ester, trimethylsilyl, alkynyl, and 2-thiophenyl substituents) (3bi-3bx). Moreover, the derivative 2bq with a 6-position bromide, was also compatible to produce the desired cross-coupling product,12 albeit with a low yield of conversion. Afterwards, 5-bromoquinolin-6-ol or simple and useful bromophenols, such as 2-bromo-4-(tert-butyl)phenol or 2-bromo-4-methoxyphenol, which possess much higher energy barriers for dearomatization, could be utilized in this cross-coupling and deliver 35–63% yields of the corresponding spiroannulative molecules (3by-3ca).
Encouragingly, notable examples including indole (N-methyl indole), carbazole, dibenzofuran and even a dibenzothiophene structure, commonly used in organic light-emitting materials, were also compatible under our conditions (3cb-3cg). To demonstrate the practicality of the reaction on more complex and biorelevant substrates, we further demonstrated the applicability of this method on a Thymol derivative in 70% yield (3ch). Besides, the vitamin E derivative with long alkyl chains was also tested as a superior candidate in this palladium-catalyzed cross-coupling strategy, delivering the target [4+1] spirofluorene product 3ci in moderate yield.
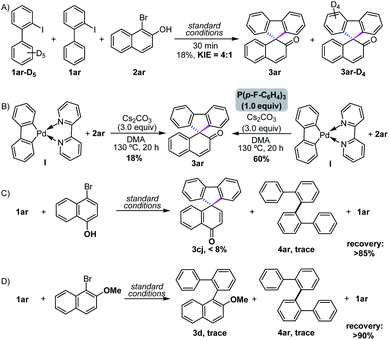
In order to gain detailed mechanistic insights into this reaction, several control experiments were carried out. Firstly, the intermolecular KIE competition experiment revealed that the ortho-C–H activation is the rate-determination step of the reaction (Scheme 2A). Subsequently, 18% yield of the [4+1] spiroannulative product was detected when 2,2′-bipyridine-coordinated cyclopalladium I was used, while the yield rapidly increased to 60% after the ligand exchange by the addition of P(4-F-Ph)3 (Scheme 2B). These outcomes implied that the reaction likely proceeded via five-membered cyclopalladation13 after the pathway of C–H activation. Moreover, reacting with 4-bromonaphthalen-1-ol only lead to <8% yield of dearomatizative spirofluorene, while >90% yield of 2-iodo-1,1′-biphenyl 1ar was recycled when 1-bromo-2-methoxynaphthalene was used as a C1 synthon, indicating that the chelation of the vicinal oxygen anion may be crucial for the control of chemo-selectivity in this cross-coupling (Scheme 2C and D).
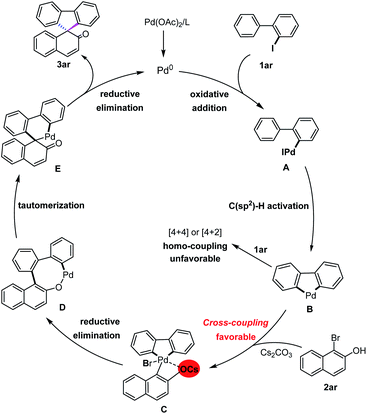
Based on the aforementioned mechanistic studies and relevant literature,7c,10a,13 a plausible mechanism of the present Pd(0)-catalyzed [4+1] spiroannulation is proposed in Scheme 3. Initially, oxidative addition of 2-iodo-1,1′-biphenyl 1ar to the Pd(0)-catalyst occurs to produce five-membered C(aryl), C(aryl)-palladacycle B by C–H bond activation in the presence of CsOPiv. Next, the naphthol oxygen anion is rapidly generated after being deprotonated with Cs2CO3 and easier to coordinate with an electron-deficient palladium center, which is beneficial to oxidative addition of the C–Br bond and furnishes the expected key intermediate C determining high chemo-selectivity in this cross-coupling. Afterwards, intermediate C would be followed by reductive elimination to form a rather strained eight-membered intermediate D, which then successfully affords palladacycle E by releasing the ring strain via enol–keto tautomerization of the phenol ring. Finally, reductive elimination of intermediate E takes place to produce the desired spiroannulative product 3ar and concomitantly regenerates the active Pd(0) for the next catalytic cycle.
