Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual catalytic enantioselective desymmetrization of allene-tethered cyclohexanones†
Lin
Zhang
a,
Ken
Yamazaki
a,
Jamie A.
Leitch
 a,
Ruben
Manzano
a,
Ruben
Manzano
 a,
Victoria A. M.
Atkinson
a,
Victoria A. M.
Atkinson
 a,
Trevor A.
Hamlin
a,
Trevor A.
Hamlin
 *b and
Darren J.
Dixon
*b and
Darren J.
Dixon
 *a
*a
aDepartment of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, UK
bDepartment of Theoretical Chemistry, Amsterdam Institute of Molecular and Life Sciences (AIMMS), Amsterdam Center for Multiscale Modeling (ACMM), Vrije Universiteit Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam, The Netherlands. E-mail: t.a.hamlin@vu.nl
First published on 24th June 2020
Abstract
The construction of enantioenriched azabicyclo[3.3.1]nonan-6-one heterocycles via an enantioselective desymmetrization of allene-linked cyclohexanones, enabled through a dual catalytic system, that provides synchronous activation of the cyclohexanone with a chiral prolinamide and the allene with a copper(I) co-catalyst to deliver the stereodefined bicyclic core, is described. Successful application to oxygen analogues was also achieved, thereby providing a new enantioselective synthetic entry to architecturally complex bicyclic ethereal frameworks. The mechanistic pathway and the origin of enantio- and diastereoselectivities has been uncovered using density functional theory (DFT) calculations.
Introduction
The morphan (azabicyclo[3.3.1]nonane) scaffold is a common and versatile subunit to many bioactive compounds, and serves as the core to a variety of natural products (Scheme 1), including himalenine C, daphniyunnine-type alkaloids, strychnine, and immunosuppressant FR901483.1 Moreover, oxygenated analogues are also found in natural products such as enterocin.2 Due to these viable biologically relevant applications, and often deficient quantities available from the natural source,3 the demand for efficient and stereocontrolled syntheses of these complex architectures has been a focus of research efforts in enantioselective synthesis in recent years.4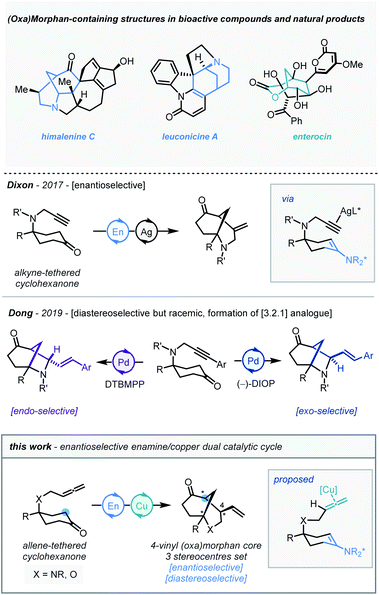 | ||
| Scheme 1 The design of an allene-linked cyclohexanone desymmetrization platform. En = enamine catalysis. | ||
The enantioselective desymmetrization of structurally simple molecules is widely considered as one of the most powerful and elegant strategies to create new stereocenters.5 In this context, the desymmetrization of carbonyl compounds through intramolecular cyclization manifolds has proven to be an excellent access point to bridged and fused bicyclic rings. Through elegant contributions from the Jia,6 Lu,7 and Zhou8 groups amongst many others – including our own9 – significant progress has been made in this field. In 2017, our group demonstrated that the silver and chiral amine co-catalyzed intramolecular desymmetrization/cyclization of alkyne-linked cyclohexanones afforded the morphan core with high enantioselectivity.10 Recently, Dong and co-workers described an intramolecular Pd-catalyzed α-allylic alkylation of alkyne-linked cyclohexanone derivatives, notably with the ability to access both exo and endo-diastereomers of the [3.2.1] morphan analogue, albeit with insignificant enantioinduction11 (Scheme 1, middle). For downstream application in complex molecule total synthesis, we were drawn towards developing a new desymmetrization methodology for the construction of the 4-vinyl-morphan and their analogous oxygenated architectures. This would require the simultaneous stereocontrolled creation of three new stereogenic centres in a bicyclic core possessing multiple key groups and functionalities.
To date, only a handful of examples of enantioselective intramolecular cyclization of allene-linked carbonyl compounds have been reported.12 Inspired by this prior art, and building on our own findings, we reasoned that a suitable dual catalyst system could be identified to furnish such target morphan structures. We envisioned that an intramolecular cyclization of allene-tethered cyclohexanones could take place via enamine catalysis and allene activation by an appropriate metal catalyst (Scheme 1, bottom), and herein we wish to report our findings.
Results & discussion
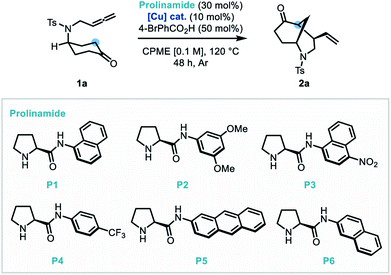
Our studies began using the nitrogen-linked allene-tethered cyclohexan-4-one derivative (1a) as a model substrate. Following preliminary co-catalyst studies, a promising copper-based system was identified where a prolinamide derivative (P1, 30 mol%), Cu(OTf)2 (10 mol%), 4-bromobenzoic acid (50 mol%) system in CPME at 120 °C, delivered the bicyclic product (2a) in a good yield (76%) and enantioselectivity (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 er, Table 1, entry 1). A survey of copper(I) and copper(II) salts which have previously found use in allene activation13 (entries 2–6) demonstrated that the copper(I) complex Cu(MeCN)4PF6 (entry 4) performed most effectively, delivering the morphan architecture in almost quantitative yield and 87
18 er, Table 1, entry 1). A survey of copper(I) and copper(II) salts which have previously found use in allene activation13 (entries 2–6) demonstrated that the copper(I) complex Cu(MeCN)4PF6 (entry 4) performed most effectively, delivering the morphan architecture in almost quantitative yield and 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 er. Following this, a study of prolinamide catalysts (entries 7–10) identified that P4 improved the enantioselectivity of the transformation to 89.5
13 er. Following this, a study of prolinamide catalysts (entries 7–10) identified that P4 improved the enantioselectivity of the transformation to 89.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 er and diastereoselectivity to >20
10.5 er and diastereoselectivity to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 9). It was also found that reducing the reaction temperature to 100 °C extinguished reactivity (entry 11). Furthermore, diluting the reaction mixture was found to be beneficial to enantioselectivity (entries 12–13). A short re-investigation of the prolinamide catalyst at this point revealed that 2-naphthylprolinamide derivative (P6) outperformed all other catalysts, and therefore was adopted as the catalyst of choice (entry 14). Finally, an exchange of the acidic additive from 4-bromobenzoic acid to trifluoroacetic acid delivered a substantial increase in enantioselectivity to 96
1 dr (entry 9). It was also found that reducing the reaction temperature to 100 °C extinguished reactivity (entry 11). Furthermore, diluting the reaction mixture was found to be beneficial to enantioselectivity (entries 12–13). A short re-investigation of the prolinamide catalyst at this point revealed that 2-naphthylprolinamide derivative (P6) outperformed all other catalysts, and therefore was adopted as the catalyst of choice (entry 14). Finally, an exchange of the acidic additive from 4-bromobenzoic acid to trifluoroacetic acid delivered a substantial increase in enantioselectivity to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er.
4 er.
| Entry | [Cu] cat. | Prolinamide | 2a (%) | drc | erd |
|---|---|---|---|---|---|
| a General conditions: 1a (0.2 mmol), [Cu] catalyst (0.02 mmol, 10 mol%), 4-bromobenzoic acid (0.1 mmol, 50 mol%), prolinamide catalyst (0.06 mmol, 30 mol%), in CPME (0.1 M, 2 mL) at 120 °C, under an argon atmosphere for 48 h. b Isolated yield. c dr calculated via1H NMR analysis of the crude reaction mixture. d er value was determined via chiral HPLC analysis of the pure product. e Reaction concentration [0.02 M]. f P6 (30 mol%) was used as prolinamide catalyst. | |||||
| 1 | Cu(OTf)2 | P1 | 76 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 2 | CuI | P1 | 44 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 3 | Cu(OAc)2 | P1 | 40 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 4 | Cu(MeCN)4PF6 | P1 | 98 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 5 | Cu(MeCN)4BF4 | P1 | 76 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 6 | Cu(acac)2 | P1 | 93 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 7 | Cu(MeCN)4PF6 | P2 | 99 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 8 | Cu(MeCN)4PF6 | P3 | 90 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 9 | Cu(MeCN)4PF6 | P4 | 99 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10:5 10:5 |
| 10 | Cu(MeCN)4PF6 | P5 | 90 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Change from entry 9 | |||||
| 11 | Reaction run at 100 °C | — | nd | nd | |
| 12 | Reaction concentration [0.04 M] | 91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|
| 13 | Reaction concentration [0.02 M] | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
|
| 14e | P6 used as organocatalyst | 82 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
|
| 15 , | TFA used instead of 4-BrPhCO 2 H | 81 |
>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|
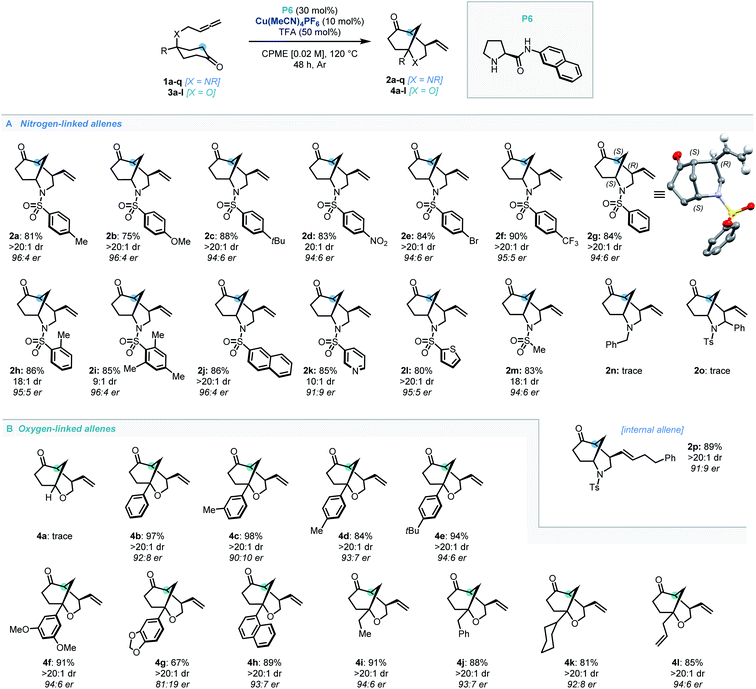
With the optimal conditions established, the scope of N-tethered substrates was explored (Scheme 2). Aryl sulfonamide-tethered allenic cyclohexanones with either electron-donating or electron-withdrawing groups at the para-position of the arene delivered morphan products in good yields with high enantioselectivity and diastereoselectivity (2b–2f, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 96
6 to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er). Phenyl substituted compound 1g afforded 2g in 78% yield and 94
4 er). Phenyl substituted compound 1g afforded 2g in 78% yield and 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er. Importantly the absolute configuration of 2g was confirmed as 1S,4R,5S by single-crystal X-ray diffraction analysis.14Ortho-methylated and multiply-substituted arene substrates were also well-tolerated, albeit with a slight drop in diastereoselectivity (2h and 2i). Naphthyl derivative 1j reacted smoothly to afford 2j in 86% yield with 96
6 er. Importantly the absolute configuration of 2g was confirmed as 1S,4R,5S by single-crystal X-ray diffraction analysis.14Ortho-methylated and multiply-substituted arene substrates were also well-tolerated, albeit with a slight drop in diastereoselectivity (2h and 2i). Naphthyl derivative 1j reacted smoothly to afford 2j in 86% yield with 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er. Substrates bearing heteroaromatic 3-pyridinyl (1k) and 2-thiophenyl (1l) substituents led to products 2k and 2l with great efficiency. Pleasingly, N-mesyl protected (1m) amines afforded the morphan bicycle with high enantioselectivity (94
4 er. Substrates bearing heteroaromatic 3-pyridinyl (1k) and 2-thiophenyl (1l) substituents led to products 2k and 2l with great efficiency. Pleasingly, N-mesyl protected (1m) amines afforded the morphan bicycle with high enantioselectivity (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er). Unfortunately, no product was observed when phenyl-substituted derivative (1o) was subjected to the optimized conditions, most likely due to excessive steric hindrance preventing the allene access to the enamine intermediate of the ketone moiety. Notably, the internal allene structure 1p also reacted smoothly under the optimized conditions, providing 2p in 89% yield with 91
6 er). Unfortunately, no product was observed when phenyl-substituted derivative (1o) was subjected to the optimized conditions, most likely due to excessive steric hindrance preventing the allene access to the enamine intermediate of the ketone moiety. Notably, the internal allene structure 1p also reacted smoothly under the optimized conditions, providing 2p in 89% yield with 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er.
9 er.
Following the success of the newly discovered dual catalytic system for the desymmetrization of nitrogen-linked allenes, our attention turned to their oxygen-substituted counterparts to construct the analogous 2-oxabicyclo[3.3.1]nonane heterocycle (oxamorphan for simplicity, herein). To the best of our knowledge, no enantioselective syntheses of such constructs via desymmetrization approaches or other stereoselective methodologies have been reported to date.15 Accordingly, application of this dual catalytic protocol would find added value in the synthesis of complex oxygen-containing heterocyclic constructs.2 Despite our first attempted application of the dual catalytic methodology to the cyclization of an oxygen-linked substrate (3a) being unsuccessful, installation of substitution at the α-position to the oxygen atom recovered reactivity, delivering the phenyl-substituted oxamorphan derivative (4b) as a single diastereomer in excellent yield and good enantioselectivity (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er).16 All further examples of the oxamorphan core were also shown to proceed with exquisite diastereoselectivity (>20
8 er).16 All further examples of the oxamorphan core were also shown to proceed with exquisite diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Substitution on the aromatic ring was shown to have little deleterious effect on the reactivity of substrates (4c–4f, 84–98% yields and 90
1 dr). Substitution on the aromatic ring was shown to have little deleterious effect on the reactivity of substrates (4c–4f, 84–98% yields and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 94
10 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er). Use of a protected catechol-derived substrate, however, led to a decrease in both the yield and enantioselectivity (4g, 67% yield and 81
6 er). Use of a protected catechol-derived substrate, however, led to a decrease in both the yield and enantioselectivity (4g, 67% yield and 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er). Pleasingly, structures with an alkyl group (ethyl, benzyl and cyclohexyl) functioned efficiently, affording 4i–4k in high yields with good enantioselectivity. Moreover, allyl substitution was well-tolerated in this desymmetrization methodology (4l).
19 er). Pleasingly, structures with an alkyl group (ethyl, benzyl and cyclohexyl) functioned efficiently, affording 4i–4k in high yields with good enantioselectivity. Moreover, allyl substitution was well-tolerated in this desymmetrization methodology (4l).
Control and deletion studies were carried out in order to probe the mechanistic pathway of our dual-catalytic entry to enantioenriched vinyl-morphan structures. A possible mechanistic pathway could proceed via an alkyne intermediate. To test this hypothesis, alkyne derivative (5a) was synthesized and subjected to the reaction conditions. However, no cyclized product was observed, hence ruling out a pathway via such an intermediate (Scheme 3A). During our preliminary scouting studies, prolinamide catalysts without an N–H bond (e.g. a tertiary amide) were found to be inferior in comparison to their secondary amide counterparts. In order to study this further, a methylated derivative of our optimized catalyst was employed under standard reaction conditions, and complete suppression of reactivity was indeed observed (Scheme 3B). Our protocol was then conducted whilst omitting each of the co-catalysts sequentially. In the absence the copper catalyst, no formation of the cyclized product was observed (Scheme 3C), whilst without the prolinamide catalyst, the morphan structure was still observed, albeit in only 16% yield and with no diastereoselectivity (Scheme 3D). This latter result points to an alternative minor pathway to racemic product presumably via an enol tautomer, which is operating under the reaction conditions. Accordingly, such background reactivity without the prolinamide catalyst is likely responsible for a slight erosion of enantioselectivity observed experimentally vs. that predicted from our computational calculations (vide infra).
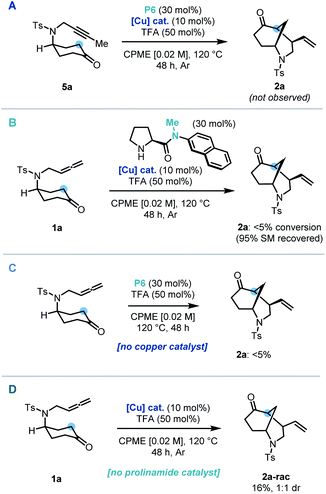 | ||
| Scheme 3 Control experiments: (A) use of an alkyne substrate. (B) Use of methylated catalyst. (C) No copper catalyst. (D) No prolinamide catalyst. | ||
In order to paint a full mechanistic picture, density functional theory analysis of the reaction pathway was performed. All the calculations reported herein were performed using the Amsterdam Density Functional (ADF) software.17 Equilibrium structures and transition structure geometries were optimized using the BLYP functional18,19 and the TZ2P basis set.20 This approach was extensively tested against ab initio reference benchmarks from hierarchical series up until CCSD(T).21 Solvent effects of Et2O (as CPME was not available) were accounted for using the conductor-like screen model (COSMO) of solvation.22 Dispersion interactions were included using Grimme's DFT-D3 correction with Becke–Johnson damping.23 The zeroth-order regular approximation (ZORA) was used to account for scalar relativistic effects.24 This level is referred to as COSMO(Et2O)-ZORA-BLYP-D3(BJ)/TZ2P. All stationary points have been verified, through vibrational analysis, to be minima (zero imaginary frequency) or transition states (one imaginary frequency). The character of the normal mode associated with the imaginary frequency has been analyzed to ensure it resembles the reaction under consideration. Optimized structures were illustrated using CYLview.25 Potential energies were refined by means of single point calculations using the M06 functional.26 This level is denoted COSMO(Et2O)-ZORA-M06/TZ2P//COSMO(Et2O)-ZORA-BLYP-D3(BJ)/TZ2P.
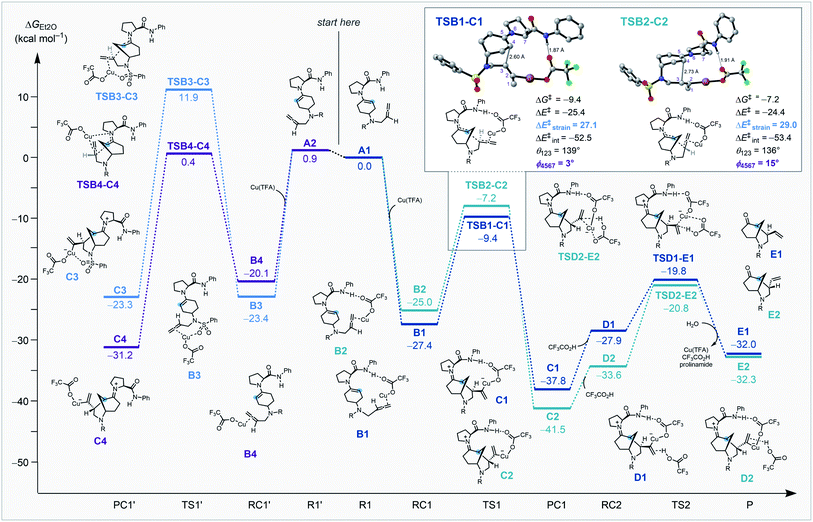
To understand the origin of enantio- and diastereocontrol of the key C–C bond forming step, we quantitatively analyzed the two competing transition structures using the activation strain model (ASM)27 of reactivity. ASM involves decomposing the electronic energy of the transition structure ΔE‡ into the strain ΔE‡strain associated with the structural deformation of the reactants from their equilibrium geometry and the interaction ΔE‡int between the deformed reactants (eqn (1)).27 The ΔE‡strain is determined by the rigidity of the reactants and by the extent to which they must deform to achieve the geometry of the transition structure. The ΔE‡int is usually stabilizing and is related to the electronic structure of the reactants and how they are mutually oriented over the course of the reaction.
| ΔE‡ = ΔE‡strain + ΔE‡int | (1) |
Our DFT studies uncovered that the enantio- and diastereoselectivities are determined during the key nucleophilic attack of an enamine28 to a copper-coordinated allene13 in the intramolecular cyclization step.
Coordination of copper to the allene along with the interaction between the prolinamide and trifluoroacetate (ligated to the copper species) play important roles in the kinetically preferred transition structure associated with selective C–C bond formation. Enantioselectivity is determined by stabilization from the hydrogen bond interaction between the prolinamide N–H bond and the O atom of trifluoroacetate. This stabilizing interaction is only accessible when the copper-coordinated allene is positioned in close proximity to the amide. Diastereoselectivity is determined by the strain energy caused by the larger dihedral angle of the enamine (φ) and the smaller angle of the allene (θ, Scheme 4, inset). Optimal geometries of the enamine and the allene are planar and linear, respectively, and the destabilizing repulsion between the allene moiety and the pyrrolidine ring causes a greater distortion that results in a higher energy barrier.
Reaction of the cyclohexanone substrate and the prolinamide catalyst forms either s-cis enamine A1 or s-transA2, and the small energy difference indicates a rapid equilibrium between these conformations. We hypothesized that the bulky substituent of the amide prevents reactivity from the top-surface of the enamine in each of the structures. The enamines and a Cu(I) species generate metal complexes and this process is highly exergonic. Reactant complexes B1 and B2 benefit from stabilizing hydrogen bonds between the amide N–H bond and an O atom on trifluoroacetate that are absent in B3 and B4. The key C–C bond formation viaTSB1-C1 proceeds with stabilizing hydrogen bonding interactions and no destabilizing steric clash between the prolinamide and allene, and is kinetically preferred by 2.2 kcal mol−1 compared to the next most favorable TSB2-C2. An activation strain analysis was performed on TSB1-C1 and TSB2-C2 to quantitatively understand the factors leading to the difference in computed reactivity and the results are summarized in (Scheme 4, inset). TSB1-C1 goes with a lower energy barrier due to a less destabilizing activation strain (ΔE‡strain = 27.1 kcal mol−1) compared to TSB2-C2 (ΔE‡strain = 29.0 kcal mol−1) that results from a smaller distortion of the enamine and the allene moieties. This step is exergonic by 10.4 kcal mol−1 and has a large Gibbs free energy barrier for the reverse reaction (28.4 kcal mol−1) that is likely irreversible. After the intramolecular cyclization, trifluoroacetic acid coordinates to the alkene of the alkylcopper(I) species C1 to form a complex D1. The protonation process through TSD1-E1 generates a vinyl group, which then regenerates Cu(TFA) and hydrolyzes the enamine to give the cyclized product E1.
Conclusion
In conclusion, a prolinamide and copper(I) catalyzed highly enantio- and diastereoselective cycloisomerization of N/O-tethered allenic cyclohexanones has been demonstrated. This dual catalytic desymmetrization strategy showed broad substrate scope with respect to both the N-tethered and O-tethered substrates, affording a range of 4-vinyl-2-morphan and 4-vinyl-2-oxamorphan derivatives in high yields and enantioselectivity. DFT studies elucidated that the reaction proceeds through a key strain-minimized transition structure containing both prolinamide and copper catalysts, which were linked through a trifluoroacetate bridge, leading to the observed high enantio- and diastereoselectivities. Efforts to apply the findings of this methodology to complex molecule synthesis are ongoing, and the results will be disclosed in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. Z. is supported by the funding from the European Union's Horizon 2020 Research and Innovation Program under the Marie Sklodowska-Curie grant agreement no. 751281-H2020-MSCA-IF-2016. K. Y. thanks the Honjo International Scholarship Foundation for funding. This work was supported by The Netherlands Organization for Scientific Research (NWO). J. A. L. thanks the Leverhulme Trust (RPG-2017-069) for a research fellowship. R. M. thanks the EU commission for IEF (PIEF-GA-2013-627232). We thank Heyao Shi for X-ray structure determination and Dr Amber L. Thompson and Dr Kirsten E. Christensen (Oxford Chemical Crystallography Service) for X-ray mentoring and help.Notes and references
- (a) K. Sakamoto, E. Tsujii, F. Abe, T. Nakanishi, M. Yamashita, N. Shigematsu, S. Izumi and M. Okuhara, J. Antibiot., 1996, 49, 37 CrossRef CAS PubMed; (b) J. Bosch, J. Bonjoch and M. Amat, The Alkaloids, ed. G. A. Cordell, Academic Press, New York, 1996, vol. 48, p. 75 Search PubMed; (c) J. Bonjoch and D. Solé, Chem. Rev., 2000, 100, 3455 CrossRef CAS PubMed; (d) J. Bonjoch and F. Diaba, Stud. Nat. Prod. Chem., 2005, 32, 3 CAS; (e) J. Bonjoch, F. Diaba and B. Bradshaw, Synthesis, 2011, 993 CrossRef CAS; (f) J. S. Cannon and L. E. Overman, Angew. Chem., Int. Ed., 2012, 51, 4288 CrossRef CAS PubMed; (g) A. K. Chattopadhyay and S. Hanessian, Chem. Rev., 2017, 117, 4104 CrossRef CAS PubMed.
- (a) A. Rizzo, R. J. Mayer and D. Trauner, J. Org. Chem., 2019, 84, 1162 CrossRef CAS PubMed; (b) A. Rizzo and D. Trauner, Org. Lett., 2018, 20, 1841 CrossRef CAS PubMed; (c) Y.-L. Wang, Y.-S. Ye, W.-W. Fu, R. Wu, Q. Xiang, Y.-Z. Lao, J.-L. Yang, H.-S. Tan, X.-W. Yang, B.-C. Yang, H.-X. Xu and G. Xu, Org. Lett., 2019, 21, 1534 CrossRef CAS PubMed.
- C.-Y. Gan, Y.-Y. Low, T. Etoh, M. Hayashi, K. Komiyama and T.-S. Kam, J. Nat. Prod., 2009, 72, 2098 CrossRef CAS PubMed.
- (a) W. He, P. Wang, J. Chen and W. Xie, Org. Biomol. Chem., 2020, 18, 1046 RSC; (b) G. Sirasani and R. B. Andrade, Org. Lett., 2011, 13, 4736 CrossRef CAS PubMed.
- For selected reviews, see: (a) X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou and J. Zhou, Chem. Rev., 2016, 116, 7330 CrossRef CAS PubMed; (b) A. Borissov, T. Q. Davies, S. R. Ellis, T. A. Fleming, M. S. W. Richardson and D. J. Dixon, Chem. Soc. Rev., 2016, 45, 5474 RSC.
- (a) R.-R. Liu, B.-L. Li, J. Lu, C. Shen, J.-R. Gao and Y.-X. Jia, J. Am. Chem. Soc., 2016, 138, 5198 CrossRef CAS PubMed; (b) B.-L. Li, W.-Y. Gao, H. Li, S.-Q. Zhang, X.-Q. Han, J. Lu, R.-X. Liang, X. Hong and Y.-X. Jia, Chin. J. Chem., 2019, 37, 63 CrossRef CAS.
- M. Wang, J. Chen, Z.-J. Chen, C. Zhong and P. Lu, Angew. Chem., Int. Ed., 2018, 57, 2707 CrossRef CAS PubMed.
- X. Huang, W. R. J. J. Oh and J. S. Zhou, Angew. Chem., Int. Ed., 2018, 57, 7673 CrossRef CAS PubMed.
- For examples, see: (a) S.-Z. Luo, L. Zhang, X. Mi, Y. Qiao and J.-P. Cheng, J. Org. Chem., 2007, 72, 9350 CrossRef CAS PubMed; (b) P. Kwiatkowski, T. D. Beeson, J. C. Conrad and D. W. C. MacMillan, J. Am. Chem. Soc., 2011, 133, 1738 CrossRef CAS; (c) A. D. Gammack Yamagata, S. Datta, K. E. Jackson, L. Stegbauer, R. S. Paton and D. J. Dixon, Angew. Chem., Int. Ed., 2015, 54, 4899 CrossRef CAS PubMed; (d) Y. Li, S. Yang, G. Wen, Q. Lin, G. Zhang, L. Qiu, X. Zhang, G. Du and X. Fang, J. Org. Chem., 2016, 81, 2763 CrossRef CAS PubMed; (e) C. Zhu, D. Wang, Y. Zhao, W.-Y. Sun and Z. Shi, J. Am. Chem. Soc., 2017, 139, 16486 CrossRef CAS PubMed; (f) T. Shu, L. Zhao, S. Li, X.-Y. Chen, C. von Essen, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2018, 57, 10985 CrossRef CAS PubMed; (g) Y. Xu, Q. Sun, T.-D. Tan, M.-Y. Yang, P. Yuan, S.-Q. Wu, X. Lu, X. Hong and L.-W. Ye, Angew. Chem., Int. Ed., 2019, 58, 16252 CrossRef CAS PubMed.
- R. Manzano, S. Datta, R. S. Paton and D. J. Dixon, Angew. Chem., Int. Ed., 2017, 56, 5834 CrossRef CAS PubMed.
- (a) P. Zheng, C. Wang, Y.-C. Chen and G. Dong, ACS Catal., 2019, 9, 5515 CrossRef CAS; (b) When using a (−)-DIOP as ligand, 11% ee was achieved.
- (a) M. Li, S. Datta, D. M. Barber and D. J. Dixon, Org. Lett., 2012, 14, 6350 CrossRef CAS PubMed; (b) T. L. N. Nguyen, C. A. Incerti-Pradillos, W. Lewis and H. W. Lam, Chem. Commun., 2018, 54, 5622 RSC; (c) X. Zhou and G. Dong, Angew. Chem., Int. Ed., 2016, 55, 15091 CrossRef CAS PubMed; (d) R. Di Sanza, T. L. N. Nguyen, N. Iqbal, S. P. Argent, W. Lewis and H. W. Lam, Chem. Sci., 2020, 11, 2401 RSC.
- For reports on allene activation using copper see: (a) B. J. Casavant, Z. M. Khoder, I. A. Berhane and S. R. Chemler, Org. Lett., 2015, 17, 5958 CrossRef CAS PubMed; (b) R. Blieck, R. A. A. Abdine, M. Taillefer and F. Monnier, Org. Lett., 2018, 20, 2232 CrossRef CAS PubMed; (c) R. Y. Liu, Y. Zhou, Y. Yang and S. L. Buchwald, J. Am. Chem. Soc., 2019, 141, 2251 CrossRef CAS PubMed; (d) W. Zhao and J. Montgomery, J. Am. Chem. Soc., 2016, 138, 9763 CrossRef CAS PubMed.
- CCDC 2000237 (2g) contain the supplementary crystallographic data for this paper. The system is numbered per IUPAC conventions, see ESI† for full numbering.
- For methodologies to access racemic oxamorphan scaffolds, see: (a) Y. Gu, P. Hu, C. Ni and X. Tong, J. Am. Chem. Soc., 2015, 137, 6400 CrossRef CAS PubMed; (b) D. Huang, S. M. Szewczyk, P. Zhang and T. R. Newhouse, J. Am. Chem. Soc., 2019, 141, 5669 CrossRef CAS PubMed; (c) M. Yoshida, M. Shibata, S. Mukae, K. Kinoshita, K. Matsumoto and T. Hirokane, Tetrahedron Lett., 2019, 60, 151262 CrossRef CAS; (d) S.-Y. Yu, Z.-L. Hu, H. Zhang, S. Wang and Z.-J. Yao, Tetrahedron Lett., 2012, 53, 2765 CrossRef CAS.
- Attempted preparation of allene S6b under the standard conditions resulted in competitive formation of cycloisomerised product S6c, which was isolated as a racemate (see supporting information, Scheme S2†).
- (a) G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS; (b) C. Fonseca Guerra, J. G. Snijders, G. te Velde and E. J. Baerends, Theor. Chem. Acc., 1998, 99, 391 Search PubMed; (c) ADF 2018.105, SCM Theoretical Chemistry, Vrije Universiteit Amsterdam, The Netherlands,http://www.scm.com Search PubMed.
- (a) J. C. Slater, Quantum Theory of Molecules and Solids, McGraw-Hill, New York, 1974 Search PubMed; (b) A. D. Becke, J. Chem. Phys., 1986, 84, 4524 CrossRef CAS; (c) A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- E. van Lenthe and E. J. Baerends, J. Comput. Chem., 2003, 24, 1142 CrossRef CAS PubMed.
- (a) G. T. de Jong, M. Solà, L. Visscher and F. M. Bickelhaupt, J. Chem. Phys., 2004, 121, 9982 CrossRef CAS PubMed; (b) G. T. de Jong and F. M. Bickelhaupt, J. Phys. Chem. A, 2005, 109, 9685 CrossRef CAS PubMed; (c) G. T. de Jong and F. M. Bickelhaupt, J. Chem. Theory Comput., 2006, 2, 322 CrossRef CAS PubMed.
- (a) A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799 RSC; (b) A. Klamt, J. Phys. Chem., 1995, 99, 2224 CrossRef CAS; (c) A. Klamt and V. Jonas, J. Chem. Phys., 1996, 105, 9972 CrossRef CAS; (d) C. C. Pye and T. Ziegler, Theor. Chem. Acc., 1999, 101, 396 Search PubMed.
- (a) S. Grimme, J. Antony, S. Ehrlich and S. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed; (b) A. D. Becke and E. R. Johnson, J. Chem. Phys., 2005, 123, 154101 CrossRef PubMed.
- (a) E. van Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1993, 99, 4597 CrossRef CAS; (b) E. van Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1994, 101, 9783 CrossRef CAS.
- C. Y. Legault, CYLview, 1.0b, Unversité de Sherbrooke, Sherbrooke, QC, Canada, 2009, http://www.cylview.org Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 Search PubMed.
- (a) P. Vermeeren, S. C. C. van der Lubbe, C. Fonseca Guerra, F. M. Bickelhaupt and T. A. Hamlin, Nat. Protoc., 2020, 15, 649 Search PubMed; (b) F. M. Bickelhaupt and K. N. Houk, Angew. Chem., Int. Ed., 2017, 56, 10070 CrossRef CAS PubMed; (c) L. P. Wolters and F. M. Bickelhaupt, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2015, 5, 324 CAS; (d) I. Fernández and F. M. Bickelhaupt, Chem. Soc. Rev., 2014, 43, 4953 RSC; (e) W.-J. van Zeist and F. M. Bickelhaupt, Org. Biomol. Chem., 2010, 8, 3118 RSC.
- For reviews/reports on mechanism in enamine catalysis see: (a) B. List, Acc. Chem. Res., 2004, 37, 548 CrossRef CAS PubMed; (b) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS PubMed; (c) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC; (d) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337 RSC; (e) J. Burés, A. Armstrong and D. G. Blackmond, Acc. Chem. Res., 2016, 49, 214 CrossRef PubMed; (f) S. Afewerki and A. Córdova, Chem. Rev., 2016, 116, 13512 CrossRef CAS PubMed; (g) J. Burés, A. Armstrong and D. G. Blackmond, Chem. Sci., 2012, 3, 1273 RSC; (h) T. Schnitzer, J. S. Möhler and H. Wennemers, Chem. Sci., 2020, 11, 1943 RSC; (i) F. R. Clemente and K. N. Houk, Angew. Chem., Int. Ed., 2004, 43, 5766 CrossRef CAS PubMed; (j) K. S. Halskov, B. S. Donslund, B. M. Paz and K. A. Jørgensen, Acc. Chem. Res., 2016, 49, 974 CrossRef CAS PubMed; (k) M. Arno and L. R. Domingo, Theor. Chem. Acc., 2002, 108, 232 Search PubMed; (l) U. Grošelj, D. Seebach, M. Badine, W. B. Schweizer, A. K. Beck, I. Krossing, P. Klose, Y. Hayashi and T. Uchimaru, Helv. Chim. Acta, 2009, 92, 1225 CrossRef; (m) J. D. Porter, E. Greve, A. Alsafran, A. R. Benoit, S. V. Lindeman and C. Dockendorff, Tetrahedron, 2018, 74, 4823 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2000237. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc02878a |
| This journal is © The Royal Society of Chemistry 2020 |