Open Access Article
Open Access ArticleModified cyclodextrins solubilize elemental sulfur in water and enable biological sulfane sulfur delivery†
Sarah G.
Bolton
 and
Michael D.
Pluth
and
Michael D.
Pluth
 *
*
Department of Chemistry and Biochemistry, Materials Science Institute, Knight Campus for Accelerating Scientific Impact, Institute of Molecular Biology, University of Oregon, Eugene, OR 97403, USA. E-mail: pluth@uoregon.edu
First published on 5th October 2020
Abstract
An important form of biological sulfur is sulfane sulfur, or S0, which is found in polysulfide and persulfide compounds as well as in elemental sulfur. Sulfane sulfur, often in the form of S8, functions as a key energy source in the metabolic processes of thermophilic Archaean organisms found in sulfur-rich environments and can be metabolized both aerobically and anaerobically by different archaeons. Despite this importance, S8 has a low solubility in water (∼19 nM), raising questions of how it can be made chemically accessible in complex environments. Motivated by prior crystallographic data showing S8 binding to hydrophobic motifs in filamentous glycoproteins from the sulfur reducing Staphylothermus marinus anaerobe, we demonstrate that simple macrocyclic hydrophobic motifs, such as 2-hydroxypropyl β-cyclodextrin (2HPβ), are sufficient to solubilize S8 at concentrations up to 2.0 ± 0.2 mM in aqueous solution. We demonstrate that the solubilized S8 can be reduced with the common reductant tris(2-carboxyethyl)phosphine (TCEP) and reacts with thiols to generate H2S. The thiol-mediated conversion of 2HPβ/S8 to H2S ranges from 80% to quantitative efficiency for Cys and glutathione (GSH). Moreover, we demonstrate that 2HPβ can catalyze the Cys-mediated reduction of S8 to H2S in water. Adding to the biological relevance of the developed systems, we demonstrate that treatment of Raw 264.7 macrophage cells with the 2HPβ/S8 complex prior to LPS stimulation decreases NO2− levels, which is consistent with known activities of bioavailable H2S and sulfane sulfur. Taken together, these investigations provide a new strategy for delivering H2S and sulfane sulfur in complex systems and more importantly provide new insights into the chemical accessibility and storage of S0 and S8 in biological environments.
Introduction
Sulfur is a long-standing critical component to life on Earth. Prior to the Great Oxidation Event approximately 2.4 billion years ago, during which time the Earth's atmosphere became rich in O2, the atmosphere on Earth was weakly reducing.1 Volcanic activity was an abundant source of sulfur-containing species, and gases including sulfur dioxide (SO2) and hydrogen sulfide (H2S) were released and dissolved in pools or lakes.2 Spark discharge experiments using a simulated atmosphere containing H2S and a mixture of reducing gases thought to be present in the early Earth atmosphere have demonstrated abiotic synthesis of diverse organic compounds including amino acids.3 In conditions reflecting those near deep-sea vents rich in iron-sulfur compounds, both H2 and organosulfur compounds were generated from a mixture of H2S and FeS under a N2/CO2 atmosphere in acidic conditions.4 Sulfane sulfur (S0), which is most commonly found as elemental octasulfur (S8), is also found in these deep-sea environments and is another important source of biologically available sulfur.Recently, interest in biological and synthetic S0 sources has increased significantly due to the connection between such species and the small biological signaling molecule H2S. H2S is produced endogenously from cysteine metabolism and serves signaling roles in diverse pathways. Along with carbon monoxide (CO) and nitric oxide (NO), H2S is now recognized as member of the family of small molecules often referred to as gasotransmitters, which are produced enzymatically and act upon specific molecular targets within cellular environments.5–7 One unique feature that distinguishes H2S from CO and NO is that sulfur has biologically-accessible oxidation states ranging from −2 to +6 and participates in a complex redox cellular landscape.8 In many eukaryotic organisms, H2S serves as a source of biologically available sulfur and is intrinsically tied to both organic and inorganic S0-containing species, including persulfides and related polysulfides/polysulfanes, in the S0 pool. This redox labile pool can generate H2S upon reduction or participate in transpersulfidation reactions to transfer S0 moieties to cysteine residues.9
Prior investigations into the reaction between elemental sulfur and H2S have demonstrated the formation of inorganic polysulfide ions (S2−n>1), which are also important intermediates in sulfur cycling in sediments.10 Interestingly, biologically produced sulfur particles from Thiobacillus sp. W5 react with HS− to generate mixed polysulfides more efficiently than when compared S8 (α-S8) directly.11 All of these examples highlight the important role and interconnected roles of the reactivity of S0 and intermediate polysulfide formation upon reaction with sulfhydryl nucleophiles. Connecting with the biological activity of H2S, the generation, action, and translocation of S0-containing species is critical to understanding the intertwined chemistry of reactive sulfur species in biology.
Despite this broad importance in both contemporary and evolutionary chemistry and biology, investigations into S0 activity in aqueous systems are challenging due to the complex reactivity of available S0 sources. Organic polysulfides, such as diallyl trisulfide (DATS) found in alliums including garlic, or other synthetic organic and inorganic polysulfides can act as sources of biologically available S0. These systems present divergent reactivity based on the polysulfide chain length and pendant alkyl group.12 Inorganic polysulfides in particular are unstable in aqueous conditions and quickly equilibrate to different polysulfide mixtures.13 Despite these fundamental challenges, available S0 sources demonstrate significant promise in different systems, ranging from anticancer properties in several human cell lines14–17 to enhanced antioxidative activity.18 In all of these cases, however, the production of byproducts obfuscates the role of S0.
An attractive approach to investigate the chemical biology of S0 is to use the most common and simplest form of sulfane sulfur: S8. However, use of S8 directly is hindered by its low solubility of 6.4 μg L−1 (19(6) nM) at 25 °C in water18 and ∼4 μg L−1 in seawater.19 Despite this low solubility, S0 found in volcanic deep-sea environments can be readily metabolized by thermophilic Archaean organisms found in these sulfur-rich habitats.5 As one example, species of the order Sulfolobales can derive energy from the metabolism of S0via both aerobic and anaerobic pathways.20,21 Similarly, Acidianus ambivalens can utilize S0 as both an electron donor and acceptor. Taken together, such organisms may provide clues into possible mechanisms of stabilizing and activating bioavailable S0 in aqueous environments.
One potential strategy for S8 solubilization and activation can be gleaned from the archaeon Staphylothermus marinus, a strict sulfur reducing anaerobe that requires S0 as its terminal electron acceptor. Found near hot deep-sea vents, S. marinus is coated in thermostable filamentous glycoprotein structure called tetrabrachion that protrude from its surface. The tetrabrachion of S. marinus is composed of a four-stranded parallel coiled-coil structure with a hydrophobic core that is particularly stable.22 The 24 kDa right-handed coiled-coil structure of the tetrabrachion contains hydrophobic cavities that have been found to encapsulate two S8 molecules (PDB: 5JR5). Closer inspection of these S8-binding cavities revealed that the sulfur motifs were held in place by van der Waals forces with aliphatic amino acid side chains leucine and isoleucine (Fig. 1).23 Additional computational investigations have investigated the transit pathways for exchange of water and S8 within these structures.24 Further supporting this observation that hydrophobic motifs can increase S8 solubility, Steudel and Holdt demonstrated that the solubility of S8 in water can be increased using surfactants, with S8 concentrations reaching up to 0.1 mM in saturated hexadecyl(trimethyl)ammonium bromide (CTAB) solutions, although reactivity studies were not reported.25 In addition, the stability of sulfur nanoparticles and sulfur sols, which can be produced both industrially and from sulfur oxidizing bacteria,26 can also be modulated by different types of surfactants.27,28 Taken together, these prior observations support that this approach to solubilize S8 in aqueous environments may be more general and could also lead to new approaches to enable chemical accessibility of S0-containing species in biological environments.
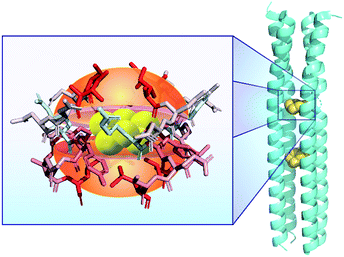 | ||
| Fig. 1 Structure of the tetrameric right-handed coiled-coil component of the tetrabrachion of the archaeon S. marinus (PDB: 5JR5) demonstrating two hydrophobic pockets in the core capable of encapsulating S8. Highlighted residues are colored according to their hydrophobicity, where red indicates stronger hydrophobicity. | ||
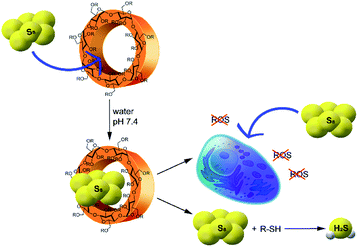
Understanding the intrinsic strategies for stabilizing simple S0-containing sources in solution remains a key unmet need that could have significant impacts in broad fields ranging from contemporary chemical biology of reactive sulfur species to greener synthetic methods for sulfur-containing compounds. Here we report that hydrophobic interactions within cavity-containing molecules, such as cyclodextrins (CDs), can be used to significantly solubilize S8 in aqueous solutions, and that this solubilized S0 is both chemically and biologically accessible. Specifically, we use 2-hydroxypropyl β-cyclodextrin (2HPβ) to generate 2HPβ/S8 solutions that are stable and quantifiable, react with thiols to generate H2S, exert antioxidant activities in cell models of oxidative stress, and increase intracellular S0 levels (Scheme 1).
Results and discussion
The simplest form of S0, S8, is readily available in high purity as a sublimed chalky yellow solid. Unfortunately, its use in biological applications is severely hampered by its hydrophobicity and low water solubility. The solubility of S8 has been calculated to be only 1.9(6) × 10−8 mol kg−1 (or 1.5(2) × 10−7 M S0), which is multiple orders of magnitude below biologically relevant S0 concentrations.18 These results suggest that even small modifications to increase S8 solubility in water can result in increased reactivity toward polysulfide formation. Moreover, efficient reduction of S8 to H2S will also require stabilization and solubilization of intermediate polysulfides or persulfide intermediates. Despite this low solubility, solid S8 has been demonstrated to be biologically accessible by erythrocytes to produce H2S,29 which highlights the potential for increasing the bioavailability of S8 in different systems. Moreover, these prior results suggest that biological pathways for sulfane sulfur activation from elemental sulfur may be accessible if S8 could be solubilized in aqueous environments.Motivated by the binding of S8 to the hydrophobic pockets of the archaeon S. marinus and increased solubility in hydrophobic environments, we envisioned that water-soluble compounds with hydrophobic interiors could enable similar S8 binding.30 Building from this hypothesis, we utilized CDs, which are cyclic oligosaccharides that contain a hydrophobic interior and that are widely utilized to bind and solubilize hydrophobic compounds.31 This solubilization is due, in part, to the hydrophobic interior of CDs, which promotes encapsulation and binding of a non-polar guest, whereas the hydrophilic exterior enables water solubility. CDs are available in different sizes/volumes, and naturally produced CDs include α-CD, β-CD, and γ-CD, which contain 6, 7, and 8 glucose units, respectively. The choice of CD depends upon the size of the nonpolar compound to be solvated and the properties of the system being studied. Although natural CDs have limited water solubility, modification of the ring periphery with hydroxypropyl groups results in significant increases in solubility. In particular, 2-hydroxypropyl β-CD (2HPβ) and 2-hydroxypropyl γ-CD (2HPγ) have been used extensively, including in drug formulations to enable delivery of otherwise hydrophobic and insoluble compounds.32
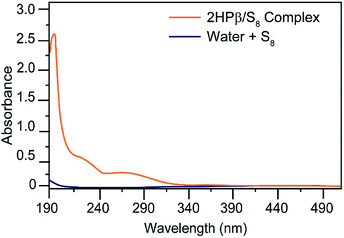
To test our general hypothesis, we treated aqueous solutions of 2HPβ with a 10-fold excess of solid S8. We chose to start our investigations with the β-CD structure because the cavity volume (262 Å3) is an ideal match for S8 (∼149 Å3; 57% cavity occupancy), based general preference for encapsulated guests to occupy ∼55% of host volume.33 After stirring as solution of 2HPβ with S8 in water for several days and subsequent filtration to remove residual insoluble S8, we observed a strong absorbance at 263 nm in the UV-vis spectrum, which is a characteristic absorbance of S8.34 By contrast, stirring S8 in water under identical conditions but in the absence of 2HPβ failed to produce a significant S8 absorbance (Fig. 2). To test the stability of the solubilized 2HPβ/S8, we next assessed whether the solution could be precipitated, filtered, and re-dissolved without loss of S8. We precipitated the 2HPβ/S8 complex with acetone, isolated the solid, and re-dissolved the resultant solid in buffer (Fig. S1†). In these experiments, we observed that the same absorbance from the original and re-dissolved solutions, confirming the stability of the solubilized system in both the liquid and solid state.
To determine which components of the 2HPβ complex were responsible for S8 solubilization, we next evaluated S8 solvation in the presence of glucose and hydroxypropyl cellulose (HPC) as models for the sugar units of the 2HPβ macrocycle and the hydroxypropyl motif, respectively. After stirring an excess of S8 to solutions of each saccharide in pH 7.4 phosphate buffered saline (PBS) buffer, the solutions were filtered, and UV-vis spectra were recorded (Fig. 3). As shown in Fig. 2, the characteristic absorbance at 263 nm corresponding to S8 was significantly larger for 2HPβ (orange, 687 μM in this solution) than for glucose (aqua) or HPC (yellow). These data suggest that the cyclic structure and cavity of the CD are key components required for S8 solvation. We next investigated the importance of the 2HPβ hydroxypropyl groups by testing S8 solubilization with β-CD (lacking the 2-HP groups). We treated β-CD in pH 7.4 PBS buffer with excess S8 and stirred for one month (Fig. S2†). After filtering the solution, we failed to observe any solubilized S8 by UV-vis spectroscopy, which suggests that the hydroxypropyl groups are required for S8 solubilization.
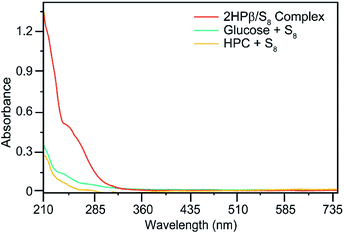 | ||
| Fig. 3 UV-vis spectra of 640 mg S8 in 10 mL pH 7.4 aqueous solutions containing 365 mg of 2HPβ, HPC, or glucose. 2HPβ solvates significantly more S8 than either HPC or glucose. | ||
To further investigate the requirement of the cyclic structure and presence of a cavity for S8 solubilization, we also investigated whether 2-hydroxypropyl γ-CD (2HPγ; cavity volume: 427 Å3) could solubilize S8. We compared the S8 solubilization in 25% w/w solutions of 2HPβ and 2HPγ in both pH. 7.4 PBS buffer and in water and observed significantly less S8 solubilization from 2HPγ than from 2HPβ (Fig. S3†). Taken together, these experiments strongly support that both the cyclic/cavity-containing structure and the presence of the hydrophobic core and hydrophilic exterior are critical for the observed S8 solvation by 2HPβ.
Building from these investigations, our next goal was to determine how much S8 was solubilized in 2HPβ solutions. However, due to the low solubility of S8 in water we used the extinction coefficient for S8 in methanol, 6730 M−1 cm−1 at 263 nm, which has been reported previously.34 To confirm that the extinction coefficient for S8 in methanol could be used to measure S8 concentrations in the 2HPβ/S8 complex in water, we first prepared a solution of S8 in MeOH at a known concentration (278 μM). We then used the known S8/methanol extinction coefficient to measure the concentration of S8 in an existing 2HPβ/S8 solution. We then diluted this solution to match the calculated value of [S8] in the methanol stock solution and compared the resulting absorbance traces. If the extinction coefficient remains constant between 2HPβ/S8 and S8 in methanol, then the calculated [S8] for the 2HPβ/S8 stock solution should be correct, and dilution from this value to the S8 concentration in the methanol stock solution should yield an identical concentration and thus an identical absorbances.
The resultant curves (Fig. S4†) are similar to the 2HPβ/S8 solution containing an S8 concentration of 250 μM, which is ∼12% different between the methanol/S8 and 2HPβ/S8 solutions. This observation confirms that the extinction coefficient does not change appreciably between these two systems. On the basis of these comparisons, we have used the molar extinction coefficient of S8 in MeOH to quantify S8 in the 2HPβ. These quantified concentrations are further supported by the quantitative S8 conversion to H2S by thiols (vide infra), which provides additional support for the value of the reported S8 concentrations in the 2HPβ system. Applying this extinction coefficient to the 50% w/w 2HPβ solution, provides an S8 concentration of 2.0 ± 0.2 mM (16 mM S0) in water. When compared to the background solubility of S8 in water, this constitutes ∼105-fold enhancement in S8 solubility.
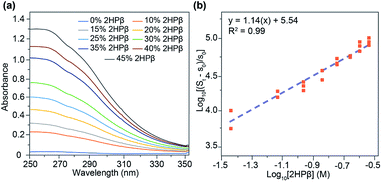
Having established that S8 is readily solubilized in aqueous solution of 2HPβ we next sought to investigate the stoichiometry and magnitude of the interaction between S8 and 2HPβ. To probe these interactions, we monitored the observed [S8] in solution as a function of increasing [2HPβ] (from 0–45% w/w) that were prepared with a 10-fold molar excess of solid S8 in solution. Under these conditions, the activity of S8 in solution remains constant, and increasing the [2HPβ] should result in a concomitant increase in [S8] in solution. After stirring each solution for several days to ensure equilibrium, the solutions were filtered, and the S8 concentration were measured by UV-vis spectrophotometry (Fig. 4a). As expected, the measured [S8] increased linearly with increasing [2HPβ], which further supports a direct interaction between S8 and 2HPβ. The above constant activity data can be used to determine the binding stoichiometry and affinity between 2HPβ and S8 through eqn (1).35 Under these conditions, the total S8 content is defined as St in eqn (1), and the concentration of unbound S8, held constant throughout experiments to ensure constant activity, is defined as s0 (1.9(6) × 10−8 mol kg−1).18 The constant n represents the binding stoichiometry.
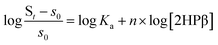 | (1) |
Generating a log–log plot with the parameters of eqn (1) demonstrates that the relationship between 2HPβ and S8 is consistent with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding (Fig. 4b). Upon performing linear regression analysis, we obtained a Ka value of 3.4 ± 0.05 × 105 M−1 for the 2HPβ/S8 complex, which is higher than the typical 102–103 M−1 binding affinities observed in βCD systems.36 Although the above analyses are supportive of a 1
1 binding (Fig. 4b). Upon performing linear regression analysis, we obtained a Ka value of 3.4 ± 0.05 × 105 M−1 for the 2HPβ/S8 complex, which is higher than the typical 102–103 M−1 binding affinities observed in βCD systems.36 Although the above analyses are supportive of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, we cannot definitively exclude equimolar higher order interactions from our data.
1 binding stoichiometry, we cannot definitively exclude equimolar higher order interactions from our data.
We repeated these binding stoichiometry experiments with 2HPγ and 2-hydroxypropyl α-CD (2HPα) (Fig. S5†). Our expectation was that these differently-sized CD hosts would not solubilize or bind S8 as efficiently as 2HPβ For example, if S8 were bound within 2HPα or 2HPγ, cavity occupancies of 86% and 32% would be observed, respectively, which is outside of the range of most host–guest interactions.30 Consistent with these expectations, 2HPγ solvated less significantly less S8 and exhibited less linear binding behavior when compared to 2HPβ (Fig. S5a†). Similarly, 2HPα solubilized very little S8 with significant deviations from a well-defined binding relationship (Fig. S5c and d†). These experiments further support that the size complementarity between S8 and 2HPβ is an important factor in solubilization.
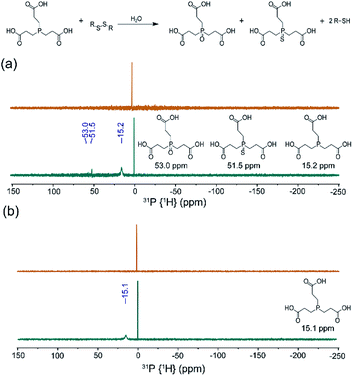
Building from our data supporting S8 solubilization with 2HPβ we next sought to determine whether the solubilized S8 is chemically accessible. To investigate this question, we first determined whether the reductant tris(2-carboxyethyl)phosphine (TCEP), which is a commonly-used and biologically compatible reductant used to reduce disulfides and other sulfane sulfur species, could access the solubilized S8 and generate the characteristic P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and P
S and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O products upon phosphine-mediated reduction and subsequent hydrolysis. Conveniently, this conversion can readily be monitored qualitatively by 31P NMR spectroscopy with TCEP (δ = 15.2–15.8 ppm), the resultant TCEP sulfide (δ = 51.5 ppm), and the associated TCEP oxide (δ = 53.0 ppm), all having characteristic NMR resonances. Prior to TCEP addition, the 31P NMR spectrum of S8 in a 25% w/w 2HPβ solution in pH 7.4 buffer only shows a peak at δ = 0 ppm from the PBS (Fig. 5a). After TCEP addition and incubation overnight at room temperature, however, the 31P NMR spectrum showed peaks corresponding to unreacted TCEP, as well as the phosphine sulfide and oxide peaks at δ = 53.0 and 51.5 ppm, respectively. To confirm that the peaks were from oxidized TCEP products, we repeated the TCEP incubation with TCEP and S8 in MeOD (Fig. S6a†) and K2S5 in D2O (Fig. S6b†). In both cases, we observed the same peaks in the 31P NMR spectrum at δ = 53.0 and 51.5 ppm, confirming product formation. In the absence of the 2HPβ/S8, only the TCEP peak is observed, which confirms that TCEP oxide is not formed from adventitious oxidation. Similarly, addition of TCEP to a solution of 2HPβ did not generate TCEP oxide (Fig. 5b). Taken together, these results demonstrate that the solubilized S8 in the 2HPβ/S8 solution is chemically accessible and can react directly with reductants.
O products upon phosphine-mediated reduction and subsequent hydrolysis. Conveniently, this conversion can readily be monitored qualitatively by 31P NMR spectroscopy with TCEP (δ = 15.2–15.8 ppm), the resultant TCEP sulfide (δ = 51.5 ppm), and the associated TCEP oxide (δ = 53.0 ppm), all having characteristic NMR resonances. Prior to TCEP addition, the 31P NMR spectrum of S8 in a 25% w/w 2HPβ solution in pH 7.4 buffer only shows a peak at δ = 0 ppm from the PBS (Fig. 5a). After TCEP addition and incubation overnight at room temperature, however, the 31P NMR spectrum showed peaks corresponding to unreacted TCEP, as well as the phosphine sulfide and oxide peaks at δ = 53.0 and 51.5 ppm, respectively. To confirm that the peaks were from oxidized TCEP products, we repeated the TCEP incubation with TCEP and S8 in MeOD (Fig. S6a†) and K2S5 in D2O (Fig. S6b†). In both cases, we observed the same peaks in the 31P NMR spectrum at δ = 53.0 and 51.5 ppm, confirming product formation. In the absence of the 2HPβ/S8, only the TCEP peak is observed, which confirms that TCEP oxide is not formed from adventitious oxidation. Similarly, addition of TCEP to a solution of 2HPβ did not generate TCEP oxide (Fig. 5b). Taken together, these results demonstrate that the solubilized S8 in the 2HPβ/S8 solution is chemically accessible and can react directly with reductants.
To further the potential biological relevance of the solubilized S8 in the 2HPβ/S8 complex, we next determined whether the solubilized S8 could be reduced by thiols to release H2S. One important feature of S0 that contributes to its role in biology is its ability to release H2S upon reduction by thiols. Polysulfides, such as DATS, are well established to release H2S after reaction with thiols and are used broadly as exogenous sources of S0. Although prior studies have investigated how different functional groups on polysulfide motifs impacts H2S release, one limitation of these systems is that all of these compounds also generate organic byproducts upon H2S release.12,37 As expected, a higher S0 content in organic polysulfides leads to greater H2S release, although tetrasulfides appear to be the largest synthetically-accessible and consistently-stable polysulfides. Using a similar logic of trying to maximize the S0 content per donor motif, we envisioned that the solubilized 2HPβ/S8 complex could also function as an entirely new approach to deliver S0 and/or H2S. Importantly, since S8 is comprised entirely of S0 it should be an effective donor, with the only byproduct being 2HPβ.
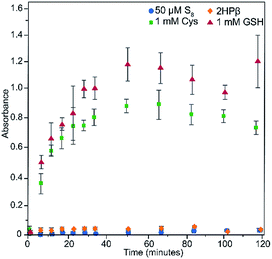
To determine whether the S8 solubilized in the 2HPβ/S8 system is accessible to thiols, and to quantify resulting H2S release, we treated a 2HPβ/S8 solution (25 μM S8, 200 μM S0 in 50% w/w 2HPβ) with 1 mM (5 equiv. with respect to S0 atoms) of cysteine or reduced glutathione (GSH) under air-free conditions in pH 7.4 PBS. We measured H2S release at different time points during the reaction by using the colorimetric methylene blue assay, which measures H2S production by the formation of the methylene blue dye (Fig. 6). Calculated efficiency values assume all S0 atoms can react to form H2S. After 45 minutes, we observed 160 ± 5 μM H2S release (80% efficiency) from the 2HPβ/S8 in the presence of cysteine (green). Under identical conditions, treatment of the complex with GSH (red), the most abundant biological thiol, yielded 220 ± 7 μM H2S release after 45 minutes, corresponding to stoichiometric reduction of each S0 atom. In the absence of S8 with only 2HPβ and 500 μM cysteine, no H2S was observed from the methylene blue assay (yellow), which confirms that 2HPβ and thiols alone do not spontaneously generate H2S or result in methylene blue formation. Similarly, in the absence of 2HPβ we did not observe any H2S release from S8 (50 μM if fully soluble) and cysteine (500 μM, blue), confirming the importance of 2HPβ to the accessibility of S8. As a whole, these results show that S8 is made chemically accessible in water by solubilization with 2HPβ, and that this sulfur can be reduced to H2S with biologically relevant thiols. Because this conversion requires the intermediate generation of persulfides and/or polysulfides en-route to H2S release, these results may also suggest that the 2HPβ facilitates the solubilization of these reactive intermediates. Alternatively, this solubilizing environment may also help stabilize other forms of elemental sulfur, such as biosulfur or sulfur sols, which could facilitate further reactivity with sulfhydryl-containing nucleophiles.
In addition to the above experiments, we also determined whether S8 needed to be pre-solubilized with 2HPβ prior to reaction with thiols, or whether 2HPβ could act as a catalyst for S8 conversion to H2S by thiols in water. To answer this question, we added 224 mg S8 solid and 500 μM cysteine to 180 mg 2HPβ in 40 mL pH 7.4 PBS buffer and monitored H2S generation using the methylene blue method. Under these conditions, we observed a faster peaking time of 15 minutes, but also a slightly lower overall efficiency of 147 μM H2S release (74% efficient) (Fig. S6†). These data indicate that S8 does not need to be pre-solvated to the 2HPβ complex prior to thiol addition in order to facilitate reaction with thiols and subsequent H2S release. These data may also support the role of 2HPβ as a phase transfer catalyst in these environments and that the rate of thiol-mediated reduction is faster than the rate of S8 encapsulation. Expanding from the present system, these results suggest that hydrophobic motifs in more complex systems may enable chemical accessibility of transiently formed S8 from different redox processes.
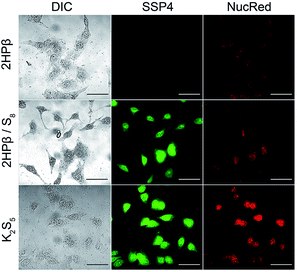
The accessibility of the solvated S8 to biological thiols prompted us to investigate whether the solvated S8 could be taken up into cells. Increasing intracellular levels of S0 induces a cytoprotective effect by reducing oxidative stress, making direct S0 donation desirable.38 Furthermore, the thiol-mediated reduction of S8 to H2S should proceed through persulfide and polysulfide formation, both of which should increase the levels of reactive sulfur species in cells. To evaluate cellular S8 uptake, we treated HeLa cells with either 10 μM S8 as 2HPβ/S8 or an equivalent amount of 2HPβ alone for 24 hours. We then treated cells with the sulfane-sulfur selective fluorescent probe SSP4.39 We observed that cells treated with 2HPβ/S8 showed a significant increase in fluorescence when compared with those treated with 2HPβ alone, which is consistent with the bioavailability of the solubilized S8 (Fig. 7).40 As a positive control, we repeated these experiments with HeLa cells that were treated with the inorganic polysulfide K2S5 as a source of S0, which also showed a significant SSP4 fluorescence response. These results support that the 2HPβ/S8 system can cause significant increases in intracellular S0 levels, though the efficiency of this uptake is not yet known.
Finally, we sought to determine whether the bioavailable solubilized S8 could be used to access protective effects associated with S0/H2S. Both H2S and S0 species play important antioxidant and anti-inflammatory roles throughout biology and are effective reducing agents able to neutralize damaging oxidants and free radical species.41,42 Polysulfides and persulfides containing S0 have demonstrated antioxidative properties greater than those attributed to H2S or thiols alone.43 The well-studied antioxidant N-acetyl cysteine has also been shown to enhance the production of S0.38 As a whole, a common theme is that polysulfides, and their role as both H2S and persulfide donor motifs, facilitates their protection against oxidative stress. The increased intracellular S8 should further produce a diverse range of polysulfides in the cells. Building from this observation, we reasoned that the high S0 content of the 2HPβ/S8 system should therefore make it an effective antioxidant in a cellular environment. With this in mind, we sought to determine whether the 2HPβ/S8 system provided antioxidant potential in cellular environments.
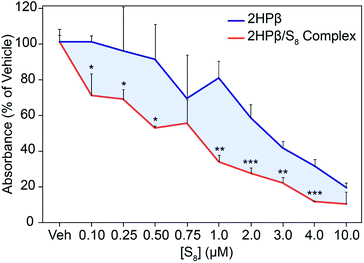
We used the colorimetric Griess reagent to track relative levels of NOx metabolites in RAW 264.7 macrophage cells pretreated with either 2HPβ/S8 or 2HPβ, and then lipopolysaccharide (LPS). In the presence of proinflammatory cytokines such as LPS, RAW 264.7 cells produce NO from inducible nitric oxide synthase (iNOS).44,45 When formed, NO is rapidly oxidized to downstream NOx species, and NO2− can be quantified directly using the colorimetric Griess assay. Importantly, H2S-releasing compounds have been previously demonstrated to significantly decrease NO2− formation in RAW 264.7 cells.46,47 To investigate the activity of 2HPβ/S8 in this system, we plated RAW 264.7 macrophage cells on 24 well plates and the following day treated with the delivery vehicle for 2HPβ/S8 (PBS), different concentrations of 2HPβ/S8, or equivalent concentrations of 2HPβ alone for 24 hours. The cells were then washed and treated with 1 μg mL−1 LPS for another 24 hours. After incubation, the media was collected from each well and the amount of NO2− was quantified using the Griess assay. The absorbance values of each treatment condition were normalized to the vehicle. As shown in Fig. 8, addition of the 2HPβ/S8 complex results in a significant and decrease in NO2− formation across a range of concentrations, as evidenced by the decrease of absorbance of the Griess product. These data are consistent with H2S and S0 release. The 2HPβ alone also results in a reduction in NO2− levels, but to a much lower extent than the 2HPβ/S8 system. It is possible that H2S, polysulfides, and sulfane sulfur species generated from the solubilized S8 all play roles in the reduction of NO2− levels in this assay, which when taken together supports prior work in the field demonstrating that both H2S and sulfane sulfur provide protective effects toward models of oxidative stress.
Conclusions
We have demonstrated that hydrophobic cyclodextrins can facilitate solubilization and chemical activity of S8 in water. In addition to providing a new and significant approach to delivering S0/sulfane sulfur to aqueous and biological environments, these results provide fundamentally new insights that impact S8 bioavailability. Building from the solubilization of S8 by the 2HPβ system, these results may suggest that pools of oxidized sulfur can stably exist in biological hydrophobic structures such as proteins. Moreover, the demonstration that the 2HPβ system can effectively catalyze S8 reduction to H2S by thiols in water also highlights this approach as a method to limit S8 accumulation. We anticipate that this and related systems currently under investigation in our lab will not only find utility as H2S and sulfane sulfur delivery systems, but also in expanding investigations into how S0 is managed in more complex biological systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSF (CHE-1454747), the Dreyfus Foundation, and the NIH (T32 GM007759, to SGB). Microscopy instrumentation was supported by the NSF (CHE-1531189).Notes and references
- K. Zahnle, L. Schaefer and B. Fegley, Cold Spring Harbor Perspect. Biol., 2010, 2, a004895 CrossRef CAS.
- S. Ranjan, Z. R. Todd, J. D. Sutherland and D. D. Sasselov, Astrobiology, 2018, 18, 1023–1040 CrossRef CAS.
- E. T. Parker, H. J. Cleaves, J. P. Dworkin, D. P. Glavin, M. Callahan, A. Aubrey, A. Lazcano and J. L. Bada, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5526–5531 CrossRef CAS.
- W. Heinen and A. M. Lauwers, Origins Life Evol. Biospheres, 1996, 26, 131–150 CrossRef CAS.
- M. Vandiver and S. H. Snyder, J. Mol. Med., 2012, 90, 255–263 CrossRef CAS.
- R. Wang, Antioxid. Redox Signaling, 2010, 12, 1061–1064 CrossRef CAS.
- A. K. Mustafa, M. M. Gadalla and S. H. Snyder, Sci. Signaling, 2009, 2, re2 Search PubMed.
- N. Lau and M. D. Pluth, Curr. Opin. Chem. Biol., 2019, 49, 1–8 CrossRef CAS.
- H. Kimura, Antioxid. Redox Signaling, 2015, 22, 347–349 CrossRef CAS.
- K. Avetisyan, T. Buchshtav and A. Kamyshny, Geochim. Cosmochim. Acta, 2019, 247, 96–105 CrossRef CAS.
- W. E. Kleinjan, A. de Keizer and A. J. H. Janssen, Colloids Surf., B, 2005, 43, 228–237 CrossRef CAS.
- S. G. Bolton, M. M. Cerda, A. K. Gilbert and M. D. Pluth, Free Radicals Biol. Med., 2019, 131, 393–398 CrossRef CAS.
- H. Liu, M. N. Radford, C. T. Yang, W. Chen and M. Xian, Br. J. Pharmacol., 2019, 176, 616–627 CrossRef CAS.
- T. Hosono, T. Fukao, J. Ogihara, Y. Ito, H. Shiba, T. Seki and T. Ariga, J. Biol. Chem., 2005, 280, 41487–41493 CrossRef CAS.
- W. Bat-Chen, T. Golan, I. Peri, Z. Ludmer and B. Schwartz, Nutr. Cancer, 2010, 62, 947–957 CrossRef CAS.
- D. Xiao and S. V. Singh, Carcinogenesis, 2006, 27, 533–540 CrossRef CAS.
- M. Murai, T. Inoue, M. Suzuki-Karasaki, T. Ochiai, C. Ra, S. Nishida and Y. Suzuki-Karasaki, Int. J. Oncol., 2012, 41, 2029–2037 CrossRef CAS.
- J. Boulegue, Phosphorus Sulfur, 2006, 5, 127–128 CrossRef.
- A. Kamyshny, Geochim. Cosmochim. Acta, 2009, 73, 6022–6028 CrossRef CAS.
- Y. Liu, L. L. Beer and W. B. Whitman, Environ. Microbiol., 2012, 14, 2632–2644 CrossRef CAS.
- A. Kletzin, T. Urich, F. Muller, T. M. Bandeiras and C. M. Gomes, J. Bioenerg. Biomembr., 2004, 36, 77–91 CrossRef CAS.
- J. Peters, W. Baumeister and A. Lupas, J. Mol. Biol., 1996, 257, 1031–1041 CrossRef CAS.
- M. McDougall, O. Francisco, C. Harder-Viddal, R. Roshko, M. Meier and J. Stetefeld, Proteins, 2017, 85, 2209–2216 CrossRef CAS.
- C. Harder-Viddal, M. McDougall, R. M. Roshko and J. Stetefeld, Comput. Struct. Biotechnol. J., 2019, 17, 675–683 CrossRef CAS.
- R. Steudel and G. Holdt, Angew. Chem., Int. Ed., 1988, 27, 1358–1359 CrossRef.
- R. Steudel, Elemental Sulfur and Sulfur-Rich Compounds I, 2003, 230, 153–166 CAS.
- R. G. Chaudhuri and S. Paria, J. Colloid Interface Sci., 2011, 354, 563–569 CrossRef CAS.
- A. A. Garcia and G. K. Druschel, Geochem. Trans., 2014, 15, 11 CrossRef.
- D. G. Searcy and S. H. Lee, J. Exp. Zool., 1998, 282, 310–322 CrossRef CAS.
- We also note that the 2008 Russian patent RU2321598C1 hypothesizes S8 binding to cyclodextrin.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS.
- T. Loftsson, P. Jarho, M. Masson and T. Jarvinen, Expert Opin. Drug Delivery, 2005, 2, 335–351 CrossRef CAS.
- S. Mecozzi and J. Rebek, Chem. – Eur. J., 1998, 4, 1016–1022 CrossRef CAS.
- R. Steudel, D. Jensen, P. Gobel and P. Hugo, Ber. Bunsen-Ges. Phys. Chem., 1988, 92, 118–122 CrossRef CAS.
- K. A. Conners, Binding Constants, The Measurement of Molecular Complex Stability, Wiley, New York, 1987 Search PubMed.
- K. A. Connors, J. Pharm. Sci., 1995, 84, 843–848 CrossRef CAS.
- M. M. Cerda, M. D. Hammers, M. S. Earp, L. N. Zakharov and M. D. Pluth, Org. Lett., 2017, 19, 2314–2317 CrossRef CAS.
- D. Ezerina, Y. Takano, K. Hanaoka, Y. Urano and T. P. Dick, Cell Chem. Biol., 2018, 25, 447–459 CrossRef CAS.
- W. Chen, C. R. Liu, B. Peng, Y. Zhao, A. Pacheco and M. Xian, Chem. Sci., 2013, 4, 2892–2896 RSC.
- The S0 appears to localizes particularly in the nucleus and nucleolus of treated cells. This observation is consistent with previous research indicating the production and accumulation of S0 in the nucleolus of cells. See: K. R. Olson, Y. Gao, A. K. Steiger, M. D. Pluth, C. R. Tessier, T. A. Markel, D. Boone, R. V. Stahelin, I. Batinic-Haberle and K. D. Straubg, Molecules, 2020, 25, 980 CrossRef CAS.
- M. Magierowski, K. Magierowska, M. Hubalewska-Mazgaj, M. Surmiak, Z. Sliwowski, M. Wierdak, S. Kwiecien, A. Chmura and T. Brzozowski, Biochem. Pharmacol., 2018, 149, 131–142 CrossRef CAS.
- L. A. Nicolau, R. O. Silva, S. R. Damasceno, N. S. Carvalho, N. R. Costa, K. S. Aragao, A. L. Barbosa, P. M. Soares, M. H. Souza and J. V. Medeiros, Braz. J. Med. Biol. Res., 2013, 46, 708–714 CrossRef CAS.
- T. Ida, T. Sawa, H. Ihara, Y. Tsuchiya, Y. Watanabe, Y. Kumagai, M. Suematsu, H. Motohashi, S. Fujii, T. Matsunaga, M. Yamamoto, K. Ono, N. O. Devarie-Baez, M. Xian, J. M. Fukuto and T. Akaike, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7606–7611 CrossRef CAS.
- C. Coletta, A. Papapetropoulos, K. Erdelyi, G. Olah, K. Modis, P. Panopoulos, A. Asimakopoulou, D. Gero, I. Sharina, E. Martin and C. Szabo, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9161–9166 CrossRef CAS.
- F. Aktan, Life Sci., 2004, 75, 639–653 CrossRef CAS.
- M. Whiteman, L. Li, P. Rose, C. H. Tan, D. B. Parkinson and P. K. Moore, Antioxid. Redox Signaling, 2010, 12, 1147–1154 CrossRef CAS.
- Y. Zhao, M. M. Cerda and M. D. Pluth, Chem. Sci., 2019, 10, 1873–1878 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, UV-vis spectra, NMR spectra, H2S measurements. See DOI: 10.1039/d0sc04137h |
| This journal is © The Royal Society of Chemistry 2020 |