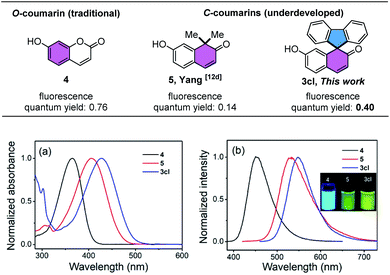
Given the broad application of unsymmetrical spirofluorene derivatives, we studied the spectroscopic properties of the synthesized spirofluorene 3cl (Fig. 1), which demonstrates the rapid construction of fluorene-based coumarins from 2-iodo-1,1′-biphenyl. Contrary to traditional O-substituted coumarins (7-hydroxy coumarins),14 the spectroscopic studies of C-coumarins are rare and underdeveloped. This new class of C-substituted coumarins exhibits special properties on the absorption/emission wavelength. Its absorption wavelength red-shifted gradually from 365 nm to 429 nm and emission wavelength red-shifted nearly 100 nm in NaHCO3–NaOH buffer (pH = 10) from the localized state, with the occurrence of yellow light and red-shift from the ultraviolet region to visible region compared to the spectral properties of 7-hydroxy coumarins (Fig. 1), which may further expand the biological application of coumarins. Besides, the fluorescence quantum yield of fluorene-based coumarin 3cl is 0.40, which is greatly improved compared to that of previous dimethyl-substituted coumarin 5 (ΦF = 0.14).15 Moreover, fluorene-based coumarin 3cl exhibited a similar pKa (7.99) with 7-hydroxycoumarin 4 (pKa = 7.56) than 5 (pKa = 8.35), indicating that 3cl has excellent performance and stability as C-coumarin derivatives. The above results implied that replacement of the oxygen atom in resonance with the pull group of the push–pull scaffold in coumarin by a less electron-donating atom could produce a significant bathochromic shift in absorption/emission wavelength, which also has been theoretically rationalized and repeatedly demonstrated in the previous work.14–16 Besides, the introduction of the rigid fluorene ring to C-coumarins will hinder the nonradiative transition and lead to the increase of fluorescence quantum yield (3cl and 5). The subsequent structural adjustment of fluorene-based coumarins continues and is expected to improve the fluorescence quantum yield of C-coumarins in the future.
Conclusions
In summary, we have developed a “two-in-one” strategy for highly chemo-selective Pd(0)-catalyzed [4+1] spiroannulation of simple o-iodobiaryl and bromonaphthols for the rapid assembly of fluorene-based spirocycles in good to high yields, with a broad substrate scope and excellent functional group tolerance. The key features of this new process are the involvements of a five-membered aryl–aryl palladacyclic intermediate and the chelation of the vicinal oxygen anion determining high chemo-selectivity in this cross-coupling. The preliminary spectroscopic studies of these structural cores for spirofluorenes exemplified the excellent properties with red-shifted absorption and emission wavelengths compared to the traditional 7-hydroxycoumarin, which provides a new perspective for the study of C-coumarins.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21925108 and 21901201), Natural Science Foundation of Shaanxi Province (2020JQ-571), the Key Science and Technology Innovation Team of Shaanxi Province (2017KCT-37), the Education Department of Shaanxi Province (18JS108, 20JS146), and the Key Laboratory Project of Xi'an (201805058ZD9CG42) for financial support.Notes and references
- For selected reviews, see: (a) T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS; (b) J. Seo, J. H. Noh and S. I. Seok, Acc. Chem. Res., 2016, 49, 562 CrossRef CAS.
- For selected applications of unsymmetrical spirofluorene skeletons, see: (a) D. Vak, B. Lim, S. Lee and D. Kim, Org. Lett., 2005, 7, 4229 CrossRef CAS; (b) J.-Y. Kim, C.-W. Lee, J. Jang and M.-S. Gong, Dyes Pigm., 2012, 94, 304 CrossRef CAS; (c) M. Romain, D. Tondelier, J.-C. Vanel, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, Angew. Chem., Int. Ed., 2013, 52, 14147 CrossRef CAS; (d) S.-J. Xiao, X.-X. Lei, B. Xia, D.-Q. Xu, H.-P. Xiao, H.-X. Xu, F. Chen, L.-S. Ding and Y. Zhou, Tetrahedron Lett., 2014, 55, 5949 CrossRef CAS; (e) P. Marinova, M. Marinov, M. Kazakova, Y. Feodorova, P. Penchev, V. Sarafian and N. Stoyanov, Biotechnol. Biotechnol. Equip., 2014, 28, 316 CrossRef CAS; (f) M. Romain, S. Thiery, A. Shirinskaya, C. Declairieux, D. Tondelier, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, Angew. Chem., Int. Ed., 2015, 54, 1176 CrossRef CAS; (g) J. Zhao, Z. Xu, K. Oniwa, N. Asao, Y. Yamamoto and T. Jin, Angew. Chem., Int. Ed., 2016, 55, 259 CrossRef CAS; (h) J. Li, D. Ding, Y. Tao, Y. Wei, R. Chen, L. Xie, W. Huang and H. Xu, Adv. Mater., 2016, 28, 3122 CrossRef CAS; (i) C. Y. Chan, Y. C. Wong, M. Y. Chan, S. H. Cheung, S. K. So and V. W. Yam, ACS Appl. Mater. Interfaces, 2016, 8, 24782 CrossRef CAS; (j) J. Wang, K. Liu, L. Ma and X. Zhan, Chem. Rev., 2016, 116, 14675 CrossRef CAS; (k) Y.-C. Chen, S.-K. Huang, S.-S. Li, Y.-Y. Tsai, C.-P. Chen, C.-W. Chen and Y. J. Chang, ChemSusChem, 2018, 11, 3225 CrossRef CAS; (l) H. Wang, Y. Liu, W. Hu, W. Xu, P. Wang, Y. Wang and X. Luan, Org. Electron., 2018, 61, 376 CrossRef CAS; (m) J. Guo, CN 109293641A, 2019.
- For selected reviews, see: (a) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS; (b) I. Cepanec. Synthesis of Biaryls, Elsevier: New York, 2004 Search PubMed; (c) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (d) G. P. McGlacken and L. M. Bateman, Chem. Soc. Rev., 2009, 38, 2447 RSC; (e) Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463 CrossRef CAS; (f) R. Pratap and V. J. Ram, Chem. Rev., 2014, 114, 10476 CrossRef CAS; (g) Y. Luo, X. Pan, X. Yu and J. Wu, Chem. Soc. Rev., 2014, 43, 834 RSC; (h) Y. Segawa, T. Maekawa and K. Itami, Angew. Chem., Int. Ed., 2015, 54, 66 CrossRef CAS; (i) H. Ito, Y. Segawa, K. Murakami and K. Itami, J. Am. Chem. Soc., 2019, 141, 3 CrossRef CAS.
- For selected examples in [4+4] annulation via o-halobiaryls, see: (a) H. Jiang, Y. Zhang, D. Chen, B. Zhou and Y. Zhang, Org. Lett., 2016, 18, 2032 CrossRef CAS; (b) C. Zhu, Y. Zhao, D. Wang, W.-Y. Sun and Z. Shi, Sci. Rep., 2016, 6, 33131 CrossRef.
- Y. Koga, T. Kaneda, Y. Saito, K. Murakami and K. Itami, Science, 2018, 359, 435 CrossRef CAS.
- C. Zhu, D. Wang, D. Wang, Y. Zhao, W.-Y. Sun and Z. Shi, Angew. Chem., Int. Ed., 2018, 57, 8848 CrossRef CAS.
- For selected examples, see: (a) M. Sickert, H. Weinstabl, B. Peters, X. Hou and M. Lautens, Angew. Chem., Int. Ed., 2014, 53, 5147 CAS; (b) S. Pan, H. Jiang, Y. Zhang, D. Chen and Y. Zhang, Org. Lett., 2016, 18, 5192 CrossRef CAS; (c) Y. Yang, B. Zhou, X. Zhu, G. Deng, Y. Liang and Y. Yang, Org. Lett., 2018, 20, 5402 CrossRef CAS; (d) W. Hagui, N. Besbes, E. Srasra, T. Roisnel, J.-F. Soulé and H. Doucet, Org. Lett., 2016, 18, 4182 CrossRef CAS; (e) D. Wei, M.-Y. Li, B.-B. Zhu, X.-D. Yang, F. Zhang, C.-G. Feng and G.-Q. Lin, Angew. Chem., Int. Ed., 2019, 58, 16543 CrossRef CAS.
- (a) B. Tan, L. Bai, P. Ding, J. Liu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2019, 58, 1474 CrossRef CAS; (b) Z. Zuo, J. Wang, J. Liu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2020, 59, 653 CrossRef CAS.
