Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Increasing protein stability by engineering the n → π* interaction at the β-turn†
Bhavesh
Khatri
 a,
Puja
Majumder
a,
Puja
Majumder
 a,
Jayashree
Nagesh
a,
Jayashree
Nagesh
 b,
Aravind
Penmatsa
b,
Aravind
Penmatsa
 *a and
Jayanta
Chatterjee
*a and
Jayanta
Chatterjee
 *a
*a
aMolecular Biophysics Unit, Indian Institute of Science, Bangalore 560012, India. E-mail: penmatsa@iisc.ac.in; jayanta@iisc.ac.in
bSolid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore, India
First published on 30th July 2020
Abstract
Abundant n → π* interactions between adjacent backbone carbonyl groups, identified by statistical analysis of protein structures, are predicted to play an important role in dictating the structure of proteins. However, experimentally testing the prediction in proteins has been challenging due to the weak nature of this interaction. By amplifying the strength of the n → π* interaction via amino acid substitution and thioamide incorporation at a solvent exposed β-turn within the GB1 proteins and Pin 1 WW domain, we demonstrate that an n → π* interaction increases the structural stability of proteins by restricting the ϕ torsion angle. Our results also suggest that amino acid side-chain identity and its rotameric conformation play an important and decisive role in dictating the strength of an n → π* interaction.
Introduction
An array of noncovalent interactions including electrostatic forces, hydrogen bonds, van der Waals interactions and hydrophobic effects in a polypeptide chain dictate its three-dimensional structure and govern its folding.1 In particular, owing to their high abundance, the noncovalent interactions originating from the backbone (main chain) atoms of a polypeptide chain,2 including the classical hydrogen bonds,3 C–H⋯O hydrogen bonds,4 C5 hydrogen bonds5 and n → π* interactions,6 play a crucial role in stabilizing protein structures. The n → π* interaction originates from the donation of the lone pair (n) electron density of the carbonyl oxygen (O)i into the empty π* orbital of the adjacent carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)i+1.7–9 The distance (d ≤ 3.2 Å) and angular criteria (θ = 109 ± 10°) defining an n → π* interaction are in agreement with the Bürgi–Dunitz trajectory for nucleophilic attack,10 which along with the associated directionality i → i + 1 (N-term → C-term) (Fig. 1) is indicative of its possible role in folding and stabilization of protein secondary structures.11–16
O)i+1.7–9 The distance (d ≤ 3.2 Å) and angular criteria (θ = 109 ± 10°) defining an n → π* interaction are in agreement with the Bürgi–Dunitz trajectory for nucleophilic attack,10 which along with the associated directionality i → i + 1 (N-term → C-term) (Fig. 1) is indicative of its possible role in folding and stabilization of protein secondary structures.11–16
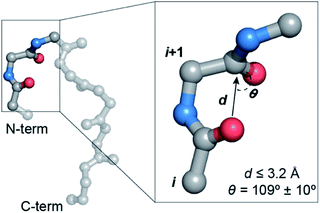 | ||
Fig. 1 A protein backbone depicting a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi → C Oi → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 n → π* interaction with the distance (d) and angular (θ) criteria used in the crystallographic analyses. Oi+1 n → π* interaction with the distance (d) and angular (θ) criteria used in the crystallographic analyses. | ||
Contribution of the n → π* interaction towards the stability of the protein structure was initially reported in collagen mimetics.17 The enhanced thermostability of a collagen mimetic with the 4R-configured proline derivative compared to that with the 4S-configured proline derivative was attributed to the stronger n → π* interaction in the 4R-configured proline derivative with the exo-pucker of the pyrrolidine ring.18 The finding was exquisitely substantiated later by the high-resolution crystal structure of the oligoproline PPII helix, where the n → π* interaction was favored by the Cγ-exo pucker and disfavored by the Cγ-endo pucker of the pyrrolidine ring.19 Furthermore, the stability of this PPII helix in the absence of intramolecular hydrogen bonds and hydration emphasizes the role of the n → π* interaction in the structural stability of collagen.
For an idealized geometry, the n → π* interaction between amides contributes ∼0.3 kcal mol−1,20 which may seem moderate. However, given the ubiquity of carbonyl groups in a polypeptide chain, n → π* interactions could have a significant collective contribution towards the overall energetics of protein stability.2 The distribution of the n → π* interaction obtained from analyses of protein crystal structures reveals that >70% of residues in α-helices, as opposed to <5% of residues in β-sheets engage in this interaction.11,12 Furthermore, since one third of all amino acids in the random coil have torsion angles in the α-helical region,21 the n → π* interaction might have an important role in restricting the conformational ensemble of unfolded proteins.22 In this context, it is worth noting that random coils and turn regions of proteins show a high abundance of reciprocal n → π* interactions (back and forth donation between adjacent carbonyl pairs).9
The evidence of the n → π* interaction has been shown by microwave and IR spectroscopy in various small molecular systems.23–27 However, despite enormous excitement in this area, so far experimental measurements of the energy of an n → π* interaction in proteins and its practical consequence on protein structural stability have been lacking. Therefore, we sought to engineer an n → π* interaction at the β-turn within a protein to understand its influence on the protein structure and its stability.
β-Turns (Fig. 2a) are the third most important protein secondary structure representing ∼20% of all protein residues28 having an important role in protein folding.29–31 Furthermore, substituting non-proline residues with proline residues in the β-turn leads to increased stabilization of the turn32 and enhanced protein stability.33,34 The increased stability results from the decreased backbone conformational entropy of the denatured state due to the restricted rotation of the N–Cα bond, also known as the ϕ torsion angle. Since an n → π* interaction also restricts the ϕ angle of an amino acid residue,11 we speculated that engineering an n → π* interaction at the β-turn would have direct consequence on the protein stability.
Here, by using bioinformatic analysis of the β-turn in proteins, we find an interplay between the conformational flexibility of the peptide backbone and the abundance of n → π* interactions at the two central residues, i + 1 and i + 2 of the β-turn. Through subsequent X-ray crystallography and computational analysis of synthetic GB1 proteins with amino acid substitutions at the i + 2 residue of the β-turn, we show that amino acid side-chain identity and its rotameric conformation have a direct influence on the strength of an n → π* interaction. Gratifyingly, the thermal denaturation of the GB1 proteins shows a good correlation between their stability and the strength of an n → π* interaction at the β-turn. Finally, we validate this observation in the Pin 1 WW domain, wherein by amplifying the strength of an n → π* interaction at the β-turn by thioamide incorporation, we could increase the thermal stability of the thioamidated Pin 1 WW domain.
Results and discussion
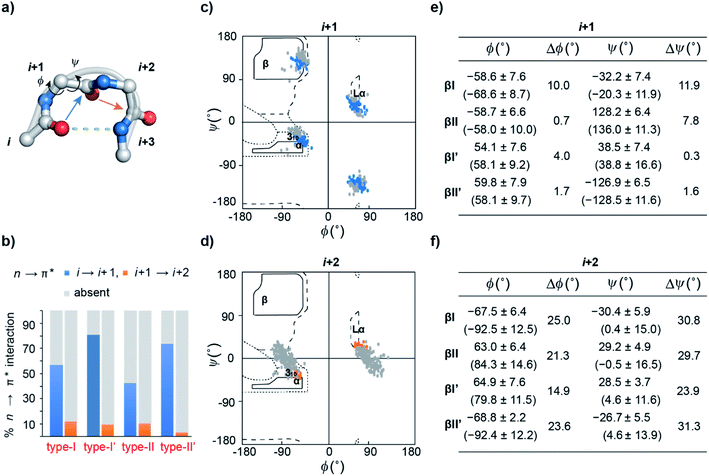
n → π* interaction and conformational flexibility at the β-turn
Previous computational analyses predicted that n → π* interactions confer conformational stability to the i + 1 residue in common type I and type II β-turns, and thus have a special role to play in the stability of turns.11 Therefore, we sought to examine the abundance of n → π* interactions and their possible correlation with the conformational flexibility of the peptide backbone in the β-turns.By analyzing a non-redundant subset of high-resolution (≤2.0 Å) protein crystal structures in the Protein Data Bank (PDB), we curated 500 β-turns (identified using Promotif) representing the common type-I, type-II, type-I′, and type-II′ turns. Next, using the distance and angular criteria defining an n → π* interaction (Fig. 1), we determined the abundance of n → π* interactions at the i + 1 and i + 2 residues in the β-turns. We noted that 40–80% of the residues engage in a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi → C
Oi → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 n → π* interaction, whereas, only 3–12% of the residues are involved in the C
Oi+1 n → π* interaction, whereas, only 3–12% of the residues are involved in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction (Fig. 2b).
Oi+2 n → π* interaction (Fig. 2b).
To identify the underlying cause of this behavior, we determined the torsion angles ϕ and ψ of i + 1 and i + 2 residues in all the β-turns and plotted them on the Ramachandran map. It was interesting to note the broader distribution of ϕ and ψ angles at the i + 2 residue (Fig. 2d) as opposed to the i + 1 residue (Fig. 2c). This is suggestive of restricted conformational freedom at the i + 1 residue, which is associated with the higher abundance of n → π* interactions at this site. We also calculated the difference in mean ϕ and ψ angles (Δϕ and Δψ) in the presence and absence of the n → π* interaction in the respective β-turns (Fig. 2e and f). The differences were significantly higher at the i + 2 residue in comparison to the i + 1 residue. This further indicates that the lower abundance of n → π* interactions at the i + 2 residue is associated with greater conformational flexibility of the peptide backbone.
Influence of the amino acid side-chain on the n → π* interaction
As β-turns are stabilized by the intramolecular hydrogen bond between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi⋯HNi+3 (Fig. 2a), the higher abundance of n → π* interactions at the i + 1 residue is perhaps linked with the conformational restriction of C
Oi⋯HNi+3 (Fig. 2a), the higher abundance of n → π* interactions at the i + 1 residue is perhaps linked with the conformational restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oivia hydrogen bonding. Thus, we surmised that the C
Oivia hydrogen bonding. Thus, we surmised that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi → C
Oi → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 n → π* interaction and the conformational space at the i + 1 position might be insensitive to amino acid substitution. Instead, the relatively flexible i + 2 residue of the β-turn (Fig. 2a), where neither the donor (n) C
Oi+1 n → π* interaction and the conformational space at the i + 1 position might be insensitive to amino acid substitution. Instead, the relatively flexible i + 2 residue of the β-turn (Fig. 2a), where neither the donor (n) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 nor the acceptor (π*) C
Oi+1 nor the acceptor (π*) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 is constrained by the intramolecular hydrogen bond, is an ideal site to probe the role of the n → π* interaction in the protein structure and its stability. Additionally, the solvent exposure of β-turns allows for amino acid substitution and examining the influence of the amino acid side-chain on the n → π* interaction. Thus, we chose to engineer the loop L1 of the 56-residue immunoglobulin-binding domain B1 of the streptococcal protein G (GB1).35–38 The solvent exposed loop L1 in wild type GB1 is a type-I β-turn (Fig. 3a) with lysine at the i + 1 position and threonine at the i + 2 position that lacks a C
Oi+2 is constrained by the intramolecular hydrogen bond, is an ideal site to probe the role of the n → π* interaction in the protein structure and its stability. Additionally, the solvent exposure of β-turns allows for amino acid substitution and examining the influence of the amino acid side-chain on the n → π* interaction. Thus, we chose to engineer the loop L1 of the 56-residue immunoglobulin-binding domain B1 of the streptococcal protein G (GB1).35–38 The solvent exposed loop L1 in wild type GB1 is a type-I β-turn (Fig. 3a) with lysine at the i + 1 position and threonine at the i + 2 position that lacks a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction. However, to have a better control over the turn conformation, we decided to introduce a type-II′ β-turn.39 Thus, we synthesized a GB1 variant where –KT– in loop L1 was substituted with D-Ala–L-Ala (1) to induce a type-II′ β-turn (Fig. 3a).
Oi+2 n → π* interaction. However, to have a better control over the turn conformation, we decided to introduce a type-II′ β-turn.39 Thus, we synthesized a GB1 variant where –KT– in loop L1 was substituted with D-Ala–L-Ala (1) to induce a type-II′ β-turn (Fig. 3a).
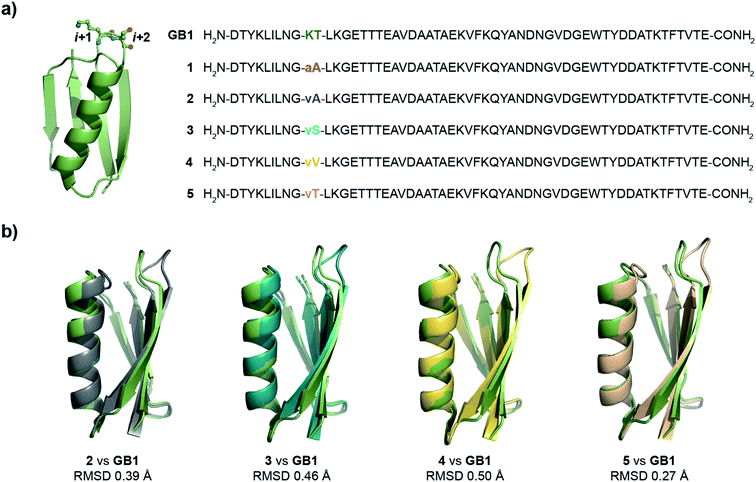 | ||
| Fig. 3 (a) Crystal structure of GB1 with –Lys–Thr– in loop L1 forming a type-I β-turn, which has been modified to a type-II′ β-turn in 1–5. The single letter code in lower case indicates D-amino acid. (b) The backbone overlay of 2 (6L9B), 3 (6L9D), 4 (6LJI), and 5 (6L91) with GB1 (2QMT). | ||
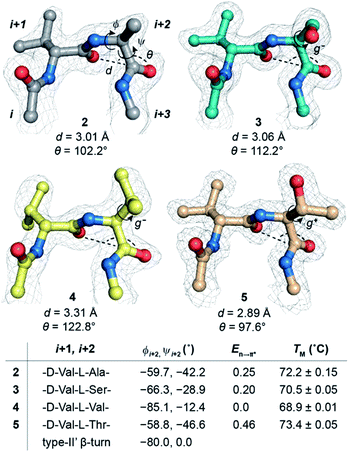
Alanine was chosen due to its preference for an n → π* interaction11 and high helix propensity40 (preference of an amino acid to be in α-helices). Although thermal denaturation of 1 using variable temperature circular dichroism (CD) showed unfolding cooperativity similarly to GB1 (Fig. S8 and S9†), multiple attempts to crystallize 1 remained unsuccessful. This is possibly a consequence of conformational flexibility introduced in the loop L1 by alanine substitution. Our earlier results indicated that the β-branched amino acid D-Val at the i + 1 site stabilizes a type-II′ β-turn more than D-Ala.41 Thus, we synthesized 2, with D-Val–L-Ala in loop L1 (Fig. 3a), which readily crystallized and X-ray diffraction data were collected to a maximum resolution of 1.9 Å. The structure of 2 overlaps closely with the tertiary structure of GB1 (backbone RMSD 0.39 Å) (Fig. 3b), although with a significant displacement of loop L1. Gratifyingly, the D-Val C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 and L-Ala C
Oi+1 and L-Ala C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 in the type-II′ β-turn engage in an n → π* interaction, where the torsion angles of L-Ala at the i + 2 site (ϕ, ψ = −59.7°, −42.2°) are remarkably close to the mean torsion angles of a right-handed α-helix (ϕ, ψ = −62°, −41°)42 (Fig. 4).
Oi+2 in the type-II′ β-turn engage in an n → π* interaction, where the torsion angles of L-Ala at the i + 2 site (ϕ, ψ = −59.7°, −42.2°) are remarkably close to the mean torsion angles of a right-handed α-helix (ϕ, ψ = −62°, −41°)42 (Fig. 4).
Encouraged by this result, we next incorporated serine with moderate helix propensity (3), valine (4) and threonine (5) with low helix propensity40 at the i + 2 site of the type-II′ β-turn (Fig. 3a). With the decreasing helix propensity of amino acids in the order Ala > Ser > Val, we noted an increase in both d and θ between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2, suggesting a gradual weakening of the n → π* interaction at the i + 2 residue (Fig. 4). Thus, to obtain a quantitative estimate of the n → π* interaction energy (En→π*) at the i + 2 residue in 2, 3, and 4, we resorted to NBO analysis,43 which clearly indicated a decreasing En→π* in the order 2 > 3 > 4 (Fig. 4).
Oi+2, suggesting a gradual weakening of the n → π* interaction at the i + 2 residue (Fig. 4). Thus, to obtain a quantitative estimate of the n → π* interaction energy (En→π*) at the i + 2 residue in 2, 3, and 4, we resorted to NBO analysis,43 which clearly indicated a decreasing En→π* in the order 2 > 3 > 4 (Fig. 4).
Despite the low helix propensity of threonine, we were surprised to note the shortest d and θ between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1⋯C
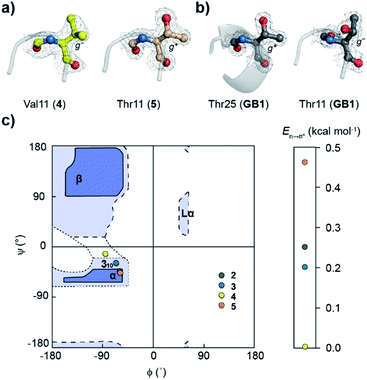
Oi+1⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 at the type-II′ β-turn in 5 with an En→π* of 0.46 kcal mol−1 (Fig. 4). An overlay of the type-II′ β-turns of both the β-branched amino acids valine (4) and threonine (5) revealed a clear difference in the side-chain rotamer conformation (Fig. 5a). Valine in 4 crystallized in a gauche− (g−) side-chain rotameric conformation, whereas threonine in 5 crystallized in a gauche+ (g+) conformation. From the statistical analyses of protein structures, it is known that β-branched amino acids favor the g+ side-chain conformation over g− in helices.44–47 Thus, Thr25 in the α-helix of GB1 with a g+ conformation engages in an n → π* interaction, whereas Thr11 at the i + 2 residue in loop L1 with a g− conformation lacks the n → π* interaction (Fig. 5b). Moreover, our dataset revealed that Thr with a g− conformation at the i + 2 residue in the β-turns does not engage in an n → π* interaction (Table S4†). Therefore, despite the low helix propensity, the n → π* interaction in threonine at the type-II′ β-turn in 5 is a result of the altered side-chain rotamer conformation.
Oi+2 at the type-II′ β-turn in 5 with an En→π* of 0.46 kcal mol−1 (Fig. 4). An overlay of the type-II′ β-turns of both the β-branched amino acids valine (4) and threonine (5) revealed a clear difference in the side-chain rotamer conformation (Fig. 5a). Valine in 4 crystallized in a gauche− (g−) side-chain rotameric conformation, whereas threonine in 5 crystallized in a gauche+ (g+) conformation. From the statistical analyses of protein structures, it is known that β-branched amino acids favor the g+ side-chain conformation over g− in helices.44–47 Thus, Thr25 in the α-helix of GB1 with a g+ conformation engages in an n → π* interaction, whereas Thr11 at the i + 2 residue in loop L1 with a g− conformation lacks the n → π* interaction (Fig. 5b). Moreover, our dataset revealed that Thr with a g− conformation at the i + 2 residue in the β-turns does not engage in an n → π* interaction (Table S4†). Therefore, despite the low helix propensity, the n → π* interaction in threonine at the type-II′ β-turn in 5 is a result of the altered side-chain rotamer conformation.
The Ramachandran plot of the i + 2 residue (Fig. 5c) in the type-II′ β-turn of 2, 3, 4, and 5 revealed that, as the strength of the n → π* interaction increases, the torsion angles of an amino acid in a non-helical region in the absence of the stabilizing intramolecular hydrogen bond are gradually altered to occupy the right-handed α-helical region. Thus, proline with a high propensity to engage in an n → π* interaction11 is a strong helix initiator.48,49 Hence, our result further supports the crucial role of the n → π* interaction in helix nucleation, as hypothesized earlier.11
Implication of the n → π* interaction on protein stability
Since, an n → π* interaction results in a restricted ϕ torsion angle (Fig. 2e and f),11 we sought to examine the influence of the n → π* interaction on the conformational stability of 2, 3, 4, and 5. The midpoint of the thermal transition (TM), which is a measure of structural stability was determined by variable temperature CD (Fig. S10–S13†). 5 with the strongest C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction displayed the maximum stability (TM) and 4 with no detectable C
Oi+2 n → π* interaction displayed the maximum stability (TM) and 4 with no detectable C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction showed the least stability (Fig. 4). We were surprised to note a very good correlation (Fig. S7†) between the TM of these proteins and En→π* between C
Oi+2 n → π* interaction showed the least stability (Fig. 4). We were surprised to note a very good correlation (Fig. S7†) between the TM of these proteins and En→π* between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2, in the absence of the stabilizing C
Oi+2, in the absence of the stabilizing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi⋯HNi+3 hydrogen bond (Fig. 4). An n → π* interaction rigidifies the β-turn by reducing the conformational entropy at the i + 2 residue, which is presumably responsible for the increased stability of the protein in solution. However, as the amino acid side-chains at the i + 2 residue of the type II′ β-turn are different in 2–5, there might be additional factors that contribute towards the stability of these proteins. Therefore, we adopted an orthogonal strategy to validate the role of the n → π* interaction in protein stability.
Oi⋯HNi+3 hydrogen bond (Fig. 4). An n → π* interaction rigidifies the β-turn by reducing the conformational entropy at the i + 2 residue, which is presumably responsible for the increased stability of the protein in solution. However, as the amino acid side-chains at the i + 2 residue of the type II′ β-turn are different in 2–5, there might be additional factors that contribute towards the stability of these proteins. Therefore, we adopted an orthogonal strategy to validate the role of the n → π* interaction in protein stability.
By employing a prolyl-based torsion balance system, Raines et al. have shown that a thioamide (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si) engages in a stronger C
Si) engages in a stronger C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si → C
Si → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 n → π* interaction than amide C
Oi+1 n → π* interaction than amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi.20,50 However, due to the longer C
Oi.20,50 However, due to the longer C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond length (1.71 Å)51 and larger van der Waals radius of sulfur (1.85 Å),52 thioamide substitution perturbs the local secondary structure of proteins where the amide oxygen participates in a shorter hydrogen bond.53–57 On the other hand, thioamide substitution at a site where the amide oxygen is involved in a longer hydrogen bond or is solvent exposed, leads to minimal perturbation of the secondary structure.53,55,57–59 Therefore, we chose to substitute the solvent exposed C
S bond length (1.71 Å)51 and larger van der Waals radius of sulfur (1.85 Å),52 thioamide substitution perturbs the local secondary structure of proteins where the amide oxygen participates in a shorter hydrogen bond.53–57 On the other hand, thioamide substitution at a site where the amide oxygen is involved in a longer hydrogen bond or is solvent exposed, leads to minimal perturbation of the secondary structure.53,55,57–59 Therefore, we chose to substitute the solvent exposed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 in the type-II′ β-turn of 2 by C
Oi+1 in the type-II′ β-turn of 2 by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1. The NBO analysis of the C
Si+1. The NBO analysis of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 to C
Oi+1 to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 substituted type-II′ β-turn in 2, 3, 4, and 5 clearly indicated a significant enhancement in En→π*, due to the amplified C
Si+1 substituted type-II′ β-turn in 2, 3, 4, and 5 clearly indicated a significant enhancement in En→π*, due to the amplified C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 → C
Si+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction (Table S3†).
Oi+2 n → π* interaction (Table S3†).
Thus, towards the synthesis of i + 1 thionated GB1 (D-Valt–L-Ala; 2a) (the thionated residue is denoted by superscript “t”), we obtained a clean 46-mer polypeptide up to the L-Alai+2. However, on completion of the 56-mer 2a on a solid support, following the acidolytic removal of protecting groups, the mass spectrum corresponded to a 45-mer fragment without the L-Alai+2 (Fig. S16B†). To circumvent the undesirable peptide cleavage, we coupled the tetrapeptide Fmoc-Asn(Trt)-Gly-D-Valt-L-Ala-COOH and Fmoc-Asn(Trt)-Gly-D-Val-L-Ala-COOH onto two individual 45-mer polypeptides. After acidolytic cleavage, although we obtained the 49-mer oxo-polypeptide, the thio-tetrapeptide coupling repeatedly resulted in the 45-mer fragment without the L-Alai+2 (Fig. S16C and D†). This suggests a spontaneous acid catalyzed cleavage of the peptide bond C-terminal to L-Alai+2 in thioamidated GB1, 2a.
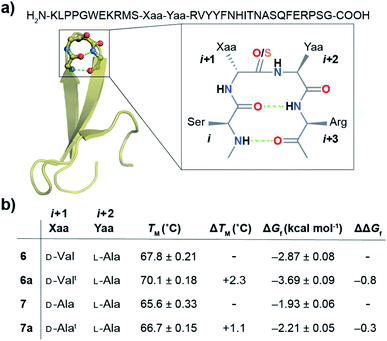
With numerous failed attempts to synthesize 2a, we focused towards the 32-mer Pin 1 WW domain, a three stranded β-sheet protein that shows a cooperative two-state folding.60,61 The Pin 1 protein is amenable to loop modification that retains the global fold with alteration in its thermodynamic stability, making it an excellent model protein for structure-folding studies.60 We selected a Pin 1 variant with a type-I′ β-turn in loop 1 and substituted the –Asn–Gly– with D-Val–L-Ala– (6) and D-Ala–L-Ala– (7) (Fig. 6a) to adopt a type-II′ β-turn that was confirmed by characteristic NOEs at the β-turn (Fig. S29†). Subsequently, we synthesized Pin 1 variants with thioamidation at the i + 1 site (D-Valt–L-Ala; 6a and D-Alat–L-Ala; 7a). Remarkably, the acidolytic removal of the protecting groups to obtain 6a resulted in both the desired product and the N- and C-terminal fragmented peptides resulting from the nucleophilic attack of D-Val C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 onto L-Ala C
Si+1 onto L-Ala C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 (Fig. 7a) as observed in 2a.
Oi+2 (Fig. 7a) as observed in 2a.
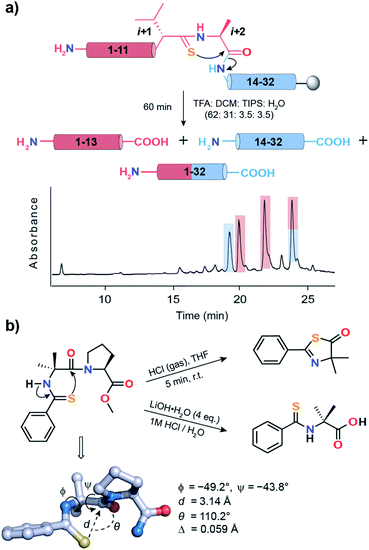 | ||
Fig. 7 (a) Acid-catalyzed cleavage of 6a yielding two N-terminal fragments (1–13), one C-terminal fragment (14–32) and the desired product (1–32). The two N-terminal fragments possibly result from the racemization of the L-Alai+2 due to keto–enol tautomerization of the thiazolone intermediate.63 The polypeptides are color coded and are shown in the HPLC chromatogram. (b) Spontaneous acid-catalyzed cleavage of the thioacylated Aib–Pro dipeptide in aqueous solution.62 The thiazolone intermediate was trapped by passing HCl gas and characterized. The crystal structure of Ph-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)-Aib-Pro-Aib-N(Me)Ph clearly depicting the n → π* interaction between C S)-Aib-Pro-Aib-N(Me)Ph clearly depicting the n → π* interaction between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si and C Si and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 of Aib. Oi+1 of Aib. | ||
An identical fragmentation was reported by Heimgartner et al. during the aqueous acidolytic workup of the thioacylated Aib–Pro dipeptide (Fig. 7b), towards the synthesis of Ph-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)-Aib-Pro-Aib-N(Me)Ph.62 However, by bubbling HCl gas through the dipeptide in THF, the thiazolone intermediate could be characterized, which results from the nucleophilic attack of Ph C
S)-Aib-Pro-Aib-N(Me)Ph.62 However, by bubbling HCl gas through the dipeptide in THF, the thiazolone intermediate could be characterized, which results from the nucleophilic attack of Ph C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si onto Aib C
Si onto Aib C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1. To our excitement, the crystal structure of the final product Ph-(C
Oi+1. To our excitement, the crystal structure of the final product Ph-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)-Aib-Pro-Aib-N(Me)Ph revealed the C
S)-Aib-Pro-Aib-N(Me)Ph revealed the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si → C
Si → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 n → π* interaction, leading to a high degree of pyramidalization, Δ = 0.059 Å at Aib C
Oi+1 n → π* interaction, leading to a high degree of pyramidalization, Δ = 0.059 Å at Aib C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1, a firm indicator of the n → π* interaction.19 Thus, the directional (i + 1 → i + 2) fragmentation observed in 2a (Fig. S16†), 6a (Fig. 7a) and 7a (Fig. S17B†) is a chemical signature of the amplified n → π* interaction between C
Oi+1, a firm indicator of the n → π* interaction.19 Thus, the directional (i + 1 → i + 2) fragmentation observed in 2a (Fig. S16†), 6a (Fig. 7a) and 7a (Fig. S17B†) is a chemical signature of the amplified n → π* interaction between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 and C
Si+1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2.
Oi+2.
Next, we assessed the folding of Pin 1 variants 6, 6a, 7, and 7a in sodium phosphate buffer (pH 7.4). All the Pin 1 proteins showed the characteristic 227 nm maximum in the CD spectrum, indicating the presence of a folded protein with β-sheets (minimum centered around 215 nm) (Fig. S22A–S25A†). The virtually identical Hα chemical shift perturbation deduced from TOCSY and NOESY experiments indicated that a single atom substitution (O to S) at the solvent exposed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 did not lead to major structural perturbation in 6a and 7a (Fig. S28†). We next performed thermal and chemical denaturation (Fig. S22–S25†) to understand the effect of the amplified n → π* interaction. The proteins showed a two-state unfolding and we were delighted to note that the C
Oi+1 did not lead to major structural perturbation in 6a and 7a (Fig. S28†). We next performed thermal and chemical denaturation (Fig. S22–S25†) to understand the effect of the amplified n → π* interaction. The proteins showed a two-state unfolding and we were delighted to note that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 → C
Si+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction enhanced the stability of 6a by 0.8 kcal mol−1 and 7a by 0.3 kcal mol−1 (Fig. 6b).
Oi+2 n → π* interaction enhanced the stability of 6a by 0.8 kcal mol−1 and 7a by 0.3 kcal mol−1 (Fig. 6b).
The increased stability arises from the reduced conformational flexibility of the amino acid residue engaged in an n → π* interaction, a feature that is analogous to the ring constraint in proline, which restricts its conformational space compared to other amino acids and increases protein stability by reducing the entropy of the unfolded state.64,65 An n → π* interaction restricts the conformational space of an amino acid residue with the adoption of torsion angles as depicted in the Ramachandran plot of the β-turn residues (Fig. 2c and d). This would also be expected in an amplified n → π* interaction by thioamide substitution. The adoption of such torsion angles is favorable at the i + 1 and i + 2 positions of a β-turn (Fig. 2a). Furthermore, since β-branched amino acids restrict the backbone conformation more than the unbranched residues,65 the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 → C
Si+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction stabilizes 6a more than 7a.
Oi+2 n → π* interaction stabilizes 6a more than 7a.
Thus, our results in the Pin 1 WW domain re-emphasize the role of the amino acid side-chain in tuning the n → π* interaction energy. Not only the side-chain rotamer of the amino acid involved in an n → π* interaction dictates its strength (Fig. 4), the steric interactions imposed by the amino acid side-chain of the donor carbonyl oxygen(i) (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si+1 in this case) (Fig. 6) can also influence an n → π* interaction.
Si+1 in this case) (Fig. 6) can also influence an n → π* interaction.
Conclusions
In summary, our bioinformatic analysis indicates that the reduced conformational freedom of the donor C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi by the intramolecular C
Oi by the intramolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi⋯HNi+3 hydrogen bond in β-turns is associated with the high abundance of n → π* interactions at the i + 1 residue, whereas, the absence of the intramolecular hydrogen bond, constraining either the C
Oi⋯HNi+3 hydrogen bond in β-turns is associated with the high abundance of n → π* interactions at the i + 1 residue, whereas, the absence of the intramolecular hydrogen bond, constraining either the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 or C
Oi+1 or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 results in conformational flexibility of the i + 2 residue, which could be restricted by introducing an C
Oi+2 results in conformational flexibility of the i + 2 residue, which could be restricted by introducing an C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+1 → C
Oi+1 → C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oi+2 n → π* interaction. The experimental results at the i + 2 residue of the type-II′ β-turn in GB1 variants suggest that amino acid side-chain identity and the rotamer conformation can modulate the strength of an n → π* interaction. Although, it is challenging to estimate the exact contribution of this energetically subtle interaction towards the global stability of the protein, we note that the altered rotamer conformation as a result of local structural changes can amplify/weaken an n → π* interaction affecting the backbone torsion angles (ϕ, ψ), thereby influencing its stability. With an enhanced n → π* interaction in the absence of the stabilizing intramolecular hydrogen bond, we observe a clear shift of amino acid torsion angles (ϕ, ψ) from a non-helical to the right-handed α-helical region. It is worth noting that the i → i + 1 directionality (N-term → C-term) associated with the n → π* interaction coincides with the formation of the productive helix nucleus at the N-terminus of a polypeptide,66–68 highlighting an important contribution of the n → π* interaction towards helix nucleation. Furthermore, the recent report of a long-range n → π* interaction in stabilizing the α-helical conformation of a synthetic peptide in water, re-emphasizes the potential of this noncovalent interaction in engineering helical structures.69
Oi+2 n → π* interaction. The experimental results at the i + 2 residue of the type-II′ β-turn in GB1 variants suggest that amino acid side-chain identity and the rotamer conformation can modulate the strength of an n → π* interaction. Although, it is challenging to estimate the exact contribution of this energetically subtle interaction towards the global stability of the protein, we note that the altered rotamer conformation as a result of local structural changes can amplify/weaken an n → π* interaction affecting the backbone torsion angles (ϕ, ψ), thereby influencing its stability. With an enhanced n → π* interaction in the absence of the stabilizing intramolecular hydrogen bond, we observe a clear shift of amino acid torsion angles (ϕ, ψ) from a non-helical to the right-handed α-helical region. It is worth noting that the i → i + 1 directionality (N-term → C-term) associated with the n → π* interaction coincides with the formation of the productive helix nucleus at the N-terminus of a polypeptide,66–68 highlighting an important contribution of the n → π* interaction towards helix nucleation. Furthermore, the recent report of a long-range n → π* interaction in stabilizing the α-helical conformation of a synthetic peptide in water, re-emphasizes the potential of this noncovalent interaction in engineering helical structures.69
To conclusively demonstrate the influence of the n → π* interaction on protein stability, we chose to amplify this weak noncovalent interaction by thioamide substitution. Since a strong n → π* interaction induces a “kink” in the polypeptide backbone by optimizing the ϕ, ψ torsion angles suitable for orbital overlap, and thereby reducing the conformational entropy at the β-turn, the thioamide substitution increased the protein stability. It is worth noting that thio-Gly465 in the natural protein methyl-coenzyme M reductase, which is suggested to stabilize the protein secondary structure near the active site, induces a kinked conformation (ϕ, ψ = −68.5°, −47.2°) by engaging in an n → π* interaction with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of Phe466 (Fig. S30†).70,71 With the recent advancement in ribosome mediated incorporation of thioamide into proteins and polypeptides, thioamide substitution could be potentially utilized to stabilize turns and enhance protein stability,72,73 aided by exogenous factors like salt concentration11 and solvation by water molecules69 that have been shown to influence the n → π* interaction in protein secondary structures.
O of Phe466 (Fig. S30†).70,71 With the recent advancement in ribosome mediated incorporation of thioamide into proteins and polypeptides, thioamide substitution could be potentially utilized to stabilize turns and enhance protein stability,72,73 aided by exogenous factors like salt concentration11 and solvation by water molecules69 that have been shown to influence the n → π* interaction in protein secondary structures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
JC acknowledge DST-SERB for funding this work through the project EMR/2016/006193. The authors acknowledge the DBT-IISc partnership program phase-I, phase-II and IISc (MHRD) for funding this work. We also acknowledge funding for infrastructural support from the following programs of the Government of India: DST-FIST, UGC-CAS, and the DBT-IISc partnership program. The X-Ray diffraction facility for Macromolecular Crystallography at IISc is funded by DST-SERB grant IR/SO/LU/0003/2010-PHASE-II. BK thanks UGC for the research fellowship. JN thanks DST-SERB for funding under the Ramanujan Faculty Fellowship scheme, Prof. E. Arunan, IISc for the access to NBO6.0 and IPC clusters to carry out the NBO calculations and Compute Canada for the use of the Niagara supercomputer resources. AP is an Intermediate fellow of the DBT-Wellcome Trust India Alliance (IA/I/15/2/502063). Sunita Prakash is acknowledged for her tremendous support with the acquisition of LC-MS at the Proteomics facility in IISc. We thank Dr Amit Choudhary for stimulating discussions.References
- K. A. Dill, Biochemistry, 1990, 29, 7133–7155 CrossRef CAS PubMed.
- R. W. Newberry and R. T. Raines, ACS Chem. Biol., 2019, 14, 1677–1686 CrossRef CAS PubMed.
- L. Pauling, R. B. Corey and H. R. Branson, Proc. Natl. Acad. Sci. U. S. A., 1951, 37, 205–211 CrossRef CAS PubMed.
- Z. S. Derewenda, L. Lee and U. Derewenda, J. Mol. Biol., 1995, 252, 248–262 CrossRef CAS PubMed.
- R. W. Newberry and R. T. Raines, Nat. Chem. Biol., 2016, 12, 1084–1088 CrossRef CAS PubMed.
- M. P. Hinderaker and R. T. Raines, Protein Sci., 2003, 12, 1188–1194 CrossRef CAS PubMed.
- S. K. Singh and A. Das, Phys. Chem. Chem. Phys., 2015, 17, 9596–9612 RSC.
- R. W. Newberry and R. T. Raines, Acc. Chem. Res., 2017, 50, 1838–1846 CrossRef CAS PubMed.
- A. Rahim, P. Saha, K. K. Jha, N. Sukumar and B. K. Sarma, Nat. Commun., 2017, 8, 78 CrossRef PubMed.
- H. B. Burgi, J. D. Dunitz and E. Shefter, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 1517–1527 CrossRef.
- G. J. Bartlett, A. Choudhary, R. T. Raines and D. N. Woolfson, Nat. Chem. Biol., 2010, 6, 615–620 CrossRef CAS PubMed.
- C. Fufezan, Proteins, 2010, 78, 2831–2838 CrossRef CAS PubMed.
- C. E. Jakobsche, A. Choudhary, S. J. Miller and R. T. Raines, J. Am. Chem. Soc., 2010, 132, 6651–6653 CrossRef CAS PubMed.
- Z. S. Shi and N. R. Kallenbach, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 3–4 CrossRef CAS PubMed.
- G. J. Bartlett, R. W. Newberry, B. VanVeller, R. T. Raines and D. N. Woolfson, J. Am. Chem. Soc., 2013, 135, 18682–18688 CrossRef CAS PubMed.
- H. R. Kilgore and R. T. Raines, J. Am. Chem. Soc., 2018, 140, 17606–17611 CrossRef CAS PubMed.
- L. E. Bretscher, C. L. Jenkins, K. M. Taylor, M. L. DeRider and R. T. Raines, J. Am. Chem. Soc., 2001, 123, 777–778 CrossRef CAS PubMed.
- M. L. DeRider, S. J. Wilkens, M. J. Waddell, L. E. Bretscher, F. Weinhold, R. T. Raines and J. L. Markley, J. Am. Chem. Soc., 2002, 124, 2497–2505 CrossRef CAS PubMed.
- P. Wilhelm, B. Lewandowski, N. Trapp and H. Wennemers, J. Am. Chem. Soc., 2014, 136, 15829–15832 CrossRef CAS PubMed.
- R. W. Newberry, B. VanVeller, I. A. Guzei and R. T. Raines, J. Am. Chem. Soc., 2013, 135, 7843–7846 CrossRef CAS PubMed.
- S. Hovmoller, T. Zhou and T. Ohlson, Acta Crystallogr., 2002, 58, 768–776 CrossRef PubMed.
- N. J. Zondlo, Nat. Chem. Biol., 2010, 6, 567–568 CrossRef CAS PubMed.
- P. Deb, G. Y. Jin, S. K. Singh, J. Moon, H. Kwon, A. Das, S. Bagchi and Y. S. Kim, J. Phys. Chem. Lett., 2018, 9, 5425–5429 CrossRef CAS PubMed.
- C. Cabezas, J. L. Alonso, J. C. Lopez and S. Mata, Angew. Chem., Int. Ed., 2012, 51, 1375–1378 CrossRef CAS PubMed.
- S. K. Singh, K. K. Mishra, N. Sharma and A. Das, Angew. Chem., Int. Ed., 2016, 55, 7801–7805 CrossRef CAS PubMed.
- S. Blanco, J. C. Lopez, S. Mata and J. L. Alonso, Angew. Chem., Int. Ed., 2010, 49, 9187–9192 CrossRef CAS PubMed.
- Q. Gou, G. Feng, L. Evangelisti and W. Caminati, Angew. Chem., Int. Ed., 2013, 52, 11888–11891 CrossRef CAS PubMed.
- A. G. de Brevern, Sci. Rep., 2016, 6, 33191 CrossRef CAS PubMed.
- I. D. Kuntz, J. Am. Chem. Soc., 1972, 94, 4009–4012 CrossRef CAS PubMed.
- G. D. Rose, L. M. Gierasch and J. A. Smith, Adv. Protein Chem., 1985, 37, 1–109 CrossRef CAS PubMed.
- P. N. Lewis, F. A. Momany and H. A. Scheraga, Proc. Natl. Acad. Sci. U. S. A., 1971, 68, 2293–2297 CrossRef CAS PubMed.
- H. E. Stanger and S. H. Gellman, J. Am. Chem. Soc., 1998, 120, 4236–4237 CrossRef CAS.
- H. L. Fu, G. R. Grimsley, A. Razvi, J. M. Scholtz and C. N. Pace, Proteins, 2009, 77, 491–498 CrossRef CAS PubMed.
- K. Watanabe, T. Masuda, H. Ohashi, H. Mihara and Y. Suzuki, Eur. J. Biochem., 1994, 226, 277–283 CrossRef CAS PubMed.
- T. Gallagher, P. Alexander, P. Bryan and G. L. Gilliland, Biochemistry, 1994, 33, 4721–4729 CrossRef CAS PubMed.
- A. M. Gronenborn, D. R. Filpula, N. Z. Essig, A. Achari, M. Whitlow, P. T. Wingfield and G. M. Clore, Science, 1991, 253, 657–661 CrossRef CAS PubMed.
- Z. E. Reinert, G. A. Lengyel and W. S. Horne, J. Am. Chem. Soc., 2013, 135, 12528–12531 CrossRef CAS PubMed.
- H. L. Schmidt, L. J. Sperling, Y. G. Gao, B. J. Wylie, J. M. Boettcher, S. R. Wilson and C. M. Rienstra, J. Phys. Chem. B, 2007, 111, 14362–14369 CrossRef PubMed.
- B. L. Sibanda and J. M. Thornton, Nature, 1985, 316, 170–174 CrossRef CAS PubMed.
- C. N. Pace and J. M. Scholtz, Biophys. J., 1998, 75, 422–427 CrossRef CAS PubMed.
- P. Lahiri, H. Verma, A. Ravikumar and J. Chatterjee, Chem. Sci., 2018, 9, 4600–4609 RSC.
- D. J. Barlow and J. M. Thornton, J. Mol. Biol., 1988, 201, 601–619 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- P. Chakrabarti and D. Pal, Prog. Biophys. Mol. Biol., 2001, 76, 1–102 CrossRef CAS.
- T. M. Gray and B. W. Matthews, J. Mol. Biol., 1984, 175, 75–81 CrossRef CAS.
- J. Janin and S. Wodak, J. Mol. Biol., 1978, 125, 357–386 CrossRef CAS PubMed.
- M. J. McGregor, S. A. Islam and M. J. Sternberg, J. Mol. Biol., 1987, 198, 295–310 CrossRef CAS PubMed.
- M. W. MacArthur and J. M. Thornton, J. Mol. Biol., 1991, 218, 397–412 CrossRef CAS.
- J. S. Richardson and D. C. Richardson, Science, 1988, 240, 1648–1652 CrossRef CAS PubMed.
- A. Choudhary, D. Gandla, G. R. Krow and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 7244–7246 CrossRef CAS PubMed.
- M. R. Truter, J. Chem. Soc., 1960, 997–1007 RSC.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- D. R. Artis and M. A. Lipton, J. Am. Chem. Soc., 1998, 120, 12200–12206 CrossRef CAS.
- R. M. Culik, H. Jo, W. F. DeGrado and F. Gai, J. Am. Chem. Soc., 2012, 134, 8026–8029 CrossRef CAS PubMed.
- R. W. Newberry, B. VanVeller and R. T. Raines, Chem. Commun., 2015, 51, 9624–9627 RSC.
- A. Reiner, D. Wildemann, G. Fischer and T. Kiefhaber, J. Am. Chem. Soc., 2008, 130, 8079–8084 CrossRef CAS PubMed.
- C. R. Walters, D. M. Szantai-Kis, Y. T. Zhang, Z. E. Reinert, W. S. Horne, D. M. Chenoweth and E. J. Petersson, Chem. Sci., 2017, 8, 2868–2877 RSC.
- J. H. Miwa, A. K. Patel, N. Vivatrat, S. M. Popek and A. M. Meyer, Org. Lett., 2001, 3, 3373–3375 CrossRef CAS PubMed.
- H. Verma, B. Khatri, S. Chakraborti and J. Chatterjee, Chem. Sci., 2018, 9, 2443–2451 RSC.
- M. S. Ardejani, E. T. Powers and J. W. Kelly, Acc. Chem. Res., 2017, 50, 1875–1882 CrossRef CAS.
- M. Jager, Y. Zhang, J. Bieschke, H. Nguyen, M. Dendle, M. E. Bowman, J. P. Noel, M. Gruebele and J. W. Kelly, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10648–10653 CrossRef CAS.
- A. Budzowski, A. Linden and H. Heimgartner, Helv. Chim. Acta, 2008, 91, 1471–1488 CrossRef CAS.
- J. H. Davies, R. H. Davis and R. A. G. Carrington, J. Chem. Soc., Perkin Trans. 1, 1972, 1983–1985 RSC.
- U. Arnold, M. P. Hinderaker, J. Koditz, R. Golbik, R. Ulbrich-Hofmann and R. T. Raines, J. Am. Chem. Soc., 2003, 125, 7500–7501 CrossRef CAS PubMed.
- B. W. Matthews, H. Nicholson and W. J. Becktel, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 6663–6667 CrossRef CAS PubMed.
- A. Acharyya, Y. H. Ge, H. F. Wu, W. F. DeGrado, V. A. Voelz and F. Gai, J. Phys. Chem. B, 2019, 123, 1797–1807 CrossRef CAS PubMed.
- L. Monticelli, D. P. Tieleman and G. Colombo, J. Phys. Chem. B, 2005, 109, 20064–20067 CrossRef CAS PubMed.
- L. Pal, P. Chakrabarti and G. Basu, J. Mol. Biol., 2003, 326, 273–291 CrossRef CAS PubMed.
- H. N. Hoang, C. Y. Wu, T. A. Hill, A. D. de Araujo, P. V. Bernhardt, L. G. Liu and D. P. Fairlie, Angew. Chem., Int. Ed., 2019, 58, 18873–18877 CrossRef CAS PubMed.
- W. G. Grabarse, F. Mahlert, S. Shima, R. K. Thauer and U. Ermler, J. Mol. Biol., 2000, 303, 329–344 CrossRef CAS PubMed.
- D. D. Nayak, N. Mahanta, D. A. Mitchell and W. W. Metcalf, eLife, 2017, 6, e29218 CrossRef PubMed.
- R. Maini, L. M. Dedkova, R. Paul, M. M. Madathil, S. R. Chowdhury, S. X. Chen and S. M. Hecht, J. Am. Chem. Soc., 2015, 137, 11206–11209 CrossRef CAS PubMed.
- R. Maini, H. Kimura, R. Takatsuji, T. Katoh, Y. Goto and H. Suga, J. Am. Chem. Soc., 2019, 141, 20004–20008 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. The coordinates for the GB1 crystal structures have been deposited in the Protein Data Bank with the following accession codes 6L9B, 6L9D, 6LJI, 6L91. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc03060k |
| This journal is © The Royal Society of Chemistry 2020 |