Open Access Article
Open Access ArticleUse of a cyclo-P4 building block – a way to networks of host–guest assemblies†
Eugenia
Peresypkina
 ,
Martin
Bielmeier
,
Alexander
Virovets
,
Martin
Bielmeier
,
Alexander
Virovets
 and
Manfred
Scheer
and
Manfred
Scheer
 *
*
Institute of Inorganic Chemistry, University of Regensburg, 93040 Regensburg, Germany. E-mail: Manfred.Scheer@ur.de
First published on 12th August 2020
Abstract
Despite the proven ability to form supramolecular assemblies via coordination to copper halides, organometallic building blocks based on four-membered cyclo-P4 ligands find only very rare application in supramolecular chemistry. To date, only three types of supramolecular aggregates were obtained based on the polyphosphorus end-deck complexes CpRTa(CO)2(η4-P4) (1a: CpR = Cp′′; 1b: CpR = Cp′′′), with none of them, however, possessing a guest-accessible void. To achieve this target, the use of silver salts of the weakly coordinating anion SbF6− was investigated as to their self-assembly in the absence and in the presence of the template molecule P3Se4. The two-component self-assembly of the building block 1a and the coinage-metal salt AgSbF6 leads to the formation of 1D or 3D coordination polymers. However, when the template-driven self-assembly was attempted in the presence of an aliphatic dinitrile, the unprecedented barrel-like supramolecular host–guest assembly P3Se4@[{(Cp′′Ta(CO)2(η4-P4))Ag}8]8+ of 2.49 nm in size was formed. Moreover, cyclo-P4-based supramolecules are connected in a 2D coordination network by dinitrile linkers. The obtained compounds were characterised by mass-spectrometry, 1H and 31P NMR spectroscopy and X-ray structure analysis.
Introduction
Complex biochemical systems, in which weak intra- and intermolecular interactions between covalent-bound macromolecules provide both rigidness and an extensive dynamic behaviour, inspire modern coordination chemistry to design discrete and extended supramolecular architectures via metal-directed metal–ligand self-assembly.1 An impressive example of mimicking enzymes was demonstrated in the group of C. A. Mirkin using so called weak-link approach.1e,f To this effect, Lewis-acidic metal-based coordination centres together with appropriate building blocks are needed that are able to design supramolecular assemblies.1g,h In addition to widely used E-donor ligands (E = N, O, S, etc.), polyphosphorus ligand complexes proved to be able to coordinate metal cations by P centres and to give, at certain conditions, supramolecular assemblies of spherical architectures possessing fullerene2 or non-fullerene3 topology. However, most of these spherical supramolecules are obtained for pentaphosphaferrocenes,4 whereas a range of end-deck cyclo-P4 complexes remain unexplored in this context.5 Only three types of supramolecules based on the {CpRTa(CO)2(η4-P4)} complexes (CpR = 1,3-C5H3tBu2 (Cp′′, 1a),5a Cp′′′ = 1,2,4-C5H2tBu3 (Cp′′′, 1b)) are known so far.6 The first type is represented by a family of [{CpRTa(CO)2(η4-P4)}6{CuX}8] supramolecules (2: CpR = Cp′′, Cp′′′; X = Cl, Br) in which the inorganic scaffold has a non-classical fullerene topology being composed exclusively of {cyclo-P4} four- and {Cu2P4} six-membered rings. Two other types of spheres demonstrate unmatched ‘peanut’-like [{1a}10{CuI}14] (3) and ‘pear’-like [{1a}5{CuI}12(CH3CN)5}] (4) scaffolds.6b Unlike many supramolecules based on cyclo-P5 building blocks, none of these four-membered ring-based spheres feature guest inclusions or even possess a guest accessible void.A way to realize host–guest chemistry is to use a larger Lewis-acidic metal cation free of any additional ligand occupying a coordination site. Moreover, the introduction of a fourth reaction component stimulating the degree of freedom in a self-organising process might create an extra chance of host–guest ability. Such a fourth component could be a linker that enables flexible aggregation. Herein we report on the self-assembly reaction of P4Se3, {Cp′′Ta(CO)2(η4-P4)} (1a), AgSbF6 and the flexible dinitrile NC(CH2)7CN to give a 2D coordination network in which a barrel-like supramolecular host–guest P3Se4@[{(Cp′′Ta(CO)2(η4-P4))Ag}8]8+ assemblies of 2.49 nm in size are connected by dinitrile linkers.
Results and discussion
To approach the task of host–guest chemistry starting with a four-membered building block, the two-component self-assembly of 1a and AgSbF6 was studied beforehand. A colourless solution of AgSbF6 in CH2Cl2 was layered with a yellow solution of 1a in toluene with a CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene middle-layer (2
toluene middle-layer (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
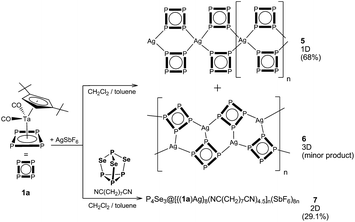
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). During the diffusion process, two different crystalline phases are formed (Fig. 1): orange plates of 5 and a few orange octahedra of 6. Unfortunately, the change of neither the stoichiometry nor the concentration of the starting materials nor the layering of the reaction solution after a complete diffusion with hexane, pentane or Et2O led to a selective formation of one of the products.
1). During the diffusion process, two different crystalline phases are formed (Fig. 1): orange plates of 5 and a few orange octahedra of 6. Unfortunately, the change of neither the stoichiometry nor the concentration of the starting materials nor the layering of the reaction solution after a complete diffusion with hexane, pentane or Et2O led to a selective formation of one of the products.
According to X-ray diffraction studies, the main product of the reaction of 1a with AgSbF6 is a 1D coordination polymer [{Cp′′Ta(CO)2(μ3,η4:η1:η1-P4)}2Ag]n(SbF6)n (5) consisting of chains with {Ag(1a)2} repeating units (Fig. 2a).‡ The Ag ions show a distorted tetrahedral coordination by four P atoms of four different cyclo-P4 ligands (P–Ag–P 90.3–140.5°), which, in turn, coordinate to another {Ag2P4} moiety in a 1,2-fashion (Fig. 2b). This structural motif, {M2P4}, has been known for other supramolecular aggregates based on polyphosphorus complexes and M(I) = Cu,2,6a,7 Ag,8 but has not been found for the cyclo-P4 derivatives. The P–P bond lengths vary in a range between 2.149(5) and 2.179(5) Å and are in agreement with those in the free complex 1a (2.150(2)–2.173(3) Å),5a whereas the Ag–P bond lengths range between 2.494(3) and 2.563(3) Å. The SbF6− counter anions and the CH2Cl2 solvent molecules separate the chains.
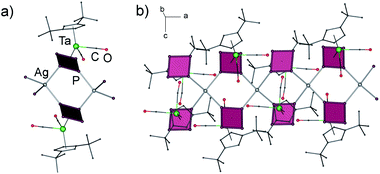 | ||
| Fig. 2 (a) Repeating unit and (b) section of 1D coordination polymer in 5. Counter anions SbF6− are omitted for clarity. | ||
Product 5 is insoluble in n-pentane and n-hexane, sparingly soluble in CH2Cl2 and fragmentises in N-donor solvents such as pyridine and CH3CN. In the ESI-MS spectrum, fragments containing 1a, Ag+ and SbF6− are visible, with the highest peak at m/z = 2604.7, which can be assigned to [{1a}4Ag2SbF6]+. The 1H and 31P NMR spectra of 5 in CD3CN show signals corresponding to free 1a that corroborates with the expected fragmentation of 5 in CD3CN.
Interestingly, the other coordination polymer [{Cp′′Ta(CO)2(μ4,η4:η1:η1:η1-P4)}2Ag]n(SbF6)n (6) was formed as a minor byproduct in the same reaction. This solvent-free phase, crystallized as orange octahedra, proved to have the same 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 1a
1 ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
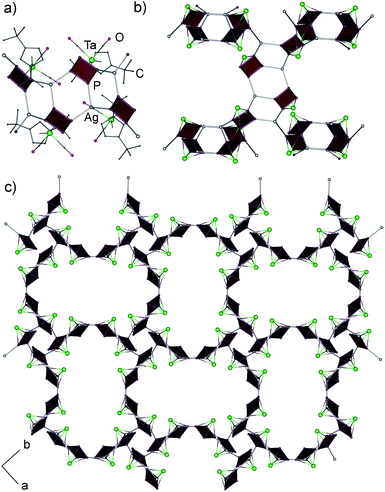
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgSbF6 as 5 and therefore represented a structural isomer of 5 which precluded a selective isolation and characterisation of the individual compounds by applying the appropriate stoichiometry of the educts. In contrast to 5, single crystal X-ray structure analysis of 6 revealed a 3D coordination polymer crystallizing as a racemic conglomerate in the chiral space groups P41212 or P43212.‡ In the 3D framework all cyclo-P4 ligands coordinate to Ag+ cations in a 1,2,3-coordination mode (Fig. 3), occurring only once in the peanut-like supramolecule.6b The tetrameric repeating {(1a)4Ag4} units in 6 are constructed by the edge-sharing of three six-membered rings of {Ag2P4}.8a The tetramers possess a curved shape due to a distorted triangular coordination environment of Ag (the P–Ag–P angles are in the range of 103.4–130.5°, Fig. 3a). Each unit is joined to four other neighbouring units (Fig. 3b) via one Ag–P coordinative bond to form a chiral 3D diamond-like network (dia9) with expanded channels (Fig. 3c). Despite being large (1.2–1.3 nm§), these channels do not provide accessible voids and are occupied by Cp′′ and CO ligands of 1a as well as by SbF6− anions. The P–P bonds lengths range from 2.146(3) to 2.180(4) Å, being in agreement with the bond lengths in 5 and 1a,5a whereas the Ag–P bond lengths vary in a range between 2.446(3) and 2.493(3) Å.
AgSbF6 as 5 and therefore represented a structural isomer of 5 which precluded a selective isolation and characterisation of the individual compounds by applying the appropriate stoichiometry of the educts. In contrast to 5, single crystal X-ray structure analysis of 6 revealed a 3D coordination polymer crystallizing as a racemic conglomerate in the chiral space groups P41212 or P43212.‡ In the 3D framework all cyclo-P4 ligands coordinate to Ag+ cations in a 1,2,3-coordination mode (Fig. 3), occurring only once in the peanut-like supramolecule.6b The tetrameric repeating {(1a)4Ag4} units in 6 are constructed by the edge-sharing of three six-membered rings of {Ag2P4}.8a The tetramers possess a curved shape due to a distorted triangular coordination environment of Ag (the P–Ag–P angles are in the range of 103.4–130.5°, Fig. 3a). Each unit is joined to four other neighbouring units (Fig. 3b) via one Ag–P coordinative bond to form a chiral 3D diamond-like network (dia9) with expanded channels (Fig. 3c). Despite being large (1.2–1.3 nm§), these channels do not provide accessible voids and are occupied by Cp′′ and CO ligands of 1a as well as by SbF6− anions. The P–P bonds lengths range from 2.146(3) to 2.180(4) Å, being in agreement with the bond lengths in 5 and 1a,5a whereas the Ag–P bond lengths vary in a range between 2.446(3) and 2.493(3) Å.
Interestingly, the curved tetrameric repeating unit in the structure of 6 can potentially be extended to some kind of a cylindrical core similar to previously reported non-classical fullerene-like cores 2.6 However, in 6 the {Cp′′Ta(CO)2} units point out alternatively on the different sides of the cyclo-P4 ligands of the concave tetramer. While in any possible cylindrical core all {Cp′′Ta(CO)2} units should point outside.
To overcome this difficulty and to direct the self-assembly process to the formation of a convex shell based on Ag cations and complexes 1a, we used a cage molecule P4Se3 as a rather small template. Moreover, to induce coordinative freedom and flexibility to form potential host–guest cages, we targeted the introduction of a flexible aliphatic linker as e.g. the dinitriles NC(CH2)nCN with n ≥ 7, because they deliver the needed distance to the next possible formed sphere. To interconnect potentially formed supramolecular assemblies of 1a and AgSbF6 templated by P4Se3, we used the linker NC(CH2)7CN in a four-component self-assembly process.
A colourless solution of AgSbF6 in CH2Cl2 is first layered with a mixture of CH2Cl2 and toluene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then with a yellow solution of 1a, P4Se3, and NC(CH2)7CN in toluene. The intermediate layer of the mixed solvents is used to slow down the diffusion and thus facilitate the growth of crystals better suitable for X-ray structure analysis. The AgSbF6, 1a, P4Se3 and NC(CH2)7CN were taken in a ratio of 1
1) and then with a yellow solution of 1a, P4Se3, and NC(CH2)7CN in toluene. The intermediate layer of the mixed solvents is used to slow down the diffusion and thus facilitate the growth of crystals better suitable for X-ray structure analysis. The AgSbF6, 1a, P4Se3 and NC(CH2)7CN were taken in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; the 10-fold excess of the dinitrile is required to avoid the formation of the insoluble polymers 5 and 6. By doing this, orange prismatic crystals of 7 in 29% yield were formed (Fig. 1). Variation of the stoichiometry only leads to the occurrence of by-products and the formation of 7 in lower yields.
10; the 10-fold excess of the dinitrile is required to avoid the formation of the insoluble polymers 5 and 6. By doing this, orange prismatic crystals of 7 in 29% yield were formed (Fig. 1). Variation of the stoichiometry only leads to the occurrence of by-products and the formation of 7 in lower yields.
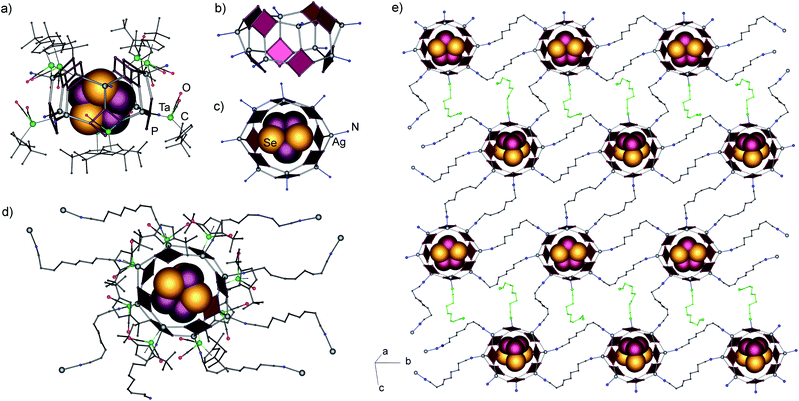
Single crystal X-ray structure analysis revealed the formation of a 2D polymeric network of interconnected supramolecular aggregates of P4Se3@[{(Cp′′Ta(CO)2(η4-P4))Ag}8(NC(CH2)7CN)4.5]n(SbF6)8n (7) (Fig. 4). In this square lattice coordination network the nodes represent novel self-assembled aggregates [{(1a)Ag}8]8+ of 2.49 nm in size (Fig. 4a, see ESI† for details).¶ In the inorganic core of the nodes, eight cyclo-P4 units and eight Ag ions are assembled in a square-antiprismatic arrangement of eight joined six-membered {Ag2P4} rings (Fig. 4b). This 40-vertex arrangement closely resembles two fused tetrameric units of 6; every cyclo-P4 ligand (P–P: 2.131(5)–2.177(5) Å) coordinates to three Ag cations, again in a 1,2,3-mode. However, the Ag environment is tetrahedral, as every silver cation, in addition to three P atoms of three 1a units, is also coordinated by one dinitrile linker (2.179(17) to 2.305(14) Å). The Ag–P bond lengths vary from 2.477(3) to 2.528 (3) Å. The similarity of the inorganic cores in 6 and 7 is underlined by P–Ag–P angles that vary from 97.3(1) to 127.4(1)° being similar to those in 6 despite the difference in the coordination number of Ag.
These supramolecular assemblies represent the first doughnut-like cyclo-Pn-based structure open at two sides. Cyclic or cylindrical molecules are rather abundant in different classes of coordination compounds. Among them, the closest analogues of cylindrical ligand-based supramolecules usually do not act as molecular containers.10 A doughnut-shaped Ti-oxo clusters with a permanent MOF-like porosity and high CO2 adsorption capacity was recently reported.11 Two bowl-like supramolecules based on pentaphosphaferrocene and Cu(I) halides were just recently obtained in our group, which are open only at one side, [{Cp*Fe(η5-P5)}11{CuX}15−x] (X = Cl, Br; x = 0.45–1.55).2d This makes the ‘doughnut’ in 7 the second example only of the open architectures among all supramolecules based on polyphosphorus cyclo-Pn ligands (n = 4, 5). Additionally, each supramolecular node contains a P4Se3 molecule as a guest (Fig. 4c) in its inner void of 0.60 × 0.68 nm.|| The guest is disordered over two positions with 80 and 20% probability. The fact that the guest is disordered suggests that there are no significant intermolecular host–guest interactions. The shortest intermolecular contacts amount to 3.54–3.77 Å for the Phost⋯Se contacts and 3.41–3.65 Å for the Phost⋯Pguest contacts11 (see the ESI† for more details). Nevertheless, the tendency of the self-assembly, without the guest, is towards the coordination polymers 5 or 6, which points to the template-driven formation of the host in 7. Perhaps, the cooperative effect of the weak van der Waals interactions between the P and Se atoms of the guest and the P atoms of the host as well as the effect of a rather spherical shape of the P4Se3 molecules enable the formation of the supramolecular host assembly.
Every Ag cation is coordinated by one dinitrile linker, however, only seven dinitriles are linked to another supramolecular node, whereas one is terminal. Three nodes are double-bridged by linkers, while the fourth one is linked by a single dinitrile ligand. The positive charge of the eight Ag(I) cations is balanced by eight SbF6− anions which occupy both the interlayer space and the meshes of the network. The presence of a terminal dinitrile possessing a non-coordinated donor cyano group shows the conceptual possibility to expand the structure in the third dimension. The separation of the nodes in the resulting layer, represented by Ag⋯Ag distances, amounts to 12.44–13.99 Å. The corresponding N⋯N distances in the bridging dinitrile ligands vary in the range between 9.24 and 10.31 Å, which demonstrates the shortening of the ligands that possess a folded conformation, as compared to 11.76 Å for the calculated length of the linear linker.12 This correlation demonstrates that the use of a shorter dinitrile with n = 6 (10.73 Å in the linear conformation) might also give similar polymers of host–guest agglomerates for 1a-based supramolecules.
Crystals of 7 are stable in the mother solution for several weeks. 7 is insoluble in hexane and pentane, sparingly soluble in CH2Cl2 and shows fragmentation in CH3CN and pyridine. In the ESI-MS spectrum of 7, fragments containing 1a, Ag, NC(CH2)7CN and SbF6− are observed, with the highest peak at m/z = 2604.7, which can be assigned to the same molecular ion [{1a}4Ag2SbF6]+ as in the case of 5. Similarly to 5 and 6, in CD3CN 7 undergoes fragmentation. Therefore, the 1H and 31P NMR spectra show only signals of the starting materials.
Conclusions
The self-assembly of the 1a complex and the coinage metal salt AgSbF6 was studied, and the possibility of forming host–guest complexes based on cyclo-P4 ligands was demonstrated for the first time. Moreover, these host–guest agglomerates can be linked in a coordination network, thus representing new polymeric matrices in which unprecedented barrel-like silver–polypnictogen-containing supramolecules serve as molecular containers. This finding opens new frontiers in the coordination chemistry of cyclo-P4 ligand complexes suggesting the design of new supramolecular architectures and exploring the influence of different coinage metal salts, the template effect of different guests as well as the variation of the nature of the linker and its lengths.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft within the project Sche 384/44-1. Parts of this research (project I-20180049) were carried out at PETRA III at DESY, a member of the Helmholtz Association (HGF). We thank Dr O. Lorbeer for his assistance regarding the use of the beamline P11.Notes and references
- (a) F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Nat. Rev. Chem., 2019, 3, 204 CrossRef; (b) Y. Sun, C. Chen and P. J. Stang, Acc. Chem. Res., 2019, 52, 802 CrossRef CAS PubMed; (c) M. Fujita, M. Tominaga, A. Aoai and B. Therrien, Acc. Chem. Res., 2005, 38, 369 CrossRef CAS PubMed; (d) M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848 RSC; (e) M. J. Wiester, P. A. Ulmann and C. A. Mirkin, Angew. Chem., Int. Ed., 2011, 50, 114 CrossRef CAS PubMed; (f) J. Mendez-Arroyo, A. I. d'Aquino, A. B. Chinen, Y. D. Manraj and C. A. Mirkin, J. Am. Chem. Soc., 2017, 139, 1368 CrossRef CAS PubMed; (g) H. Ruffin, S. A. Baudron, D. Salazar-Mendoza and M. W. Hosseini, Chem.–Eur. J., 2014, 20, 2449 CrossRef CAS PubMed; (h) D. W. Agnew, I. M. DiMucci, A. Arroyave, M. Gembicky, C. E. Moore, S. N. MacMillan, A. L. Rheingold, K. M. Lancaster and J. S. Figueroa, J. Am. Chem. Soc., 2017, 139, 17257 CrossRef CAS PubMed.
- (a) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781 CrossRef CAS PubMed; (b) M. Scheer, A. Schindler, C. Gröger, A. Virovets and E. V. Peresypkina, Angew. Chem., Int. Ed., 2009, 48, 5046 CrossRef CAS PubMed; (c) M. Scheer, A. Schindler, R. Merkle, B. P. Johnson, M. Linseis, R. Winter, C. E. Anson and A. V. Virovets, J. Am. Chem. Soc., 2007, 129(44), 13386 CrossRef CAS PubMed; (d) H. Brake, E. Peresypkina, C. Heindl, A. V. Virovets and M. Scheer, Chem. Sci., 2019, 10, 29402 RSC.
- E. V. Peresypkina, C. Heindl, A. Virovets, E. Mädl, H. Brake and M. Scheer, Chem.–Eur. J., 2018, 24, 2503 CrossRef CAS PubMed; F. Dielmann, M. Fleischmann, C. Heindl, E. Peresypkina, A. V. Virovets, R. M. Gschwind and M. Scheer, Chem.–Eur. J., 2015, 21, 6208 CrossRef PubMed; C. Heindl, E. V. Peresypkina, A. V. Virovets, W. Kremer and M. Scheer, J. Am. Chem. Soc., 2015, 137, 10938 CrossRef PubMed; C. Schwarzmaier, A. Schindler, C. Heindl, S. Scheuermayer, E. Peresypkina, A. Virovets, M. Neumeier, R. Gschwind and M. Scheer, Angew. Chem., Int. Ed., 2013, 52, 10896 CrossRef PubMed; F. Dielmann, C. Heindl, F. Hastreiter, E. V. Peresypkina, A. V. Virovets, R. M. Gschwind and M. Scheer, Angew. Chem., Int. Ed., 2014, 53, 13605 CrossRef PubMed; C. Heindl, E. Peresypkina, A. V. Virovets, I. S. Bushmarinov, M. G. Medvedev, B. Krämer, B. Dittrich and M. Scheer, Angew. Chem., Int. Ed., 2017, 56, 13237 CrossRef PubMed.
- E. Peresypkina, C. Heindl, A. Virovets and M. Scheer, Inorganic Superspheres, in Clusters – Contemporary Insight in Structure and Bonding, ed. S. Dehnen, 2016, vol. 174, pp. 321–373 Search PubMed.
- (a) O. J. Scherer, R. Winter and G. Wolmershäuser, Z. Anorg. Chem., 1993, 619, 827 CrossRef CAS; (b) K. A. Mandla, M. L. Neville, C. E. Moore, A. L. Rheingold and J. S. Figueroa, Angew. Chem., Int. Ed., 2019, 131, 15473 CrossRef.
- (a) B. P. Johnson, F. Dielmann, G. Balazs, M. Sierka and M. Scheer, Angew. Chem., Int. Ed., 2006, 45, 2473 CrossRef CAS PubMed; (b) F. Dielmann, E. V. Peresypkina, B. Kraemer, F. Hastreiter, B. P. Johnson, M. Zabel, C. Heindl and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 14833 CrossRef CAS PubMed.
- M. Elsayed Moussa, M. Piesch, M. Fleischmann, A. Schreiner, M. Seidl and M. Scheer, Dalton Trans., 2018, 47, 16031 RSC; A. Cavaille, N. Saffon-Merceron, N. Nebra, M. Fustier-Boutignon and N. Mezailles, Angew. Chem., Int. Ed., 2018, 57, 1874 CrossRef CAS PubMed.
- (a) S. Deng, C. Schwarzmaier, M. Zabel, J. F. Nixon, M. Bodensteiner, E. V. Peresypkina, G. Balazs and M. Scheer, Eur. J. Inorg. Chem., 2011, 2011, 2991 CrossRef CAS; (b) M. Elsayed Moussa, M. Seidl, G. Balazs, M. Zabel, A. V. Virovets, B. Attenberger, A. Schreiner and M. Scheer, Chem.–Eur. J., 2017, 23, 16199 CrossRef PubMed.
- (a) V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CrossRef CAS; (b) V. A. Blatov, M. O'Keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44 RSC; (c) M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782 CrossRef PubMed.
- (a) T. C. Stamatatos, S. Mukherjee, K. A. Abboud and G. Christou, Chem. Commun., 2009, 62 RSC; (b) F. Xu, H. N. Miras, R. A. Scullion, D.-L. Long, J. Thiel and L. Cronin, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11609 CrossRef CAS PubMed; (c) J.-D. Leng, J.-L. Liu and M.-L. Tong, Chem. Commun., 2012, 48, 5286 RSC; (d) P. Klufers and J. Schuhmacher, Angew. Chem., Int. Ed., 1994, 33, 1863 CrossRef.
- C. Zhao, Y.-Z. Han, S. Dai, X. Chen, J. Yan, W. Zhang, H. Su, S. Lin, Z. Tang, B. K. Teo and N. Zheng, Angew. Chem., Int. Ed., 2017, 56, 16252 CrossRef CAS PubMed.
- CHEM3D Ultra by Perkin Elmer, 2017, version 17.0.0.206 Search PubMed.
- M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details on X-ray structural and spectroscopic characterization. CCDC 2009904–2009906. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc03437a |
‡ X-ray structure analysis: compound 5: C30.35H42.70AgCl0.70F6O4P8SbTa2, orange plate, Mr = 1449.64 g mol−1, triclinic, sp. gr. P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.4796(5), b = 14.2670(6), c = 17.0118(8) Å, α = 96.742(4), β = 90.464(4), γ = 101.225(4)°, V = 2476.3(2) Å3, Z = 2, T = 90 K, R1 = 0.067, wR2 = 0.182, GOF = 1.00; compound 6: C15H21AgF6O2P4SbTa, orange octahedron, Mr = 881.77 g mol−1, tetragonal, sp. gr. P41212, a = b = 19.22628(9), c = 26.5819(2) Å, V = 9825.98(13) Å3, Z = 16, T = 90 K, R1 = 0.030, wR2 = 0.062, GOF = 0.95, Flack parameter = −0.009(5); compound 7: C328.5H477N18O32F96P72Cl15Ag16Sb16Ta16Se6, orange prism, Mr = 16 , a = 10.4796(5), b = 14.2670(6), c = 17.0118(8) Å, α = 96.742(4), β = 90.464(4), γ = 101.225(4)°, V = 2476.3(2) Å3, Z = 2, T = 90 K, R1 = 0.067, wR2 = 0.182, GOF = 1.00; compound 6: C15H21AgF6O2P4SbTa, orange octahedron, Mr = 881.77 g mol−1, tetragonal, sp. gr. P41212, a = b = 19.22628(9), c = 26.5819(2) Å, V = 9825.98(13) Å3, Z = 16, T = 90 K, R1 = 0.030, wR2 = 0.062, GOF = 0.95, Flack parameter = −0.009(5); compound 7: C328.5H477N18O32F96P72Cl15Ag16Sb16Ta16Se6, orange prism, Mr = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 818.72 g mol−1, triclinic, sp. gr. P 818.72 g mol−1, triclinic, sp. gr. P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 17.9549(1), b = 22.5635(2), c = 38.3495(4) Å, α = 81.1581(7), β = 78.4160(7), γ = 73.7720(7)°, V = 14 , a = 17.9549(1), b = 22.5635(2), c = 38.3495(4) Å, α = 81.1581(7), β = 78.4160(7), γ = 73.7720(7)°, V = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 534.6(2) Å3, Z = 2, T = 80 K, R1 = 0.0895, wR2 = 0.287, GOF = 1.09. See ESI for more information. 534.6(2) Å3, Z = 2, T = 80 K, R1 = 0.0895, wR2 = 0.287, GOF = 1.09. See ESI for more information. |
| § The size of the channel in 6 is estimated as the minimal distance between the closest non-bonded P atoms minus doubled van der Waals radius of P.13 |
| ¶ The outer diameter in 7 is calculated as the distance between the H atoms of two of the furthermost Cp′′ ligands plus twice the van der Waals radii of H.13 |
| || The size of the void in 7 is calculated as the distance between the centroids of every individual cyclo-P4 unit and the centroid of the node minus twice the van der Waals radius of P (1.8 Å).13 |
| This journal is © The Royal Society of Chemistry 2020 |