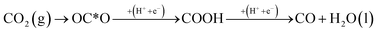Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Why do RuO2 electrodes catalyze electrochemical CO2 reduction to methanol rather than methane or perhaps neither of those?†
Ebrahim
Tayyebi
 a,
Javed
Hussain
a,
Javed
Hussain
 a and
Egill
Skúlason
a and
Egill
Skúlason
 *ab
*ab
aScience Institute, University of Iceland, VR-III, 107 Reykjavík, Iceland. E-mail: egillsk@hi.is
bFaculty of Industrial Engineering, Mechanical Engineering and Computer Science, University of Iceland, VR-III, 107 Reykjavík, Iceland
First published on 30th July 2020
Abstract
The electrochemical CO2 reduction reaction (CO2RR) on RuO2 and RuO2-based electrodes has been shown experimentally to produce high yields of methanol, formic acid and/or hydrogen while methane formation is not detected. This CO2RR selectivity on RuO2 is in stark contrast to copper metal electrodes that produce methane and hydrogen in the highest yields whereas methanol is only formed in trace amounts. Density functional theory calculations on RuO2(110) where only adsorption free energies of intermediate species are considered, i.e. solvent effects and energy barriers are not included, predict however, that the overpotential and the potential limiting step for both methanol and methane are the same. In this work, we use both ab initio molecular dynamics simulations at room temperature and total energy calculations to improve the model system and methodology by including both explicit solvation effects and calculations of proton–electron transfer energy barriers to elucidate the reaction mechanism towards several CO2RR products: methanol, methane, formic acid, CO and methanediol, as well as for the competing H2 evolution. We observe a significant difference in energy barriers towards methane and methanol, where a substantially larger energy barrier is calculated towards methane formation than towards methanol formation, explaining why methanol has been detected experimentally but not methane. Furthermore, the calculations show why RuO2 also catalyzes the CO2RR towards formic acid and not CO(g) and methanediol, in agreement with experimental results. However, our calculations predict RuO2 to be much more selective towards H2 formation than for the CO2RR at any applied potential. Only when a large overpotential of around −1 V is applied, can both formic acid and methanol be evolved, but low faradaic efficiency is predicted because of the more facile H2 formation.
Introduction
The electrochemical CO2 reduction reaction (CO2RR) has received a lot of attention recently, as it can be a technology to convert carbon dioxide to particular alcohols and hydrocarbons.1–14 The CO2 gas can be captured from both industrial point sources as well as from the atmosphere which would mitigate the rising levels of anthropomorphic CO2 emissions. Electrochemical processes are an appealing approach since the electricity can come from renewable sources (such as wind or solar energy) to make synthetic fuels for the existing infrastructure that uses fuels from fossil fuel sources. In this application, selective methanol formation would be highly desirable since it can be used directly as a liquid fuel.The electrochemical CO2RR catalyzed by metallic electrodes has been studied extensively in recent years. Insight into the mechanisms or the reaction pathways for reducing CO2 to different products including HCOOH, CO, CH3OH, CH4, C2H4, C2H5OH etc., has been obtained by both theoretical calculations15–25 and experimental work.7,9 Therein, it has been shown that previous theoretical studies using a thermochemical approach26 where only the adsorption free energies of intermediate species are included, are quite successful in predicting experimental overpotentials (or onset potentials).14,16 However, energy barriers of proton–electron transfer steps are required to elucidate the reaction mechanism and reaction pathways and capture the trends in product distribution as a function of applied potential seen in the experiments on pure metals.21,23
As a solvent, water usually plays an important role in various electrocatalytic reactions such as the CO2RR. For electrocatalytic reactions carried out in water, including the effect of solvation of reactants, intermediates, and products at the surface may be essential as the mechanism may be different.27–30 However, density functional theory (DFT) calculations reveal that modeling solid–liquid interfaces is complex and requires efficient functionals describing hydrogen bonding interactions.31–34 Despite this complexity, several groups have developed reasonable solid–liquid interface models suitable to study electrochemical processes where some electrochemical phenomena can be explained, e.g. catalytic trends, rates, reaction pathways and mechanisms.23–25,35–44
A crucial step towards a rational design of new catalysts that are selective and efficient in reducing CO2 to specific hydrocarbons and alcohols is to determine the detailed reaction mechanism for the process. To accomplish this, a detailed description of the electrochemical solid–liquid interface is required. Recently, it has been observed that a detailed description of the electrochemical solid–liquid interface model is successful in capturing the experimental trends of product distribution for the CO2RR as a function of applied potential on pure metals23 whereas the more approximated thermochemical model (TCM) and the implicit computational hydrogen electrode (CHE)26 cannot capture these trends, but do capture the overpotentials for the CO2RR and other electrochemical reactions quite well.14–16,45,46 The reason for this achievement is that the molecular level structure of water on noble transition metals is qualitatively well known and therefore realistic model systems can be used. Unfortunately, for transition metal oxide (TMO) surfaces, the molecular scale structure of water is still controversial, and therefore, realistic model systems have not been fully developed yet. However, it has been shown that TMO surfaces strongly chemisorb molecular water via interaction between the lone pair of the oxygen atom in the water molecule and the 5-fold coordinately unsaturated site (CUS)28,29,47 while on noble metal surfaces, a water bilayer is usually physisorbed on top of the metal surface.23,33,34,37,40 The interaction of water with a RuO2(110) surface has been studied experimentally by using high resolution electron energy loss spectroscopy (HREELS) and thermal desorption spectroscopy (TDS).28 Using HREELS, it was shown that H2O molecules chemisorb on Ru CUS sites through the oxygen atom of the water molecules. From HREELS and TDS results it was also found that water dissociation does not occur on the perfect RuO2(110) surface while a small amount of water dissociation observed in HREELS is due to some vacant Ru bridge sites.
Copper has been shown to be the only pure metal electrode catalyzing the CO2RR to hydrocarbons and alcohols where 15 carbon-containing products have been detected and where methane is the major product at high overpotentials (>40%).9,48 Other pure metal electrodes form H2(g), CO(g) or HCOOH(aq) as major products. For all these pure metal electrodes, methanol has been detected but in very low yields (<0.1%). Transition metal oxide surfaces, however, have been shown to catalyze the CO2RR to methanol in high yields (2–76%) as well as to formic acid (1–78%), depending on the applied potential and other reaction conditions, where RuO2-based electrodes have been studied the most.49–53 Methane, CO and methanediol have not been detected, except in one study where trace amounts of methane and CO were reported.52
Recently, RuO2 has been revisited experimentally for the CO2RR by Mezzavilla et al.54 where hydrogen gas (via the hydrogen evolution reaction, HER) was reported to be the main product at all applied potentials. This contradicts the previous experimental studies49–53 where methanol and/or formic acid were observed to be the major products. At very high overpotentials (−0.75 and −0.9 V vs. RHE), some trace amounts of formate and CO were observed. By alloying RuO2 with Ti, Cu or Sn, the faradaic efficiency of formate and CO increased to around 20% and 10%, respectively, at high overpotentials (−0.75 and −1 V vs. RHE). Furthermore, they show that even though RuO2 is not an active catalyst for the CO2RR to methanol, adsorbed CO is detected on the surface, when either CO or CO2 are introduced into the electrolyte. To the best of our knowledge, all experimental results on RuO2 and RuO2-based electrodes are summarized in Table S1 in the ESI.†
Reaction pathways have been derived from DFT calculations using the TCM and CHE models for the CO2RR on RuO2 towards formic acid, methanediol, methanol and methane and are summarized in Table 1.55–59 Methane and methanol are predicted to go through the same reaction pathway until the OCH3 intermediate is formed. The next hydrogenation results in either methanol or methane formation, but in the latter process the adsorbed oxygen atom is reduced further to water to complete the catalytic cycle. The same reaction step is predicted to be the potential limiting step (PLS) for both products, or the OCHO to HCOOH step.55–59 However, other elementary steps such as protonation of OCH3 and OH removal to form water need similar applied potentials according to thermodynamics.
| Number of H+ + e− steps | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pathways | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Formic acid | OCHO | HCOOH(aq) | ||||||
| Methanediol | OCHO | HCOOH | H2COOH | H2C(OH)2(aq) | ||||
| Methanol | OCHO | HCOOH | H2COOH | CH3O + OH | CH3O + H2O(l) | CH3OH(aq) | ||
| Methane | OCHO | HCOOH | H2COOH | CH3O + OH | CH3O + H2O(l) | CH4(g) + O | OH | H2O(l) |
As described above, high yields of hydrogen,54 formic acid or methanol49–53 are detected experimentally whereas methane formation has not been reported in any studies. Clearly, the results from simple thermodynamic models (TCM and CHE) do not capture the experimental observations of these products. Therefore, a more detailed model is required, including explicit solvent effects and calculations of the proton–electron transfer energy barriers for all possible reaction steps. Within this study, the energy barriers for the HER and CO2RR are calculated towards various products using the (110) facet of RuO2 as the model system using one layer of co-adsorbed water to elucidate the reaction mechanisms to form hydrogen, methanol, methane, formic acid, methanediol and CO.
Model systems and computational details
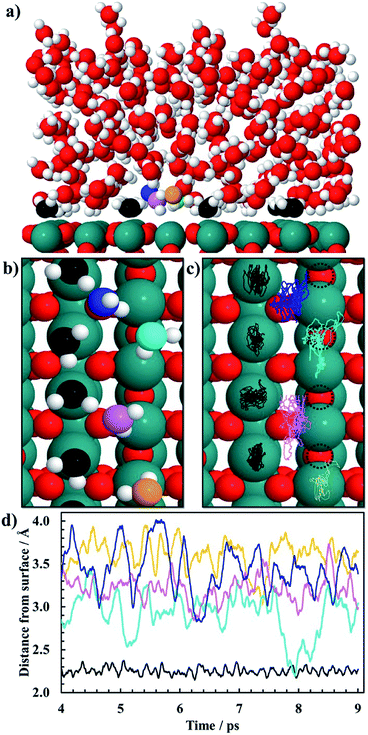
Ab initio molecular dynamics (AIMD) simulations are carried out for the (110) facet of RuO2 in reduced form with five layers of water above the surface (Fig. 1a). Born–Oppenheimer AIMD simulations are carried out using the freely available program package CP2K/Quickstep.60,61 The density functional implementation in Quickstep is based on a hybrid Gaussian plane wave (GPW) scheme. Orbitals are described by an atom-centered Gaussian-type basis set, while an auxiliary plane wave basis is used to re-expand the electron density.62 Analytic Goedecker–Teter–Hutter (GTH) pseudopotentials are employed to represent the core electrons.63,64 The basis sets for the valence electrons consist of short-ranged (less diffuse) double-ζ basis functions with one set of polarization functions (DZVP).65 The plane wave basis for the electron density is cut off at 400 Ry while the plane wave cut off of a reference grid covered by a Gaussian is 60 Ry. All our AIMD simulations only use the Γ point of the supercell for expansion of the orbitals. The GGA–PBE functional is utilized for all AIMD calculations.66,67 The Grimme's D3 method is also used to include van der Waals interactions of water–water, water–Ru and water–surface oxygen.68 The slab consists of three atomic layers with 16 metal atoms and 32 oxygen atoms in each layer, and the exposed liquid phase is presented by 84 H2O molecules. The simulation box is 44 Å along the z-axis with a vacuum of 20 Å. The system is subject to periodic boundary conditions in all directions. Atoms in the slab along with the water molecules are allowed to reconstruct during the AIMD simulation. The simulations are performed using the Verlet algorithm with a time step of 1 fs at a temperature of 300 K within the canonical ensemble for a total simulation period of 9 ps. Generalized Langevin equation thermostats are used to enhance molecular dynamics simulations.69,70 The convergence of the vertical energy gap can be monitored by the time accumulative averages, as shown in Fig. S1.† Since we perform the simulation at coverage significantly close to unity, which corresponds to bulk water in contact with the RuO2 surface, a specific well-defined geometry is observed in the simulation during a 9 ps AIMD run of the system with 84 H2O molecules at room temperature. A snapshot of the equilibrated system is shown in Fig. 1a where chemisorbed water molecules decorate the CUS Ru sites (oxygen atoms of those water molecules are black).To estimate the free energy of an adsorbate in the CO2RR and HER, static geometry optimization calculations are done using a plane-wave based pseudopotential formalism with a generalized gradient approximation (GGA) to describe the exchange–correlation effects within the BEEF–vdW functional71 implemented in the periodic DFT package VASP.72,73 All these calculations are done using a model system shown in Fig. 2 which will be further elaborated below. A plane wave basis set with a cutoff energy of 350 eV is utilized to expand the valence electron orbitals and the PAW method is used to represent the core electrons.74 In two separate studies, similar calculations for the CO2RR on RuO2(110) were conducted using two different cutoff values (one 350 eV (ref. 59) and the other 500 eV (ref. 56)) and they obtained similar results. Therefore, this indicates that a cutoff energy of 350 eV is accurate enough for the current study. The RuO2(110) slab consists of four atomic layers with four metal atoms and eight oxygen atoms in each layer. The system is subject to periodic boundary conditions in all directions. Dipole correction is applied to decouple the electrostatic interaction between the periodically repeated slabs. Atoms in the bottom two layers are fixed while the atoms in the top two layers along with adsorbed intermediates are allowed to relax during optimization. The atomic structures are determined using a 4 × 4 × 1 Monkhorst–Pack mesh for Brillouin-zone sampling until the energies are converged to within 10−5 eV and atomic forces drop below 0.03 eV Å−1. The climbing image nudged elastic band (CI-NEB)75,76 method is used to calculate activation energies for the HER and CO2RR towards different intermediates and products on the RuO2(110) surface.
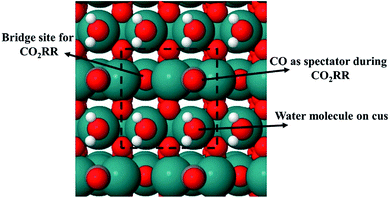
In order to calculate the activation energy for proton–electron transfer of different intermediates for the CO2RR and HER on RuO2 with an explicit description of water, we consider only two water molecules (one monolayer, ML) on the 5-fold CUS metal site of RuO2 (Fig. 2). Siahrostami and Vojvodic have shown this model to be sufficient to capture the electrostatic contributions from water in the case of the oxygen evolution reaction on RuO2, IrO2 and TiO2.44 Furthermore, we justify the choice of that model system with AIMD simulations at room temperature in Fig. 1 and we apply this model when calculating activation energies towards different intermediates and products in the CO2RR and HER on the RuO2(110) electrode. When a proton–electron transfer has been carried out from one of the co-adsorbed water molecules to reduce H or CO2RR intermediates, an OH group is formed and adsorbed on the CUS site. This OH group should be easily protonated from the reservoir of protons in the bulk solution to regenerate co-adsorbed water molecules on the surface needed for the subsequent reduction steps.77 We do not calculate the energy barriers to regenerate the co-adsorbed water molecules, but we assume that the co-adsorbed water is in equilibrium with adsorbed OH and solvated proton in the bulk of electrolyte solution. We therefore only calculate the thermodynamics of the reaction:
| A + OH + ½H2(g) → A + H2O |
Previously, the concept of a CO spectator species in the CO2RR on RuO2(110) was introduced by Karamad et al.55 and later used by Bhowmik et al.56–58 and Tayyebi et al.59 Therefore it is assumed that the CO2RR takes place on a bridge site in the presence of a CO spectator which is located on every other bridge site as shown in Fig. 2. The source of adsorbed CO on the surface may therefore come from the reaction:
In the Results section below we indeed show that a low CO coverage will be formed on the RuO2(110) electrode, justifying this model system and the role of CO as a spectator species during the CO2RR.
Results
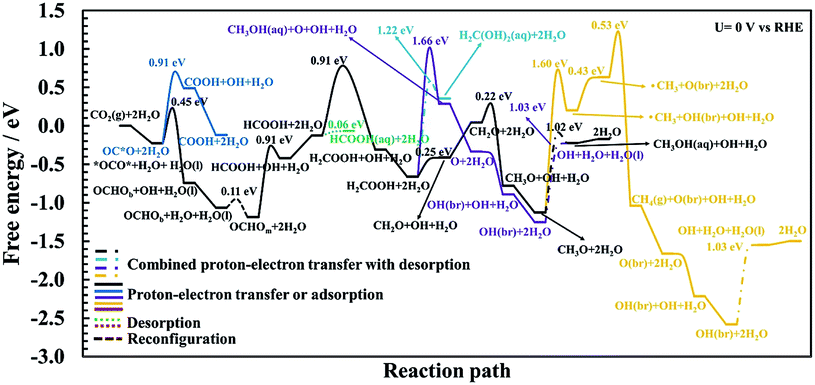
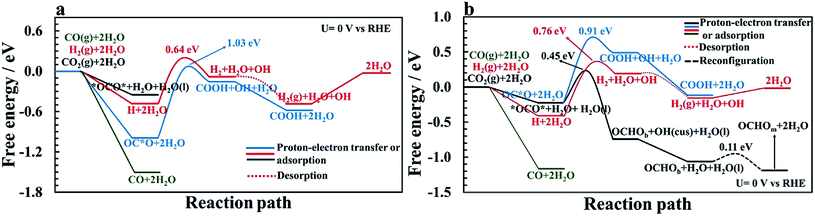
AIMD simulations of equilibrated structures of 84 water molecules above the RuO2(110) surface are shown in Fig. 1. Snapshots of the molecular structure are shown in Fig. 1a and b, where chemisorbed water molecules decorate the CUS Ru sites (black oxygen atoms). These results are in very good agreement with the HREELS and TDS studies for adsorption of water on RuO2(110).28 To obtain some further insight into the movement of the water molecules closest to the RuO2(110) surface, trajectories of their oxygen atoms in the xy-plane and the z-direction are plotted in Fig. 1c and d, respectively. Fig. 1c shows that the water molecules on CUS Ru atoms stay relatively localized and remain close to their initial positions, although they exhibit some small fluctuations in the xy-plane. They are also found to be rather localized in the z-direction at around 2.25 ± 0.1 Å from the surface (black trajectory in Fig. 1d). The water molecules in the bilayer above the bridge sites of the RuO2(110) surface become, however, much more disordered and delocalized in the xy-plane as shown with the colored trajectories in Fig. 1c. They are also highly mobile in the z-direction and located between 2.5 and 4 Å above the surface where each molecule fluctuates by around 1 Å (colored trajectories in Fig. 1d). This shows a very weak interaction of the water molecules above the bridge Ru sites, leaving them empty to catalyze the CO2RR. These results support the choice of the reduced model system (Fig. 2) used to calculate the various elementary steps needed to obtain insight into the reaction mechanism.Proton–electron transfer reaction barriers are calculated for the CO2RR to CO(g), HCOOH(aq), H2C(OH)2(aq), CH3OH(aq) and CH4(g) on RuO2(110) and compared with that of the competing H2(g) formation. Free energy reaction pathways along with energy barriers, Ea, are presented in Fig. 3 and 4 for the CO2RR and in Fig. 5 for the HER at U = 0 V vs. RHE, for a range of proton–electron transfer steps, reconfiguration steps and desorption steps. The minimum energy paths (MEPs) along with configurations of the initial states, saddle points and final states for a range of possible intermediates related to the CO2RR and HER on RuO2(110) can be found in the ESI.†
 | ||
| Fig. 5 Free energy diagrams and energy barriers for hydrogen evolution reaction pathways on RuO2(110) at U = 0 V vs. RHE (a) without CO as a spectator and (b) with CO as a spectator (25% CO coverage). The first couple of reaction steps of the CO2RR (from Fig. 3 and 4) are included for comparison of these competing reactions. Adsorption free energy of CO(g) is also included for comparison. | ||
In the main part of this paper it is assumed that CO2 is reduced on a bridge site of the surface in the presence of adsorbed CO as a spectator species which is located on every other bridge site, but water molecules are adsorbed on all CUS sites forming one ML of water coverage as explained above (Fig. 2). To start with in this paper, however, we do more detailed calculations than have been done so far in order to show that adsorbed CO is indeed a spectator species in the CO2RR where it neither desorbs from the surface nor reduces further to adsorbed COH or CHO species (Fig. 3). Thereafter, a low CO coverage is used as a model system to investigate the reaction pathways of the CO2RR towards formic acid, methanediol, methanol and methane on RuO2(110) in more detail (Fig. 4).
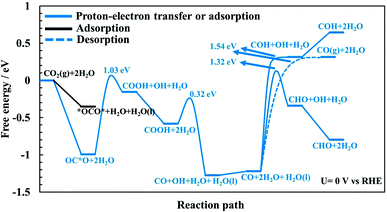
CO formation on RuO2(110): why CO is a spectator species
The CO2RR is conducted on the RuO2(110) surface with 1 ML of adsorbed H2O molecules on the CUS Ru sites but without any adsorbed CO molecules. This represents the initial state of the surface we may expect at the time when CO2 is introduced in the system of the experiments. The free energies of the intermediates from CO2 to CO(g), CHO and COH are shown in Fig. 3 along with energy barriers of the elementary steps. We find that the way CO2 binds to the surface can change the reaction mechanism towards different CO2RR products but that is also discussed further below in relation with Fig. 4 (when we have the CO spectator species). Under these initial conditions, we obtain two different configurations for adsorption of CO2 on the RuO2(110) surface. CO2 can either bind where the carbon atom (OC*O) binds to a bridge site (Fig. S2a†) or where the two oxygen atoms (*OCO*) bind bidentate to a bridge site and the adjacent CUS site where one of the water molecule desorbs from the surface (Fig. S2b†). OC*O is found to be a more stable intermediate compared to *OCO* (see Fig. 3). Therefore, the next step in the CO2RR is to transfer a proton to the oxygen atom in OC*O to form COOH, and then further reduction of COOH leads to the formation of adsorbed CO on the bridge site. The highest energy barrier in the CO2RR to form adsorbed CO is 1.03 eV at 0 V vs. RHE (Fig. 3). Since our results predict that methanol formation needs an overpotential of around −1 V vs. RHE (discussed below) this energy barrier will become around 0.5 eV at that potential assuming a transfer coefficient of 0.5. Such a negative applied potential increases the chance of building up some CO coverages on the surface. This is supported in the recent experiments by Mezzavilla et al.54 where both adsorbed CO2 and CO were detected on the RuO2(110) surface at large negative potentials where it is concluded that adsorbed CO acts solely as a spectator species in the CO2RR.54 These detailed calculations, therefore, support earlier assumptions that adsorbed CO molecules may result from reduced CO2 molecules during the CO2RR experiment. There it was also shown that 25% CO coverage reproduces appropriate overpotentials for methanol formation on RuO2(110).55–59In all of the calculation done so far for the CO2RR on RuO2(110) using the TCM model, further desorption or reduction of adsorbed CO is assumed to be slow compared to the main pathway of the CO2RR. Therefore, CO2 will be reduced all the way to e.g. methanol while CO remains on the surface as a spectator.55–59 Here we justify this assumption by calculating the energy barriers for desorption of CO to CO(g) (Fig. S6†) as well as for proton–electron transfer to CO to form either COH (Fig. S7†) or CHO (Fig. S8†) intermediates. Fig. 3 shows significant barriers for all these reaction steps (1.32–1.44 eV) predicting slow kinetics for further CO desorption/reduction which shows the validity of the assumption that has been made before in TCM simulations for the CO2RR on RuO2(110).
Reaction pathways of the CO2RR on RuO2(110) towards formic acid, methanediol, methanol and methane
The first proton–electron transfer step can either lead to OCHOb or COOH, depending on which intermediate gets protonated, *OCO* or O*CO, respectively. Here we have distinguished the bidentate configuration (OCHOb) where it is bonded through two oxygen atoms to a bridge site and the closest CUS site from the monodentate configuration (OCHOm) where it is bonded through only one of the oxygen atoms, which is discussed further below. Our calculated energy barriers in Fig. 4 show that the required barrier for protonation of *OCO* to OCHOb is 0.45 eV (Fig. S9†) while for OC*O to COOH is estimated to be 0.91 eV (Fig. S4†). Therefore, OCHOb is predicted to form on RuO2(110) instead of COOH in the presence of the CO spectator species. Here it is noted that *OCO* and OCHOb are the only intermediates that bind to the surface in a bidentate form. In both cases, one of the co-adsorbed water molecules needs to be moved approximately 1.3 Å above the CUS site to provide an empty site for those species to bind to the surface. In fact, we find that the only feasible way to transfer a proton to the carbon atom in CO2 is that if it is adsorbed on the surface in a bidentate form (see Fig. S2b and S9†). When OCHOb is formed, the next step is to rearrange OCHOb to OCHOm (Fig. S10†) to continue the reduction further. This is a monodentate adsorbate that binds through only one oxygen atom to the bridge site of the surface and the two water molecules are chemisorbed to the CUS sites (see final state in Fig. S10† or initial state in Fig. S11†). The energy barrier for this reconfiguration is very low (0.11 eV) and this step is slightly downhill in free energy. This rearrangement will therefore be easily overcome at room temperature.
In order to investigate methane formation, several possible pathways and mechanisms are considered. The mechanism with the lowest overall barrier is considered first but alternative mechanisms are explored below. The protonation of adsorbed CH3O may also result in adsorbed CH3OH and subsequent cleavage of the O–C bond in HO–CH3, resulting in a planar ˙CH3 radical desorbed slightly from the surface and leaving an OH species adsorbed at the bridge site on the surface, OH(br). This mechanism has a significantly higher barrier than when directly desorbing methanol as discussed above, with an energy barrier of 1.60 eV, and a considerably large additional barrier above the thermodynamics (yellow curve in Fig. 4 and S21†). The subsequent reduction of this ˙CH3 radical to desorbed CH4(g) has also a significantly large overall barrier as described further here. After the planar ˙CH3 radical is formed, the hydrogen atom in the adsorbed OH(br) intermediate is transferred back to adsorbed OH on the CUS site, forming co-adsorbed water on the surface (yellow curve in Fig. 4 and S22†). The additional barrier is insignificant, but the thermochemical barrier is uphill by 0.43 eV. This co-adsorbed water then transfers a proton–electron to the ˙CH3 radical to form methane. The additional barrier is moderately high or 0.53 eV (Fig. S23†) but the overall barrier for this mechanism is large, or around 2.3 eV (yellow curve in Fig. 4). The difference in the overall barrier height between methanol formation (1.02 eV) and methane formation via this mechanism (2.3 eV) results in a large difference in selectivity towards these products. This difference in energy barriers between these two products corresponds to around a 1022 order of magnitude difference in rates at U = 0 V and under standard conditions.23
Since the mechanism of methane formation is a crucial step in the overall work and the mechanism presented above resulted in an overall high energy barrier it requires further investigation. The proton transfer can also be to the carbon atom of the O–CH3 admolecule to form a desorbed CH4(g) molecule directly (not shown in Fig. 4, but shown Fig. S20†). The calculation of the minimum energy pathway for this mechanism results in the O–C bond in the adsorbed O–CH3 being broken first, resulting in this same planar ˙CH3 radical above the surface which is then protonated to form methane. The calculated activation energy for this combined mechanism is 2.77 eV, with a large additional barrier of more than 2 eV. Comparing the overall barrier of 2.77 eV for this mechanism with the one presented above (2.3 eV) shows that a lower barrier can be achieved by forming first methanol and then breaking the O–C bond before the ˙CH3 radical is reduced. However, in both cases, an insignificant rate of methane formation is predicted at room temperature compared to the rate of methanol formation.
Because in both mechanisms presented above for methane formation a ˙CH3 radical is first formed before it can be reduced to a CH4(g) molecule, yet another alternative mechanism is considered. Here we consider whether a water molecule in the first bilayer can assist in this reaction. The proton is shuttled from the co-adsorbed water to this additional water molecule via a Grotthuss mechanism and transferred to the methyl group of the O–CH3 admolecule to form methane directly. A mechanism like this would bypass forming the ˙CH3 radical first and could lower the overall barrier because this concerted mechanism should weaken the O–CH3 bond. During simulation of this reaction path, however, a proton–electron transfer from the co-adsorbed water to the oxygen atom of the CH3O admolecule results in an initial formation of a methanol molecule that desorbs from the surface instead of favoring the initially set reaction path. Therefore, it rather favors the mechanism presented initially having a barrier around 2.3 eV and the one included in Fig. 4. This shows that the proton–electron transfer is carried out with the co-adsorbed water rather than from a water molecule in the first bilayer.
The high barriers for methane formation presented above provide insight and reasoning as to why methane is not detected experimentally despite the fact that the simple TCM calculations predict the same overpotentials and PLS for both methane and methanol formation as introduced earlier in this work. It should be noted that we predict here the OCH3 to CH3OH(aq) step to be the PLS whereas the HCOOH to H2COOH is the RLS for methanol formation as explained above.
Fig. 5a shows the comparison of the competing HER with the CO2RR on a clean RuO2(110) surface at U = 0 V vs. RHE. The adsorption free energy of OC*O is around −1 eV and binds stronger to the surface than a H adatom of around −0.5 eV where both intermediates adsorb to a bridge vacant site. Since in a CO2-saturated solution the proton concentration is much higher than the CO2 concentration it is reasonable to assume that these sites will have a higher coverage of H than adsorbed CO2. However, when CO2 comes into the double layer it will adsorb stronger than protons. Since the applied potential affects the proton adsorption much more strongly than the CO2 adsorption, these results indicate that the adsorption free energies of H and CO2 become equal at −0.5 V vs. RHE. After adsorption of hydrogen on the surface, the next step is to protonate hydrogen using co-adsorbed water and produce adsorbed H2 on the surface. The calculated activation energy for this step is estimated to be 0.64 eV at U = 0 V. In the next step, H2 leaves the surface without any energy barrier. This is compared with the CO2RR pathway towards forming adsorbed CO on the surface, where the highest barrier is 1.03 eV. These results indicate that the HER is much more facile than the CO2RR on the clean RuO2(110) surface at reducing potentials, but when very negative potential is applied, some CO2 will be reduced to adsorbed CO, in agreement with recent experimental results.54 Another way to introduce adsorbed CO as a spectator species on the surface is to include some CO gas into the electrolyte before CO2RR experiments. Fig. 5a shows that CO adsorbs much stronger to the surface than either protons or CO2.
The same competing processes of the HER and CO2RR are compared in Fig. 5b when a CO spectator is adsorbed on the surface. The presence of CO on the surface destabilizes the binding free energy of hydrogen slightly, or to a value of around −0.4 eV. While the adsorption free energy of *OCO* is only slightly affected by the presence of CO, the adsorption of OC*O is largely affected, and binds weaker than H. This results in even higher possibilities of the catalytic sites being occupied with H rather than CO2, especially when reducing potentials are applied. The energy barrier for H2 formation increases to around 0.76 eV at 0 V vs. RHE with the CO spectator (Fig. S28†), compared to a barrier of around 0.64 eV without CO (Fig. S27†). The H2 formation steps are concluded to be the RLS for the HER as the energy barriers are higher than for the Volmer step presented above. Fig. 5b shows that the competing energy barrier for the formate pathway in the CO2RR is only 0.45 eV at 0 V vs. RHE. As shown in Fig. 4, the PLS for formic acid formation is the reduction of the formate intermediate which requires −0.9 V. At these reducing potentials, most of the bridge sites will be covered with protons, resulting in much higher rates of the HER than the CO2RR, in good agreement with recent experimental findings.54
Discussion
In this work, the proton–electron transfer barriers can be classified into four groups:1. Proton–electron transfer to oxygen (e.g. O–CH–O to O–CH–OH)
2. Proton–electron transfer to the hydroxyl group (e.g. O–CH2–OH to O–CH2 + H2O(l))
3. Proton–electron transfer to carbon (e.g. O–C–O to O–CH–O)
4. Proton–electron transfer to hydrogen
Overall, the proton–electron transfer steps presented in this paper show that attacking the –O or –OH groups of CO2RR intermediates results in low additional barriers on the order of 0.0 to 0.3 eV while attacking the carbon atom results in higher additional barriers. For highly exergonic reactions they are around 0.2 to 0.5 eV. For the reaction having around thermoneutral or endergonic reaction free energies, the additional energy barrier is around 0.5 to 0.9 eV, except in the case of methane formation, which is over 2 eV. This is not surprising since oxygen is more electronegative than carbon which should favor a proton transfer. Finally, in the case of adsorbed H, our results show low additional barriers, or 0.34 and 0.15 eV, without and with a CO spectator, respectively. The energy barriers presented in this work are summarized in Table 2 where they are classified based on which atom of the CO2RR or H intermediates protonation takes place.
| Reactions | Classification | ΔE | E a | Additional barrier |
|---|---|---|---|---|
| CO2 + H2O → COOH + OH | Transfer to O | 0.78 | 0.88 | 0.10 |
| CO2 + H2O → OCHOb + OH (S) | Transfer to C | −0.87 | 0.45 | 0.45 |
| OCHOb → OCHOm (S) | Reconfiguration | −0.11 | 0.11 | 0.11 |
| OCHO + H2O → HCOOH + OH (S) | Transfer to O | 0.72 | 0.86 | 0.14 |
| HCOOH → HCOOH(aq) (S) | Desorption | 0.20 | 0.20 | 0.00 |
| HCOOH + H2O → H2COOH + OH (S) | Transfer to C | −0.33 | 0.91 | 0.91 |
| H2COOH + H2O → H2C(OH)2 + OH (S) | Transfer to O | 0.52 | 0.72 | 0.20 |
| H2C(OH)2 → H2C(OH)2(aq) (S) | Desorption | 0.60 | 0.60 | 0.00 |
| H2COOH + H2O → CH2O + H2O(l) + OH (S) | Transfer to OH | 0.78 | 0.78 | 0.00 |
| H2COOH + H2O → CH3OH(aq) + O + OH (S) | Transfer to C | 1.13 | 1.66 | 0.53 |
| CH2O + H2O → CH3O + OH (S) | Transfer to C | −0.91 | 0.22 | 0.22 |
| CH3O + H2O → CH3OH(aq) + OH (S) | Transfer to O | 0.94 | 1.06 | 0.12 |
| CH3O + H2O → ˙CH3 + OH(br) + OH(CUS) (S) | Transfer to O | 1.50 | 1.97 | 0.47 |
| OH(br) + OH(CUS) → O(br) + H2O(CUS) (S) | Reconfiguration | 0.74 | 0.74 | 0.00 |
| ˙CH3 + O(br) + H2O → CH4(g) + O(br) + OH(CUS) (S) | Transfer to C | −1.42 | 0.53 | 0.53 |
| CH3O + H2O → CH4(g) + O + OH (S) | Transfer to C | 0.64 | 2.77 | 2.13 |
| O + H2O → OH + OH (S) | Transfer to O | −0.62 | 0.00 | 0.00 |
| COOH + H2O → CO + H2O(l) + OH | Transfer to OH | −0.33 | 0.32 | 0.32 |
| CO + H2O → COH + OH | Transfer to O | 1.57 | 1.57 | 0.00 |
| CO + H2O → CHO + OH | Transfer to C | 0.97 | 1.41 | 0.44 |
| CO → CO(g) | Desorption | 1.98 | 1.98 | 0.00 |
| H + H2O → H2(g) + OH | Transfer to H | 0.30 | 0.64 | 0.34 |
| H + H2O → H2(g) + OH (S) | Transfer to H | 0.61 | 0.76 | 0.15 |
In the case of pure metal electrodes, the reaction pathways are very different depending on whether a detailed solvation and kinetic model is used or a thermochemical one.23 Despite a much more detailed modeling of the reaction paths presented in this work for RuO2(110) than in previous studies using the TCM and CHE models,55–59 both methods predict the same adsorbed intermediates between proton–electron transfer steps, except in one case. Table 3 summarizes the theoretical reaction pathways based on our current work for the CO2RR towards methanol and methane and should be compared with the results in Table 1 which summarizes the pathways predicted by the TCM–CHE models. After the fourth proton–electron transfer step we predict with the detailed modeling that the H2COOH admolecule is reduced to the CH2O intermediate on the surface and an H2O(l) molecule that desorbs from the surface (Table 3), whereas the simpler TCM model predicts the CH3O + OH intermediates on the surface (Table 1). This is however not because of the negligible kinetic barrier we find for this step, but because of the presence of co-adsorbed water on the surface that imposes the formation of adsorbed CH2O and H2O(l) instead of forming the CH3O and OH intermediates. The CH3O and OH intermediates will be formed when CH2O is protonated further by co-adsorbed water (Fig. 4 and Table 3).
| Pathways | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Methanol | OCHO | HCOOH | H2COOH | CH2O + H2O(l) | CH3O | CH3OH(aq) | ||
| Methane | OCHO | HCOOH | H2COOH | CH2O + H2O(l) | CH3O | CH4(g) + O | OH | H2O(l) |
Finally, we address the question of why Cu is more selective towards methane than methanol while the opposite trend is observed and predicted here on RuO2; i.e. more selective towards methanol than methane. There is a fundamental difference between these two types of catalysts which alters the binding configurations of the CO2RR intermediates, which changes the selectivity. While all intermediates bind through the carbon atom on the Cu(111) surface,23 they all bind through their oxygen atom(s) on the RuO2(110) surface. As an example, on Cu(111) the first two intermediates are predicted to be COOH and CO where both bind to the surface through the carbon atom, while OCHO and HCOOH are predicted to be the first two intermediates on RuO2(110), both binding through their oxygen atom(s). This is presumably because of the oxygen vacant bridge sites on RuO2(110), which tend to bind intermediates through their oxygen atoms rather than through their carbon atom. Furthermore, we observe that when an intermediate binds to the surface through an oxygen atom, a very high activation energy is needed to break the O–C bond; as shown in the case of O–CH3 reduction to O + CH4 in Table 2. Therefore, O–CH3 is much rather reduced to methanol than methane on RuO2. In contrast, when intermediates bind through the carbon atom to the surface, breaking the C–O bond requires a very low activation energy, as shown in the case of COOH reduction to CO + H2O in Table 2 for RuO2 and has been shown previously for Cu(111).23 This is why Cu is more selective towards methane than methanol where all intermediates bind through the carbon atom.
Conclusions
In this work, we calculate proton–electron transfer energy barriers for the CO2RR and HER on RuO2(110) using 1 ML of co-adsorbed water molecules on the CUS and low CO coverages on the surface to gain further insight into the reaction mechanisms towards hydrogen, methanol, methane, methanediol, formic acid and CO evolution. We obtained two substantial additional energy barriers on top of the thermodynamics. One is related to a proton–electron transfer to the O–CH–OH species to form O–CH2–OH with an additional energy barrier of 0.91 eV which is the RLS towards methanol and methanediol. The highest energy barrier we find towards methanol is, however, 1.02 eV, for the reduction of the OCH3 intermediate, but since the barrier mainly comes from the thermodynamics of that reaction step it is easily surmounted at room temperature at the required overpotential for methanol formation which is calculated to be around −1 V vs. RHE. The other substantial additional energy barrier we find in this work is related to the protonation of the O–CH3 intermediate to form methane with an overall barrier of 2.3 eV or more. A few mechanisms are considered where they all resulted in having more than one elementary step. In all cases, high barriers for breaking the O–C bond are found to be limiting. These results show clearly from the energy barriers why methanol can be formed on RuO2 electrodes at high overpotentials while methane formation seems impossible. The calculations predict, however, that hydrogen is the main product at all potentials which is consistent with the most recent experimental results.Generally, we find that the additional energy barriers on top of the thermodynamics for proton–electron transfer to –O and –OH groups in CO2RR intermediates are low. This indicates that these elementary steps are easily surmountable at the overpotential required for the reaction and they are not kinetically limited. In contrast, proton–electron transfer steps to the carbon atom in CO2RR intermediates and for O–C scission steps are predicted to be kinetically limited in some cases even if the thermodynamics are favorable. Furthermore, we find that the required barriers to desorb CO from the RuO2(110) surface or to further reduce it to COH or CHO are relatively high and this supports the hypothesis regarding CO as a spectator species on RuO2 electrodes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Icelandic Research Fund (grant no. 196437-051), the Research Fund of the University of Iceland and the ‘Nordic Consortium for CO2 Conversion’ (NordForsk project no. 85378, http://site.uit.no/nordco2).References
- Y. Hori, A. Murata and R. Takahashi, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 2309–2326 RSC.
- Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Electrochim. Acta, 1994, 39, 1833–1839 CrossRef CAS.
- M. R. Singh, Y. Kwon, Y. Lum, J. W. Ager and A. T. Bell, J. Am. Chem. Soc., 2016, 138, 13006–13012 CrossRef CAS PubMed.
- A. S. Varela, W. Ju, A. Bagger, P. Franco, J. Rossmeisl and P. Strasser, ACS Catal., 2019, 9, 7270–7284 CrossRef CAS.
- A. Vasileff, X. Zhi, C. Xu, L. Ge, Y. Jiao, Y. Zheng and S. Z. Qiao, ACS Catal., 2019, 9, 9411–9417 CrossRef CAS.
- H. Zhang, Y. Zhang, Y. Li, S. Ahn, G. T. R. Palmore, J. Fu, A. A. Peterson and S. Sun, Nanoscale, 2019, 11, 12075–12079 RSC.
- K. J. P. Schouten, Y. Kwon, C. J. M. Van Der Ham, Z. Qin and M. T. M. Koper, Chem. Sci., 2011, 2, 1902–1909 RSC.
- K. J. P. Schouten, Z. Qin, E. P. Gallent and M. T. M. Koper, J. Am. Chem. Soc., 2012, 134, 9864–9867 CrossRef CAS PubMed.
- K. P. Kuhl, E. R. Cave, D. N. Abram and T. F. Jaramillo, Energy Environ. Sci., 2012, 5, 7050–7059 RSC.
- W. Tang, A. A. Peterson, A. S. Varela, Z. P. Jovanov, L. Bech, W. J. Durand, S. Dahl, J. K. Nørskov and I. Chorkendorff, Phys. Chem. Chem. Phys., 2012, 14, 76–81 RSC.
- R. Reske, M. Duca, M. Oezaslan, K. J. P. Schouten, M. T. M. Koper and P. Strasser, J. Phys. Chem. Lett., 2013, 4, 2410–2413 CrossRef CAS.
- A. S. Varela, C. Schlaup, Z. P. Jovanov, P. Malacrida, S. Horch, I. E. L. Stephens and I. Chorkendorff, J. Phys. Chem. C, 2013, 117, 20500–20508 CrossRef CAS.
- K. J. P. Schouten, E. Pérez Gallent and M. T. M. Koper, J. Electroanal. Chem., 2014, 716, 53–57 CrossRef CAS.
- K. P. Kuhl, T. Hatsukade, E. R. Cave, D. N. Abram, J. Kibsgaard and T. F. Jaramillo, J. Am. Chem. Soc., 2014, 136, 14107–14113 CrossRef CAS PubMed.
- A. A. Peterson, F. Abild-Pedersen, F. Studt, J. Rossmeisl and J. K. Nørskov, Energy Environ. Sci., 2010, 3, 1311–1315 RSC.
- A. A. Peterson and J. K. Nørskov, J. Phys. Chem. Lett., 2012, 3, 251–258 CrossRef CAS.
- X. Liu, P. Schlexer, J. Xiao, Y. Ji, L. Wang, R. B. Sandberg, M. Tang, K. S. Brown, H. Peng, S. Ringe, C. Hahn, T. F. Jaramillo, J. K. Nørskov and K. Chan, Nat. Commun., 2019, 32 CrossRef PubMed.
- X. Nie, M. R. Esopi, M. J. Janik and A. Asthagiri, Angew. Chem., Int. Ed., 2013, 52, 2459–2462 CrossRef CAS PubMed.
- F. Calle-Vallejo and M. T. M. Koper, Angew. Chem., Int. Ed., 2013, 52, 7282–7285 CrossRef CAS PubMed.
- H. Xiao, T. Cheng, W. A. Goddard and R. Sundararaman, J. Am. Chem. Soc., 2016, 138, 483–486 CrossRef CAS PubMed.
- J. Hussain, H. Jónsson and E. Skúlason, Faraday Discuss., 2016, 195, 619–636 RSC.
- M. R. Singh, J. D. Goodpaster, A. Z. Weber, M. Head-Gordon and A. T. Bell, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E8812–E8821 CrossRef CAS PubMed.
- J. Hussain, H. Jónsson and E. Skúlason, ACS Catal., 2018, 8, 5240–5249 CrossRef CAS.
- A. Bagger, L. Arnarson, M. H. Hansen, E. Spohr and J. Rossmeisl, J. Am. Chem. Soc., 2019, 141, 1506–1514 CrossRef CAS PubMed.
- A. Bagger, R. M. Arán-Ais, J. Halldin Stenlid, E. Campos dos Santos, L. Arnarson, K. Degn Jensen, M. Escudero-Escribano, B. Roldan Cuenya and J. Rossmeisl, ChemPhysChem, 2019, 20, 3096–3105 CrossRef CAS PubMed.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- M. A. Henderson, Surf. Sci. Rep., 2002, 46, 1–308 CrossRef CAS.
- A. Lobo and H. Conrad, Surf. Sci., 2003, 523, 279–286 CrossRef CAS.
- R. Mu, Z. J. Zhao, Z. Dohnálek and J. Gong, Chem. Soc. Rev., 2017, 46, 1785–1806 RSC.
- A. Rendón-Calle, S. Builes and F. Calle-Vallejo, Appl. Catal., B, 2020, 276, 119147 CrossRef.
- P. J. Feibelman, Science, 2002, 295, 99–102 CrossRef CAS PubMed.
- H. Ogasawara, B. Brena, D. Nordlund, M. Nyberg, A. Pelmenschikov, L. G. M. Pettersson and A. Nilsson, Phys. Rev. Lett., 2002, 89, 276102 CrossRef CAS PubMed.
- S. Schnur and A. Groß, New J. Phys., 2009, 11, 125003 CrossRef.
- X. Lin and A. Groß, Surf. Sci., 2012, 606, 886–891 CrossRef CAS.
- M. Van Den Bossche, E. Skúlason, C. Rose-Petruck and H. Jónsson, J. Phys. Chem. C, 2019, 123, 4116–4124 CrossRef CAS.
- P. Lindgren, G. Kastlunger and A. A. Peterson, ACS Catal., 2020, 10, 121–128 CrossRef CAS.
- E. Skúlason, V. Tripkovic, M. E. Björketun, S. Gudmundsdóttir, G. Karlberg, J. Rossmeisl, T. Bligaard, H. Jónsson and J. K. Nørskov, J. Phys. Chem. C, 2010, 114, 18182–18197 CrossRef.
- M. H. Hansen and J. Rossmeisl, J. Phys. Chem. C, 2016, 120, 29135–29143 CrossRef CAS.
- T. Cheng, H. Xiao and W. A. Goddard, J. Phys. Chem. Lett., 2015, 6, 4767–4773 CrossRef CAS PubMed.
- E. Tayyebi, Y. Abghoui and E. Skúlason, ACS Catal., 2019, 9, 11137–11145 CrossRef CAS.
- C. Zhang, J. Hutter and M. Sprik, J. Phys. Chem. Lett., 2019, 10, 3871–3876 CrossRef CAS PubMed.
- X. Liu, J. Xiao, H. Peng, X. Hong, K. Chan and J. K. Nørskov, Nat. Commun., 2017, 8, 15438 CrossRef CAS PubMed.
- J. D. Goodpaster, A. T. Bell and M. Head-Gordon, J. Phys. Chem. Lett., 2016, 7, 1471–1477 CrossRef CAS PubMed.
- S. Siahrostami and A. Vojvodic, J. Phys. Chem. C, 2015, 119, 1032–1037 CrossRef CAS.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS.
- P. M. Gíslason and E. Skúlason, Nanoscale, 2019, 11, 18683–18690 RSC.
- J. Lee, D. C. Sorescu, X. Deng and K. D. Jordan, J. Phys. Chem. Lett., 2013, 4, 53–57 CrossRef CAS PubMed.
- Y. Hori, in Modern Aspects of Electrochemistry, Springer New York, New York, NY, 2008, pp. 89–189 Search PubMed.
- A. Bandi, J. Electrochem. Soc., 1990, 137, 2157 CrossRef CAS.
- A. Bandi, J. Electrochem. Soc., 1992, 139, 1605 CrossRef CAS.
- J. P. Popić, M. L. Avramov-Ivić and N. B. Vuković, J. Electroanal. Chem., 1997, 421, 105–110 CrossRef.
- N. Spataru, K. Tokuhiro, C. Terashima, T. N. Rao and A. Fujishima, J. Appl. Electrochem., 2003, 33, 1205–1210 CrossRef CAS.
- J. Qu, X. Zhang, Y. Wang and C. Xie, Electrochim. Acta, 2005, 50, 3576–3580 CrossRef CAS.
- S. Mezzavilla, Y. Katayama, R. Rao, J. Hwang, A. Regoutz, Y. Shao-Horn, I. Chorkendorff and I. E. L. Stephens, J. Phys. Chem. C, 2019, 123, 17765–17773 CrossRef CAS.
- M. Karamad, H. A. Hansen, J. Rossmeisl and J. K. Nørskov, ACS Catal., 2015, 5, 4075–4081 CrossRef CAS.
- A. Bhowmik, T. Vegge and H. A. Hansen, ChemSusChem, 2016, 9, 3230–3243 CrossRef CAS PubMed.
- A. Bhowmik, H. A. Hansen and T. Vegge, J. Phys. Chem. C, 2017, 121, 18333–18343 CrossRef CAS.
- A. Bhowmik, H. A. Hansen and T. Vegge, ACS Catal., 2017, 7, 8502–8513 CrossRef CAS.
- E. Tayyebi, J. Hussain, Y. Abghoui and E. Skúlason, J. Phys. Chem. C, 2018, 122, 10078–10087 CrossRef CAS.
- J. Vandevondele, M. Krack, F. Mohamed, M. Parrinello, T. Chassaing and J. Hutter, Comput. Phys. Commun., 2005, 167, 103–128 CrossRef CAS.
- J. Hutter, M. Iannuzzi, F. Schiffmann and J. Vandevondele, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 15–25 CAS.
- G. Lippert, J. Hutter and M. Parrinello, Mol. Phys., 1997, 92, 477–488 CrossRef CAS.
- S. Goedecker and M. Teter, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 1703–1710 CrossRef CAS PubMed.
- C. Hartwigsen, S. Goedecker and J. Hutter, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 3641–3662 CrossRef CAS.
- J. VandeVondele and J. Hutter, J. Chem. Phys., 2007, 127, 114105 CrossRef PubMed.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 CrossRef CAS PubMed.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 4978 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- M. Ceriotti, G. Bussi and M. Parrinello, Phys. Rev. Lett., 2009, 102, 020601 CrossRef PubMed.
- M. Ceriotti, G. Bussi and M. Parrinello, J. Chem. Theory Comput., 2010, 6, 1170–1180 CrossRef CAS.
- J. Wellendorff, K. T. Lundgaard, A. Møgelhøj, V. Petzold, D. D. Landis, J. K. Nørskov, T. Bligaard and K. W. Jacobsen, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 235149 CrossRef.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- G. Henkelman and H. Jónsson, J. Chem. Phys., 2000, 113, 9978–9985 CrossRef CAS.
- V. Tripković, E. Skúlason, S. Siahrostami, J. K. Nørskov and J. Rossmeisl, Electrochim. Acta, 2010, 55, 7975–7981 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc01882a |
| This journal is © The Royal Society of Chemistry 2020 |