- For selected reviews and examples, see: (a) S. P. Roche and J. Porco Jr, Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS; (b) C. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef; (c) W. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC; (d) C. Zheng and S.-L. You, Chem, 2016, 1, 830 CrossRef CAS ; for pioneering examples, see:; (e) M. Bao, H. Nakamura and Y. Yamamoto, J. Am. Chem. Soc., 2001, 123, 759 CrossRef CAS; (f) B. M. Trost and J. Quancard, J. Am. Chem. Soc., 2006, 128, 6314 CrossRef CAS; (g) J. García-Fortanet, F. Kessler and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 6676 CrossRef; (h) Q. Wu, H. He, W. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418 CrossRef CAS; (i) T. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno and Y. Hamada, Org. Lett., 2010, 12, 5020 CrossRef CAS; (j) Q. Wu, W. Liu, C. Zhuo, Z. Rong, K. Ye and S.-L. You, Angew. Chem., Int. Ed., 2011, 50, 4455 CrossRef CAS.
- (a) J. Nan, Z. Zuo, L. Luo, L. Bai, H. Zheng, Y. Yuan, J. Liu, X. Luan and Y. Wang, J. Am. Chem. Soc., 2013, 135, 17306 CrossRef CAS; (b) J. Nan, J. Liu, H. Zheng, Z. Zuo, L. Hou, H. Hu, Y. Wang and X. Luan, Angew. Chem., 2015, 127, 2386 CrossRef; (c) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, J. Am. Chem. Soc., 2015, 137, 4876 CrossRef CAS; (d) L. Bai, Y. Yuan, J. Liu, J. Wu, L. Han, H. Wang, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2016, 55, 6946 CrossRef CAS; (e) L. Fan, J. Liu, L. Bai, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2017, 56, 14257 CrossRef CAS; (f) Z. Zuo, H. Wang, L. Fan, J. Liu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2017, 56, 2767 CrossRef CAS; (g) Z. Zuo, H. Wang, Y. Diao, Y. Ge, J. Liu and X. Luan, ACS Catal., 2018, 8, 11029 CrossRef CAS; (h) L. Bai, J. Liu, W. Hu, K. Li, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2018, 57, 5151 CrossRef CAS.
- (a) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS; (b) M. Lafrance, S. I. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2007, 129, 14570 CrossRef CAS; (c) S. Rousseaux, S. I. Gorelsky, B. K. W. Chung and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 10692 CrossRef CAS.
- CCDC 2010458 (3ax) and 2010459 (3by)† contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- For selected examples of five-membered cyclopalladium, see: (a) G. Dyker, Angew. Chem., Int. Ed., 1992, 31, 1023 CrossRef; (b) G. Dyker, Angew. Chem., Int. Ed., 1994, 33, 103 CrossRef; (c) B. Martín-Matute, C. Mateo, D. J. Cárrdenas and A. M. Echavarren, Chem.–Eur. J, 2001, 7, 2341 CrossRef; (d) R. C. Larock and Q. Tian, J. Org. Chem., 2001, 66, 7372 CrossRef CAS; (e) J. Cámpora, P. Palma, D. del Río, J. A. López and P. Valerga, Chem. Commun., 2004, 1490 RSC; (f) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem.–Eur. J., 2010, 16, 2654 CrossRef CAS; (g) Z. Wu, D. Ma, B. Zhou, X. Ji, X. Ma, X. Wang and Y. Zhang, Angew. Chem., Int. Ed., 2017, 56, 12288 CrossRef CAS; (h) J. Ye, Z. Shi, T. Sperger, Y. Yasukawa, C. Kingston, F. Schoenebeck and M. Lautens, Nat. Chem., 2017, 9, 361 CrossRef CAS; (i) H. Yoon, M. Rölz, F. Landau and M. Lautens, Angew. Chem., Int. Ed., 2017, 56, 10920 CrossRef CAS; (j) A. Lu, X. Ji, B. Zhou, Z. Wu and Y. Zhang, Angew. Chem., Int. Ed., 2018, 57, 3233 CrossRef CAS; (k) Q. Zhao, W. C. Fu and F. Y. Kwong, Angew. Chem., Int. Ed., 2018, 57, 3381 CrossRef CAS.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Chem. Rev., 2019, 119, 10403 CrossRef CAS.
- S. Qiu, D. Dai, C. Guo, Z. Sun, H. Li, X. Qian and Y. Yang, Dyes Pigm., 2019, 163, 55 CrossRef CAS.
- (a) C. J. Tredwell and C. M. Keary, Chem. Phys., 1979, 43, 307 CrossRef CAS; (b) P. F. Corey, R. W. Trimmer and W. G. Biddlecom, Angew. Chem., Int. Ed., 1991, 30, 1646 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2010458 (3ax) and CCDC 2010459 (3by). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc04386a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |