Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rendering hydrophobic nanoclusters water-soluble and biocompatible†
Xi
Kang‡
 a,
Xiao
Wei‡
a,
Pan
Xiang
b,
Xiaohe
Tian
b,
Zewen
Zuo
cd,
Fengqi
Song
cd,
Shuxin
Wang
a,
Xiao
Wei‡
a,
Pan
Xiang
b,
Xiaohe
Tian
b,
Zewen
Zuo
cd,
Fengqi
Song
cd,
Shuxin
Wang
 *a and
Manzhou
Zhu
*a and
Manzhou
Zhu
 *a
*a
aDepartment of Chemistry and Centre for Atomic Engineering of Advanced Materials, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Key Laboratory of Structure and Functional Regulation of Hybrid Materials of Ministry of Education, Anhui University, Hefei 230601, P. R. China. E-mail: ixing@ahu.edu.cn; zmz@ahu.edu.cn
bSchool of Life Sciences, Anhui University, Hefei 230601, P. R. China
cNational Laboratory of Solid State Microstructures, Collaborative Innovation Center of Advanced Microstructures, School of Physics, Nanjing University, Nanjing 210093, P. R. China
dAtomic Manufacture Institute, Nanjing 211805, P. R. China
First published on 22nd April 2020
Abstract
Hydrophobic and hydrophilic nanoclusters embody complementary superiorities. The means to amalgamate these superiorities, i.e., the atomic precision of hydrophobic clusters and the water dissolvability of hydrophilic clusters, remains challenging. This work presents a versatile strategy to render hydrophobic nanoclusters water-soluble—the micellization of nanoclusters in the presence of solvent-conjoined Na+ cations—which overcomes the above major challenge. Specifically, although [Ag29(SSR)12(PPh3)4]3− nanoclusters are absolutely hydrophobic, they show good dissolvability in aqueous solution in the presence of solvent-conjoined Na+ cations (Na1(NMP)5 or Na3(DMF)12). Such cations act as both counterions of these nanoclusters and surface cosolvent of cluster-based micelles in the aqueous phase. A combination of DLS (dynamic light scattering) and aberration-corrected HAADF-STEM (high angle annular dark field detector scanning transmission electron microscopy) measurements unambiguously shows that the phase-transfer of hydrophobic Ag29 into water is triggered by the micellization of nanoclusters. Owing to the excellent water solubility and stability of [Ag29(SSR)12(PPh3)4]3−[Na1(NMP)5]3+ in H2O, its performance in cell staining has been evaluated. Furthermore, the general applicability of the micellization strategy has been verified. Overall, this work presents a convenient and efficient approach for the preparation of cluster-based, biocompatible nanomaterials.
1 Introduction
Metal nanoclusters, benefiting from their atomically precise structures, fascinating properties, and potential applications, are an emerging class of modular nanomaterials.1–11 Recent years have witnessed significant research efforts on the controllable synthesis and the structural determination of metal nanoclusters.12–34 Typically, the great nanocluster family encompasses both hydrophobic and hydrophilic clusters.(i) Owing to their precise structures, hydrophobic nanoclusters are mostly researched for fathoming structural evolutions and structure–property correlations at the atomic level.1 Based on such knowledge and understanding of mechanisms underlying cluster performances, several efficient approaches have been put forward to accurately dictate the chemical–physical performances of metal nanoclusters.1–11,35–42 However, in consideration of their hydrophobicity, these nanoclusters are less likely directly applicable to water-phase applications, such as chemical sensing, bio-imaging, bio-labeling, and biotherapy, to name a few.
(ii) On the other hand, although several hydrophilic nanoclusters are not that precise in terms of structures and compositions (even some of them are poly-dispersed), they are much more suitable for the aforementioned bio-applications owing to their water dissolvability.43–51 In addition, hydrophilic nanoclusters benefit from ultra-small sizes, non-toxicity, strong photo-stability, good biocompatibility, and potential anti-cancer activity, and their use in biological applications is thus highly promising.43–51 However, drawbacks of known hydrophilic nanoclusters also exist—their properties are even harder to precisely control than in hydrophobic nanoclusters, and their imprecise structures preclude quantitative tracking in cells or organisms. These drawbacks greatly impede the practical uses of hydrophilic nanoclusters in bio-applications.
Indeed, hydrophobic and hydrophilic nanoclusters embody complementary superiorities. Accordingly, the means to transfer atomically precise, hydrophobic nanoclusters into water, and then exploit them for aqueous-phase applications, is anticipated to overcome the aforementioned drawbacks, and should be an important goal in nanocluster science. Herein, we report a versatile strategy to render hydrophobic nanoclusters water-soluble—the micellization of these nanoclusters in the presence of solvent-conjoined Na+ cations. Specifically, the [Ag29(SSR)12(PPh3)4]3− (SSR = 1,3-benzene dithiol) nanocluster is absolutely hydrophobic; however, in the presence of solvent-conjoined cations (Na1(NMP)5 or Na3(DMF)12) as counterions, Ag29@Na compounds ([Ag29(SSR)12(PPh3)4]3−[Na1(NMP)5]+3 or [Ag29(SSR)12(PPh3)4]3−[Na3(DMF)12]3+, Ag29-Na1 or Ag29-Na3 hereafter because they contain three Na+ monomers or one [Na3]3+ trimer, respectively) show good dissolvability in aqueous solution. A combination of DLS (dynamic light scattering) and aberration-corrected HAADF-STEM (high angle annular dark field detector scanning transmission electron microscopy) measurements unambiguously shows that the phase-transfer of hydrophobic Ag29 into water is triggered by the micellization of nanoclusters. Owing to the excellent water dissolvability and stability of Ag29-Na1, its performance in cell staining is evaluated, and such cluster-based micelles show specific selectivity in staining lysosomes. Furthermore, the general applicability of this micellization method in the nanocluster field has been verified, based on several other negative-charged nanoclusters, which further demonstrates the significance of this method in the preparation of cluster-based, biocompatible nanomaterials.
2 Experimental methods
Materials
All reagents were purchased from Sigma-Aldrich and used without further purification: silver nitrate (AgNO3, 99%, metal basis), triphenylphosphine (PPh3, 99%), 1,3-benzene dithiol (SSR, 99%), sodium borohydride (NaBH4, 99.9%), sodium acetate (CH3COONa, 99%), methylene chloride (CH2Cl2, HPLC, Aldrich), methanol (CH3OH, HPLC, Aldrich), N,N-dimethylformamide (DMF, HPLC, Aldrich), N-methyl-2-pyrrolidone (NMP, HPLC, Aldrich), and ethyl ether ((C2H5)2O, HPLC, Aldrich).Synthesis of [Ag29(SSR)12(PPh3)4]3−
The preparation of [Ag29(SSR)12(PPh3)4]3− was based on the reported method of the Bakr and the Pradeep groups.52,53Synthesis of [Ag29(SSR)12(PPh3)4]3−[Na1(NMP)5]+3 (i.e., Ag29-Na1)
20 mg of [Ag29(SSR)12(PPh3)4]3− was dissolved in 5 mL of NMP, and 2 mg of CH3COONa was added under vigorous stirring at 273 K (ice-bath). After 30 min, the organic layer was separated, which produced the Ag29-Na1 nanocluster. The yield was 95% based on the Ag element (calculated from the Ag29(SSR)12(PPh3)4). This NMP solution of Ag29-Na1 was directly used for the crystallization and characterization.Synthesis of [Ag29(SSR)12(PPh3)4]3−[Na3(DMF)12]3+ (i.e., Ag29-Na3)
50 mg of the Ag29-Na1 crystal was dissolved in 5 mL of DMF under vigorous stirring at 273 K (ice-bath). This DMF solution was poured into 200 mL of CH2Cl2, and the precipitate was collected and further dissolved in 5 mL of DMF, producing the Ag29-Na3 nanocluster. The yield was 95% based on the Ag element (calculated from the Ag29-Na1). This DMF solution of Ag29-Na3 was directly used for the crystallization and characterization.Crystallization of Ag29-Na1 and Ag29-Na3
Single crystals of Ag29-Na1 or Ag29-Na3 were cultivated at room temperature by vapor-diffusing ethyl ether into the NMP solution of Ag29-Na1 or the DMF solution of Ag29-Na3. After 2 weeks, red crystals were collected, and the structure of Ag29-Na1 or Ag29-Na3 was determined.Micellization of Ag29-Na1 and Ag29-Na3
Specifically, 3 mg of Ag29-Na1 or Ag29-Na3 was dissolved in 3 mL H2O. In this process, Ag29-Na1 and Ag29-Na3 cluster-based micelles were formed in their aqueous solutions. For measuring the sizes of Ag29-Na1 or Ag29-Na3 micelles in different stages (Fig. 3), 100*n μL of the aforementioned aqueous solution of Ag29-based micelles was injected into (3–0.1*n) mL H2O each time (n is the number of the stage), and the obtained H2O solution was directly used for the DLS analysis.The general nanocluster micellization method
Specifically, 30 mg of each negative-charged nanocluster was mixed with 1 mg of CH3COONa and 30 μL DMF, and the obtained mixture was dissolved in 1 mL H2O. The precipitate was then removed to produce the cluster-based micelle in H2O. Notably, DLS measurements in Fig. 6 were performed in saturated aqueous solutions of these nanoclusters. The syntheses of negative-charged nanoclusters (including [Au25(SC2H4Ph)18]−, [Ag25(SPhMe2)18]−, [Pt1Ag24(SPhMe2)18]2−, [Ag44(SPhF2)30]4−, [Au12Ag32(SPhF2)30]4−, and [Ag28Cu12(SPhCl2)24]4−) were based on the reported methods.54–58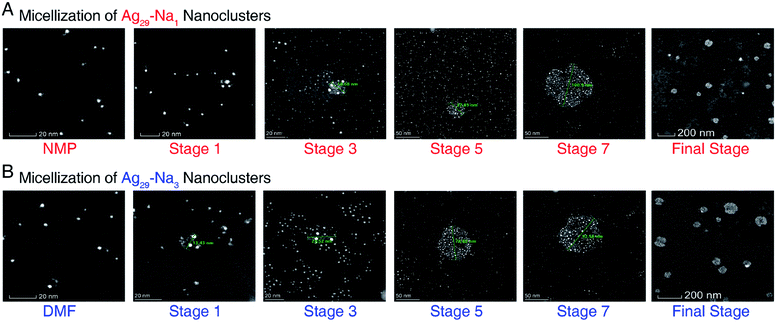 | ||
| Fig. 4 The aberration-corrected HAADF-STEM images of Ag29-Na1 and Ag29-Na3 micelles. (A) Micellization of Ag29-Na1 nanoclusters, corresponding to the different states in Fig. 3A and B. (B) Micellization of Ag29-Na3 nanoclusters, corresponding to the different states in Fig. 3D and E. Only images of selected stages are shown here, and the whole process images are depicted in ESI Fig. S5 and S6.† Scale bar = 20 nm for NMP/DMF, stage 1, and stage 3; scale bar = 50 nm for stage 5 and stage 7; scale bar = 200 nm for the final stage. | ||
The solubility of different nanoclusters in aqueous solution
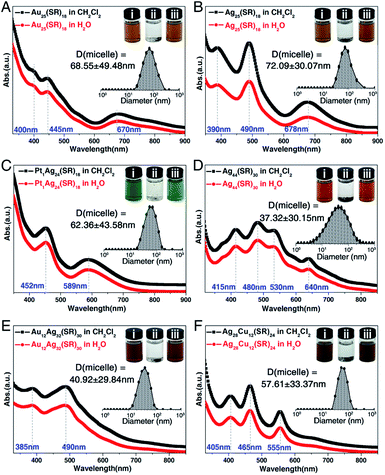
The nanocluster@solvent-conjoined cation compound was dissolved in H2O, and the solubility of each nanocluster in the aqueous solution was determined. Specifically, the solubility of Ag29-Na1 in H2O was 6.76 mg mL−1; the solubility of Ag29-Na3 in H2O was 7.88 mg mL−1; the solubility of Au25(SC2H4Ph)18@Na–DMF in H2O was 5.35 mg mL−1; the solubility of Ag25(SPhMe2)18@Na–DMF in H2O was 5.78 mg mL−1; the solubility of Pt1Ag24(SPhMe2)18@Na–DMF in H2O was 13.42 mg mL−1; the solubility of Ag44(SPhF2)30@Na–DMF in H2O was 27.12 mg mL−1; the solubility of Au12Ag32(SPhF2)30@Na–DMF in H2O was 28.34 mg mL−1; the solubility of Ag28Cu12(SPhCl2)24@Na–DMF in H2O was 25.32 mg mL−1.Characterization
All UV-vis absorption spectra of nanoclusters were recorded using an Agilent 8453 diode array spectrometer, whose background correction was made using a pure solution blank.PL spectra were measured on a FL-4500 spectrofluorometer with the same optical density of 0.1.
Electrospray ionization mass spectrometry (ESI-MS) measurements were performed using a MicrOTOF-QIII high resolution mass spectrometer. The sample was directly infused into the chamber at 5 μL min−1. For preparing the ESI samples, nanoclusters were dissolved in NMP/DMF (0.1 mg mL−1) and diluted (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with methanol.
2) with methanol.
23Na nuclear magnetic resonance (NMR) spectra were acquired using a Bruker 600 Avance III spectrometer equipped with a Bruker BBO multinuclear probe (BrukerBioSpin, Rheinstetten, Germany).
Dynamic light scattering (DLS) was performed with a Malvern Zetasizer Nano ZS instrument. For preparing the DLS samples, the nanocluster@solvent-conjoined cation compounds were dissolved in H2O to produce the cluster-based micelles. The DLS result of each nanocluster was repeated 40 times to remove the error.
The Ag29-based micelles were imaged with an aberration-corrected HAADF-STEM (high angle annular dark field scanning transmission electron microscope) after the solvent that contained Ag29-based micelles was drop-cast onto ultrathin carbon film TEM grids. The microscope employed was a FEI Themis Z. The electron beam energy was 200 kV. The collection angle of the HAADF detector was adjusted to collect signals scattered between 52 (inner angle) and 200 (outer angle) mrad (camera length of 146 mm). The HAADF-STEM image was obtained with Thermo Scientific Velox software using 1024 × 1024 pixels and the dwell time is set to 10 us.
X-ray crystallography
For Ag29-Na1, the data collection for single crystal X-ray diffraction was carried out on a Stoe Stadivari diffractometer under nitrogen flow, using graphite-monochromatized Cu Kα radiation (λ = 1.54186 Å). For Ag29-Na3, the data collection for the single-crystal X-ray diffraction was carried out on a Bruker Smart APEX II CCD diffractometer under nitrogen flow, using graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å). Data reductions and absorption corrections were performed using the SAINT and SADABS programs, respectively. The electron density was squeezed by Platon. The structure was solved by direct methods and refined with full-matrix least squares on F2 using the SHELXTL software package. All non-hydrogen atoms were refined anisotropically, and all the hydrogen atoms were set in geometrically calculated positions and refined isotropically using a riding model.Cell culture
HepG2 (liver hepatocellular carcinoma) cells were purchased from Shanghai Bioleaf BioBiotech. Co. Ltd. Specifically, the cells were incubated in Dulbecco's Modified Eagle's medium (DMEM) containing 10% FBS and 1% antibiotics (penicillin and streptomycin), and the cells were further maintained at 37 °C in an atmosphere of 5% CO2 and 95% air.Confocal microscopic imaging
Confocal microscopy imaging was performed with a Leica TCS SP8 confocal microscope with an adjustable white laser (470–700 nm) and 63X/100X oil-immersion objective lens. The incubated cells were excited at 470 nm for Ag29-Na1, and 633 nm for Lysotracker Red. The emission signals were collected at 550 nm for Ag29-Na1, and 650–700 nm for Lysotracker Deep Red.STED super-resolution image
Stimulated emission depletion nanoscopy (STED) experiments were performed under a Leica DMi8 confocal microscope equipped with a Leica TCS SP8 STED-ONE unit. The compound was excited under a STED laser (donut laser: 590 nm), and the emission signals were collected using HyD reflected light detectors (RLDs) with 2048 × 2048 pixel and ×100 scanning speed. The STED micrographs were further processed “deconvolution wizard” function using the Huygens Professional software (version: 16.05) under an authorized license. The area radii were estimated under 0.02 micros with exclusion of 100 absolute back-ground values. The following measurement settings were used: maximum iterations: 40 times; signal-to-noise ratio: 20; quality threshold: 0.05; iteration mode: optimized; brick layout: auto.3 Results and discussion
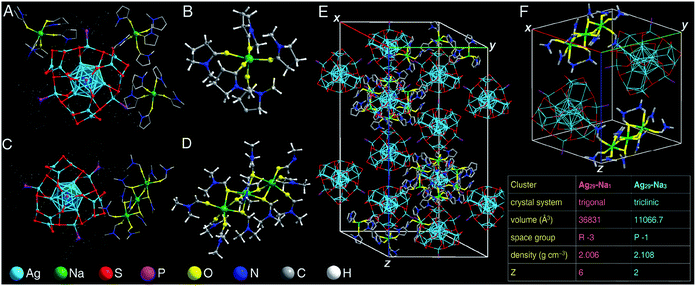
The preparations of both Ag29-Na1 and Ag29-Na3 nanoclusters are shown in the Experimental methods section, and the crystallization of Ag29-Na1 or Ag29-Na3 nanoclusters was conducted by vapor-diffusing ethyl ether into the NMP solution of Ag29-Na1 or the DMF solution of Ag29-Na3. Structurally, the overall configuration of the Ag29(SSR)12(PPh3)4 framework in both Ag29-Na1 and Ag29-Na3 was much like that of the previously reported one;52,53 however, there were still subtle differences among these Ag29 frameworks in terms of the corresponding bond lengths (Table S1†). Specifically, except for the bonds between Ag(core shell) and S(motif), all bonds including Ag(core)–Ag(core shell), Ag(core shell)–Ag(core shell), Ag(core shell)–Ag(motif), Ag(motif)–S(motif), and Ag(motif)–P(motif) of Ag29-Na1 and Ag29-Na3 nanoclusters were much longer than those in the bare Ag29(SSR)12(PPh3)4 nanocluster (Ag29-Na0 hereafter). Accordingly, the overall Ag29 core structures of both Ag29-Na1 and Ag29-Na3 were more expanding than that in Ag29-Na0.For the counterions of these [Ag29(SSR)12(PPh3)4]3− nanoclusters, the Ag29-Na1 nanocluster comprised three NMP-conjoined [Na1(NMP)5]+ cations per Ag29 compound (Fig. 1A and B), whereas each Ag29 compound matched only one DMF-conjoined [Na3(DMF)12]3+ cation in the structure of Ag29-Na3 (Fig. 1C and D). It should be noted that no Na+ cation (or other cations) has been observed in the crystal lattice of Ag29-Na0 in both crystals of Ag29(SSR)12(PPh3)4 reported by the Bakr and the Pradeep groups, which might result from the high disorder of these Na+ counterions.52,53 Indeed, induced by the fixation of the crown ether, Chakraborty et al. captured these Na+ cations in a form of Na+@dibenzo-18-crown-6.59 In this work, for both Ag29-Na1 and Ag29-Na3 nanoclusters, the Na+ counterions were fixed by oxygen-carrying solvents such as NMP and DMF to generate the solvent-conjoined cations.
Specifically, for the [Na1(NMP)5]+ of Ag29-Na1, each Na+ cation was surrounded by five NMP solvent molecules through Na–O interactions (Fig. 1B), whereas for the [Na3(DMF)12]3+ of Ag29-Na3, the three Na+ cations were tied with a linear pattern by twelve DMF solvent molecules (Fig. 1D).
The crystal lattice of Ag29-Na1 contained six [Ag29(SSR)12(PPh3)4]3− anions and 18 [Na1(NMP)5]+ cations (Fig. 1E); by comparison, two [Ag29(SSR)12(PPh3)4]3− anions and two [Na3(DMF)12]3+ cations were observed in the unit cell of Ag29-Na3 (Fig. 1F). In this context, in terms of the molecular charge, each “−3”-charged Ag29 matched three “+1”-charged [Na1(NMP)5]+ in Ag29-Na1, or one “+3”-charged [Na3(DMF)12]3+ in Ag29-Na3 to realize the charge balance.
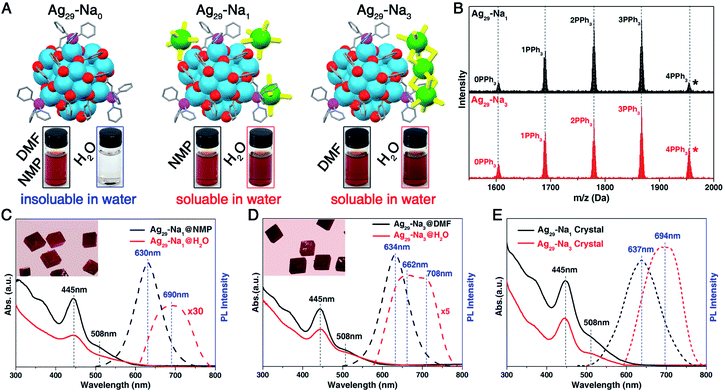
Although the Ag29-Na0 nanocluster displayed good solubility in NMP or DMF, it was absolutely hydrophobic. However, owing to the presence of the solvent-conjoined cations such as Na1(NMP)5 and Na3(DMF)12, Ag29-Na1 and Ag29-Na3 nanoclusters were perfectly soluble in both organic reagents (NMP and DMF) and the aqueous solution (Fig. 2A). Previous research has investigated the phase transfer of hydrophilic nanoclusters from aqueous to organic phases induced by the addition of counterions (e.g., phase transfer of the Au22 nanocluster in the presence of tetraoctylammonium cations);60–62 however, the study of the reverse process (i.e., phase transfer of hydrophobic nanoclusters from organic to aqueous phases) is rather limited in the nanocluster research field. In this work, based on the Ag29(SSR)12(PPh3)4 cluster template, the transfer of hydrophobic nanoclusters from the organic phase to water has been accomplished.
ESI-MS results of both Ag29-Na1 and Ag29-Na3 nanoclusters exhibited five peaks corresponding to the Ag29(SSR)12(PPh3)n compounds where n was 0–4 (Fig. 2B and S1‡), demonstrating the dissociation–aggregation pattern of PPh3 ligands on the Ag29 nanocluster surface.63 However, the [Na1(NMP)5]+ and [Na3(DMF)12]3+ were undetectable in the ESI-MS, which was proposed to result from the weak interactions between Na+ cations and NMP/DMP molecules causing such solvent-conjoined cations to decompose in mass spectroscopy. 23Na NMR was performed to verify the presence of Na+ or Na+-conjoined cations in these nanoclusters. As shown in ESI Fig. S2A,† the 23Na NMP signals of CH3COONa were 0.55 and 1.36 ppm in DMF-D7 and NMP-D9, respectively, whereas the signals of Ag29-Na0 (in DMF-D7), Ag29-Na1 (in NMP-D9) and Ag29-Na3 (in DMF-D7) were −3.08, −0.88, and −1.06 ppm, respectively. Such differences also suggested the distinct existing form of each Na+-based cation in the corresponding nanocluster. Besides, the 23Na NMP signal of CH3COONa in D2O is located at −0.12 ppm, which was remarkably different from those of Ag29-Na1 or Ag29-Na3 in D2O (−3.75 or −3.36 ppm, respectively; Fig. S2B†). In this context, throughout the micellization of Ag29 clusters, Na+/solvent counterions would not dissociate from the nanoclusters. However, it still remained unknown whether the structures of [Na1(NMP)5]+ and [Na3(DMF)12]3+ cations retained in the cluster-based micelles because these micelles were hard to analyze at the atomic level.
The optical absorptions and emissions of the Ag29 nanoclusters in different solutions were compared. As depicted in Fig. 2C, the UV-vis spectrum of the NMP solution of Ag29-Na1 showed an intense absorption at 445 nm and three shoulder bands at 320, 365, and 508 nm, whereas all of these peaks were attenuated when the nanocluster was dissolved in aqueous solution; such a phenomenon was also observed for the Ag29-Na3 nanocluster (Fig. 2D). Despite this attenuation, the optical absorptions of both Ag29-Na1 and Ag29-Na3 in aqueous solution were actually quite similar to those in NMP or DMF. However, remarkable differences existed in terms of the emission wavelength and the photoluminescence (PL) intensity (Fig. 2C and D). Specifically, the Ag29-Na1@NMP emitted at 630 nm, whereas the emission peak of Ag29-Na1@H2O is located at 690 nm along with the broadening of the emission wavelength. Besides, the emission of Ag29-Na3@DMF was centered at 634 nm, and the broadened emission of Ag29-Na3@H2O displayed two peaks at 662 and 708 nm. Of note, compared with the Ag29-Na1 in NMP or Ag29-Na3 in DMF, significant attenuation on PL intensity was monitored when these Ag29 nanoclusters were dissolved in H2O.
Although all types of Ag29 nanoclusters (Ag29-Na0, Ag29-Na1, and Ag29-Na3) presented cubic-like crystals at the macro-level (Fig. 2C,D, insets),52,53 the emissions of them were entirely different. It has been demonstrated that the crystal of Ag29-Na0 emitted at 670 or 700 nm with different crystalline patterns.53 However, the emissions of Ag29-Na1 and Ag29-Na3 luminesced at 637 and 694 nm, respectively (Fig. 2E; and see Fig. S3† for the emission of Ag29-Na0). Besides, the PL intensity of the Ag29-Na3 crystal was slightly stronger than that of the Ag29-Na1 crystal. Such differences in emission wavelength and PL intensity of these Ag29 crystals resulted from their different crystal lattices (or different inter-cluster interactions).
It has been demonstrated that the hydrophobic Ag29(SSR)12(PPh3)4 nanoclusters can be transferred into water in the presence of solvent-conjoined Na+ cations; however, the existence of these Ag29 nanoclusters in aqueous solution remains mysterious. Herein, the DLS (dynamic light scattering) and aberration-corrected HAADF-STEM techniques have been performed for monitoring their real-time existence.
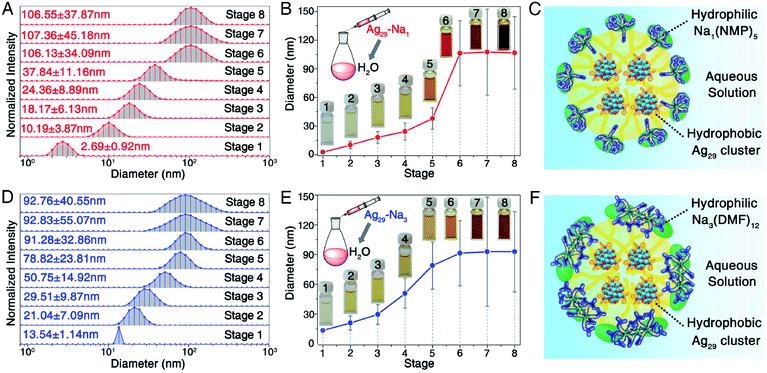
With the help of DLS, the sizes of these Ag29 nanoclusters (Ag29-Na1 and Ag29-Na3) in aqueous solution were monitored (Fig. 3). The concentration of Ag29-Na1 (or Ag29-Na3) in H2O of each stage was controlled as (0.1*n/3) mg mL−1 (n is the stage number in Fig. 3, and see the corresponding optical absorptions in Fig. S4†). For Ag29-Na1, the measured sizes of these clusters were quite small (∼2.69 nm) when their concentration was low (0.03 mg mL−1). When the concentration of nanoclusters increased, a remarkable size growth was observed. Finally, the Ag29-Na1 cluster size soared to a plateau of ∼106 nm (Fig. 3A and B). A similar variation tendency has been observed for the Ag29-Na3 nanocluster, whose sizes in the aqueous solutions grew from ∼13.54 nm to ∼92.76 nm (Fig. 3D and E). Considering that it is not possible for the hydrophobic Ag29(SSR)12(PPh3)4 clusters to form the contact surface with H2O molecules, we proposed that the phase-transfer of Ag29 into water resulted from the micellization of such nanoclusters. The critical micelle concentration (CMC) of Ag29-Na1 and Ag29-Na3 micelles should both occur at stage 6 (Fig. 3), and thus the CMC values of both Ag29 micelles were determined as 0.2 mg mL−1.
As depicted in Fig. 3C and F, the Ag29 cluster-based micelle was composed of a hydrophobic Ag29 interior and a hydrophilic Na–NMP (or Na–DMF) surface. That is, owing to the water-soluble Na–NMP (or Na–DMF) surface, the cluster-based micelle displayed good dissolvability and stability in aqueous solution.
The aberration-corrected HAADF-STEM measurements were further performed to verify the generation of the cluster-based micelles. Fig. 4, S5, and S6† show the selected images of these Ag29-carrying micelles. As depicted in Fig. 4A and S5,†Ag29-Na1 cluster entities were discrete in NMP, and were gradually assembled in aqueous solution. With the increased concentration of the dissolved Ag29-Na1 in H2O, the sizes of micelles increased gradually. Finally, the sizes of the Ag29-Na1 micelles were stabilized at about 100 nm. Similar size variations have also been observed for the Ag29-Na3 micellization process, where the final-stage sizes were also determined as about 100 nm (Fig. 4b and S6†). Such a size growth trend and the final-stage size excellently matched with those derived from the DLS measurement (Fig. 3), further confirming the cluster micellization process. Based on the DLS and STEM results, the aggregation numbers in cluster-based micelles were proposed (Fig. S7†)—111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 of Ag29-Na1 in each micelle with a 106 nm diameter and 72
360 of Ag29-Na1 in each micelle with a 106 nm diameter and 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 of Ag29-Na3 in each micelle with a 92 nm diameter.
810 of Ag29-Na3 in each micelle with a 92 nm diameter.
Of note, although the structure of the Ag29(SSR)12(PPh3)4 molecule is retained during cluster micellization, the structures of [Na1(NMP)5]+ and [Na3(DMF)12]3+ cations may be altered; however, the water solubility of Ag29-Na1 and Ag29-Na3 activated by the presence of these solvent-conjoined cations indeed renders these hydrophobic clusters biocompatible to some extent, which sheds light on the preparation of atomically precise cluster-based, biocompatible nanomaterials.
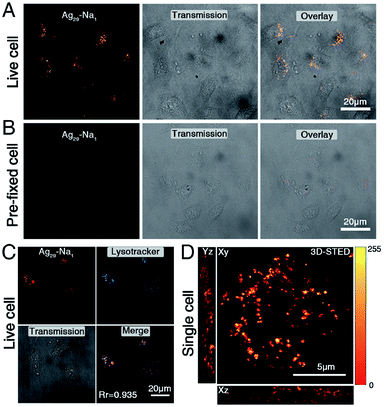
Although both Ag29-Na1 and Ag29-Na3 exhibited excellent solubility in water (6.76 mg mL−1 for the Ag29-Na1 and 7.88 mg mL−1 for Ag29-Na3), the Ag29-Na3 micelles were prone to coagulation; by comparison, the Ag29-Na1 micelles were quite stable in the aqueous phase (Fig. S8†). Due to the excellent stability of Ag29-Na1 micelles, their performance in cell staining was evaluated. Specifically, 5 mg mL−1Ag29-Na1 was incubated with live HepG2 cells and imaged directly under a laser confocal microscope. As shown in Fig. 5A, after incubation for 2 hours, Ag29-Na1 enabled effective uptake in the cytosolic region and displayed a punctate signal. In a parallel experiment (Fig. 5B), the same concentration of Ag29-Na1 incubated with pre-fixed cells displayed neglected uptake. These results demonstrated that Ag29-Na1 was not a cell permeable probe, but might be internalized with live cells via an energy-dependent uptake pathway, such as endocytosis. To precisely determine the intracellular compartment where Ag29-Na1 is stained, a colocalization experiment was performed. Live cells were incubated with Ag29-Na1 and labelled with a lysosomal commercial dye, LysoTracker Deep Red. The micrograph in Fig. 5C suggested that the Ag29-Na1 signal highly overlapped with the LysoTracker signal with a Pearson correlation coefficient (Rr) of 0.935, which further confirmed that the cell entry of Ag29-Na1 might follow an endocytosis pathway. These observations also demonstrated that the Ag29-Na1 micelles could stain lysosomes.
Furthermore, the bio-application of Ag29-Na1 in super-resolution imaging was evaluated. The chosen single cell was incubated with Ag29-Na1 as described above and imaged under a stimulated emission depletion nanoscope (STED). The three-dimensional (3D) micrographs revealed a whole cell lysosome distribution at an unprecedented resolution at both the x-axis and z-axis. This strongly demonstrated the ultra-high photon-stability of Ag29-Na1 and reflected that such cluster micelles could be utilized for super-resolution-based imaging. Of note, due to the existence of the lipophilic phospholipid bilayer in the cell, the structures of [Na1(NMP)5]+ and [Na3(DMF)12]3+ cations were hard to retain in the cell staining process. However, the significant role of these solvent-conjoined cations in corresponding Ag29 nanoclusters was the phase-transfer effect that rendered these hydrophobic nanoclusters water-soluble, and thus their bio-applications appear to be promising.
In previous studies concerning cation-containing micelles, the role of Na+ cations in micellization has been thoroughly researched (e.g., the micellization of alkyl sulfates or the ionic micelles).64–68 However, the cation-induced micellization has not been reported in the nanocluster field. Considering that the solvent-conjoined Na+ cations can act as general counterions for negatively charged nanoclusters, we perceive a good opportunity to render such hydrophobic nanoclusters water-soluble. Herein, several negative-charged nanoclusters including [Au25(SC2H4Ph)18]−, [Ag25(SPhMe2)18]−, [Pt1Ag24(SPhMe2)18]2−, [Ag44(SPhF2)30]4−, [Au12Ag32(SPhF2)30]4−, and [Ag28Cu12(SPhCl2)24]4− nanoclusters were used for evaluating the general applicability of the nanocluster micellization strategy.54–58 For the preparation, each nanocluster was mixed with CH3COONa and minute quantities of DMF, which produced the [cluster]−[Na–solvent]+ compounds. As shown in the digital photos in Fig. 6, in the absence of [Na–solvent]+ cations, these nanoclusters were well soluble in CH2Cl2 (photo i) but insoluble in H2O (photo ii); that is, they were absolutely hydrophobic. By comparison, in the presence of solvent-conjoined Na+ cations, all of the obtained compounds showed good dissolvability in aqueous solution (photo iii). Specifically, the water solubility of Au25(SC2H4Ph)18@Na–DMF, Ag25(SPhMe2)18@Na–DMF, Pt1Ag24(SPhMe2)18@Na–DMF, Ag44(SPhF2)30@Na–DMF, Au12Ag32(SPhF2)30@Na–DMF, Ag28Cu12(SPhCl2)24@Na–DMF was 5.35, 5.78, 13.42, 27.12, 28.34, and 25.32 mg mL−1, respectively. As depicted in Fig. 6, for each nanocluster, the optical absorptions in CH2Cl2 and in H2O were the same, demonstrating the stability of the nanocluster in the aqueous phase. Furthermore, DLS measurements were performed in the saturated aqueous solutions of these nanoclusters, and all of the size-distribution results demonstrated the generation of cluster-based micelles (Fig. 6, insets). Consequently, the micellization of nanoclusters triggered by the addition of solvent-conjoined cations is indeed a generally applicable strategy for rendering hydrophobic nanoclusters water-soluble, at least for the negatively charged nanoclusters.
4 Conclusions
In summary, we presented a versatile strategy to render hydrophobic nanoclusters water-soluble—the micellization of nanoclusters; such a dissolvability variation was triggered by the addition of solvent-conjoined Na+ cations. Specifically, although several negative-charged nanoclusters (such as Ag29(SSR)12(PPh3)4, Au25(SR)18, Ag25(SR)18, etc.) were absolutely hydrophobic, they showed good dissolvability in aqueous solution in the presence of solvent-conjoined Na+ cations. Crystal structures of Ag29-Na1 and Ag29-Na3 demonstrated that such Na+ cations were capped by oxygen-carrying solvent molecules, and existed as [Na1(NMP)5]3+ or [Na3(DMF)12]3+, acting as both counterions of negatively charged nanoclusters and surface cosolvent of cluster-based micelles in the aqueous phase. A combination of DLS and aberration-corrected HAADF-STEM unambiguously identified the generation of micelles of such nanoclusters. Owing to the excellent water solubility and stability of Ag29-Na1, its performance in cell staining has been evaluated—Ag29-Na1 cluster-based micelles can stain lysosomes in both general imaging and super-resolution-based imaging. Overall, this work hopefully sheds light on the preparation of atomically precise cluster-based, biocompatible nanomaterials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the NSFC (U1532141, 21631001, 21871001, and 21803001), the Ministry of Education, the Education Department of Anhui Province (KJ2017A010), and the 211 Project of Anhui University.Notes and references
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208 CrossRef CAS PubMed.
- Q. Yao, X. Yuan, T. Chen, D. T. Leong and J. Xie, Adv. Mater., 2018, 30, e1802751 CrossRef PubMed.
- J. Yan, B. K. Teo and N. Zheng, Acc. Chem. Res., 2018, 51, 3084 CrossRef CAS PubMed.
- M. Agrachev, M. Ruzzi, A. Venzo and F. Maran, Acc. Chem. Res., 2019, 52, 44 CrossRef CAS PubMed.
- K. Kwak and D. Lee, Acc. Chem. Res., 2019, 52, 12 CrossRef CAS PubMed.
- B. Bhattarai, Y. Zaker, A. Atnagulov, B. Yoon, U. Landman and T. P. Bigioni, Acc. Chem. Res., 2018, 51, 3104 CrossRef CAS PubMed.
- B. Nieto-Ortega and T. Bürgi, Acc. Chem. Res., 2018, 51, 2811 CrossRef CAS PubMed.
- Q. Tang, G. Hu, V. Fung and D.-e. Jiang, Acc. Chem. Res., 2018, 51, 2793 CrossRef CAS PubMed.
- N. A. Sakthivel and A. Dass, Acc. Chem. Res., 2018, 51, 1774 CrossRef CAS PubMed.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Acc. Chem. Res., 2018, 51, 2465 CrossRef CAS PubMed.
- S. Takano, H. Hirai, S. Muramatsu and T. Tsukuda, J. Am. Chem. Soc., 2018, 140, 12314 CrossRef CAS PubMed.
- S. Sharma, K. K. Chakrahari, J.-Y. Saillard and C. W. Liu, Acc. Chem. Res., 2018, 51, 2475 CrossRef CAS PubMed.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Acc. Chem. Res., 2018, 51, 3114 CrossRef CAS PubMed.
- Z. Gan, N. Xia and Z. Wu, Acc. Chem. Res., 2018, 51, 2774 CrossRef CAS PubMed.
- M. Sugiuchi, Y. Shichibu and K. Konishi, Angew. Chem., Int. Ed., 2018, 57, 7855 CrossRef CAS PubMed.
- C. A. Hosier and C. J. Ackerson, J. Am. Chem. Soc., 2019, 141, 309 CrossRef CAS.
- S. Kenzler, C. Schrenk and A. Schnepf, Angew. Chem., Int. Ed., 2017, 56, 393 CrossRef CAS PubMed.
- N. Yan, N. Xia, L. Liao, M. Zhu, F. Jin, R. Jin and Z. Wu, Sci. Adv., 2018, 4, eaat7259 CrossRef CAS PubMed.
- S. Zhuang, D. Chen, L. Liao, Y. Zhao, N. Xia, W. Zhang, C. Wang, J. Yang and Z. Wu, Angew. Chem., Int. Ed., 2020, 59, 3073 CrossRef CAS PubMed.
- X.-S. Han, X. Luan, H.-F. Su, J.-J. Li, S.-F. Yuan, Z. Lei, Y. Pei and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 2309 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399 CrossRef CAS PubMed.
- T.-A. D. Nguyen, Z. R. Jones, B. R. Goldsmith, W. R. Buratto, G. Wu, S. L. Scott and T. W. Hayton, J. Am. Chem. Soc., 2015, 137, 13319 CrossRef CAS PubMed.
- R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689 CrossRef CAS PubMed.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922 CrossRef CAS PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A.-H. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749 CrossRef CAS PubMed.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Acc. Chem. Res., 2018, 51, 3094 CrossRef CAS PubMed.
- S.-S. Zhang, F. Alkan, H.-F. Su, C. M. Aikens, C.-H. Tung and D. Sun, J. Am. Chem. Soc., 2019, 141, 4460 CrossRef CAS PubMed.
- M. van der Linden, A. J. van Bunningen, L. Amidani, M. Bransen, H. Elnaggar, P. Glatzel, A. Meijerink and F. M. F. de Groot, ACS Nano, 2018, 12, 12751 CrossRef CAS.
- F. Tian and R. Chen, J. Am. Chem. Soc., 2019, 141, 7107 CrossRef CAS.
- M. J. Alhilaly, R.-W. Huang, R. Naphade, B. Alamer, M. N. Hedhili, A.-H. Emwas, P. Maity, J. Yin, A. Shkurenko, O. F. Mohammed, M. Eddaoudi and O. M. Bakr, J. Am. Chem. Soc., 2019, 141, 9585 CrossRef CAS PubMed.
- X. Yuan, C. Sun, X. Li, S. Malola, B. K. Teo, H. Häkkinen, L.-S. Zheng and N. Zheng, J. Am. Chem. Soc., 2019, 141, 11905 CrossRef CAS PubMed.
- M. Bodiuzzaman, A. Ghosh, K. S. Sugi, A. Nag, E. Khatun, B. Varghese, G. Paramasivam, S. Antharjanam, G. Natarajan and T. Pradeep, Angew. Chem., Int. Ed., 2019, 58, 189 CrossRef CAS PubMed.
- K. Kim, K. Hirata, K. Nakamura, H. Kitazawa, S. Hayashi, K. Koyasu and T. Tsukuda, Angew. Chem., Int. Ed., 2019, 58, 11637 CrossRef CAS.
- H. Yoshida, M. Ehara, U. D. Priyakumar, T. Kawai and T. Nakashima, Chem. Sci., 2020, 11, 2394 RSC.
- K. K. Chakrahari, R. P. B. Silalahi, J.-H. Liao, S. Kahlal, Y.-C. Liu, J.-F. Lee, M.-H. Chiang, J.-Y. Saillard and C. W. Liu, Chem. Sci., 2018, 9, 6785 RSC.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- L. Liao, C. Wang, S. Zhuang, N. Yan, Y. Zhao, Y. Yang, J. Li, H. Deng and Z. Wu, Angew. Chem., Int. Ed., 2020, 59, 731 CrossRef CAS.
- H. Peng, Z. Huang, H. Deng, W. Wu, K. Huang, Z. Li, W. Chen and J. Liu, Angew. Chem., Int. Ed., 2019 DOI:10.1002/anie.201913445.
- B. Varnholt, M. J. Guberman-Pfeffer, P. Oulevey, S. Antonello, T. Dainese, J. A. Gascón, T. Bürgi and F. Maran, J. Phys. Chem. C, 2016, 120, 25378 CrossRef CAS.
- K. Kwak, W. Choi, Q. Tang, M. Kim, Y. Lee, D.-e. Jiang and D. Lee, Nat. Commun., 2017, 8, 14723 CrossRef PubMed.
- Y.-Z. Li, R. Ganguly, K. Y. Hong, Y. Li, M. E. Tessensohn, R. Webster and W. K. Leong, Chem. Sci., 2018, 9, 8723 RSC.
- B. Du, X. Jiang, A. Das, Q. Zhou, M. Yu, R. Jin and J. Zheng, Nat. Nanotechnol., 2017, 12, 1096 CrossRef CAS PubMed.
- X. Jiang, B. Du and J. Zheng, Nat. Nanotechnol., 2019, 14, 874 CrossRef CAS PubMed.
- Y. Lin, P. Charchar, A. J. Christofferson, M. R. Thomas, N. Todorova, M. M. Mazo, Q. Chen, J. Doutch, R. Richardson, I. Yarovsky and M. M. Stevens, J. Am. Chem. Soc., 2018, 140, 18217 CrossRef CAS PubMed.
- Y. Liu, K. Ai, X. Cheng, L. Huo and L. Lu, Adv. Funct. Mater., 2010, 20, 951 CrossRef CAS.
- J. Yu, S. A. Patel and R. M. Dickson, Angew. Chem., Int. Ed., 2007, 46, 2028 CrossRef CAS PubMed.
- X.-D. Zhang, J. Chen, Z. Luo, D. Wu, X. Shen, S.-S. Song, Y.-M. Sun, P.-X. Liu, J. Zhao, S. Huo, S. Fan, F. Fan, X.-J. Liang and J. Xie, Adv. Healthcare Mater., 2014, 3, 133 CrossRef CAS PubMed.
- L. Shang, F. Stockmar, N. Azadfar and G. U. Nienhaus, Angew. Chem., Int. Ed., 2013, 52, 11154 CrossRef CAS PubMed.
- H. Zhang, H. Liu, Z. Tian, D. Lu, Y. Yu, S. Cestellos-Blanco, K. K. Sakimoto and P. Yang, Nat. Nanotechnol., 2018, 13, 900 CrossRef PubMed.
- M. A. H. Muhammed, L. K. Cruz, A.-H. Emwas, A. M. El-Zohry, B. Moosa, O. F. Mohammed and N. M. Khashab, Angew. Chem., Int. Ed., 2019, 58, 15665 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. D. Gobbo, R. G. AbdulHalim, M. Eddaoudi, D.-e. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970 CrossRef CAS PubMed.
- A. Nag, P. Chakraborty, M. Bodiuzzaman, T. Ahuja, S. Antharjanam and T. Pradeep, Nanoscale, 2018, 10, 9851 RSC.
- J. F. Parker, J. E. F. Weaver, F. McCallum, C. A. Fields-Zinna and R. W. Murray, Langmuir, 2010, 26, 13650 CrossRef CAS PubMed.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578 CrossRef CAS PubMed.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 11880 CrossRef CAS PubMed.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun., 2013, 4, 2422 CrossRef PubMed.
- J. Yan, H. Su, H. Yang, C. Hu, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2016, 138, 12751 CrossRef CAS PubMed.
- P. Chakraborty, A. Nag, K. S. Sugi, T. Ahuja, B. Varghese and T. Pradeep, ACS Mater. Lett., 2019, 1, 534 CrossRef CAS.
- K. Pyo, V. D. Thanthirige, K. Kwak, P. Pandurangan, G. Ramakrishna and D. Lee, J. Am. Chem. Soc., 2015, 137, 8244 CrossRef CAS PubMed.
- K. Pyo, V. D. Thanthirige, S. Y. Yoon, G. Ramakrishna and D. Lee, Nanoscale, 2016, 8, 20008 RSC.
- J. W. Padelford, T. Wang and G. Wang, ChemElectroChem, 2016, 3, 1201 CrossRef CAS.
- X. Kang, S. Wang and M. Zhu, Chem. Sci., 2018, 9, 3062 RSC.
- M. H. Ropers, G. Czichocki and G. Brezesinski, J. Phys. Chem. B, 2003, 107, 5281 CrossRef CAS.
- F. S. Lima, I. M. Cuccovia, R. Buchner, F. E. Antunes, B. Lindman, M. G. Miguel, D. Horinek and H. Chaimovich, Langmuir, 2015, 31, 2609 CrossRef CAS PubMed.
- F. Talens-Alesson, J. Phys. Chem. B, 2009, 113, 9779 CrossRef CAS PubMed.
- L. Moreira and A. Firoozabadi, Langmuir, 2010, 26, 15177 CrossRef CAS PubMed.
- S. Mondal, S. Ghosh and S. De, Langmuir, 2012, 28, 11329 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S8 and Tables S1–S3 for the ESI-MS, NMR, UV-vis, and HAADF-STEM results of nanoclusters, and the structural comparison between nanoclusters. CCDC 1941155 and 1889356. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc01055c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |