Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic pH responsivity of triazole-based self-immolative linkers†
Derrick A.
Roberts
 *ab,
Ben S.
Pilgrim
*ab,
Ben S.
Pilgrim
 c,
Tristan N.
Dell
c,
Tristan N.
Dell
 d and
Molly M.
Stevens
d and
Molly M.
Stevens
 *bd
*bd
aKey Center for Polymers and Colloids, School of Chemistry, The University of Sydney, Sydney, NSW 2006, Australia. E-mail: derrick.roberts@sydney.edu.au
bDepartment of Medical Biochemistry and Biophysics, Karolinska Institutet, 171 77 Stockholm, Sweden
cSchool of Chemistry, The University of Nottingham, Nottingham NG7 2RD, UK
dDepartment of Materials, Department of Bioengineering, Institute for Biomedical Engineering, Imperial College London, London SW7 2AZ, UK. E-mail: m.stevens@imperial.ac.uk
First published on 3rd March 2020
Abstract
Gating the release of chemical payloads in response to transient signals is an important feature of ‘smart’ delivery systems. Herein, we report a triazole-based self-immolative linker that can be reversibly paused or slowed and restarted throughout its elimination cascade in response to pH changes in both organic and organic-aqueous solvents. The linker is conveniently prepared using the alkyne–azide cycloaddition reaction, which introduces a 1,4-triazole ring that expresses a pH-sensitive intermediate during its elimination sequence. Using a series of model compounds, we demonstrate that this intermediate can be switched between active and dormant states depending on the presence of acid or base, cleanly gating the release of payload in response to a fluctuating external stimulus.
Introduction
Chemists have long pursued stimuli-specific strategies for activating latent molecules, motivated by applications in controlled release,1 sensing,2 imaging,3 and signal amplification.4 To this end, ‘self-immolation’ has emerged as a powerful tool. Originally conceived in the 1980s to improve prodrug activation,5 self-immolation is the spontaneous and irreversible fragmentation of a multicomponent compound into small molecules through a cascade of cyclisation or elimination reactions—a process often likened to the falling of dominoes or the burning of a fuse.6 Molecules that undergo self-immolation have been adapted as linkers for temporarily connecting a cleavable protecting group (designated as the ‘trigger’) to a chemical payload.7 These linkers maintain space between the trigger and payload and enhance the entropic driving force that promotes complete and traceless deprotection.8 These unique qualities have inspired numerous studies into the development of self-immolative linkers for the controlled release of small molecules9 and, more recently, for constructing self-immolative polymers that undergo complete head-to-tail depolymerisation upon trigger cleavage.10Self-immolative linkers are ideally activated by removal of the trigger group in response to a specific chemical or biological stimulus.11 After trigger cleavage, the ensuing self-immolation sequence proceeds along an uninterrupted kinetics trajectory that is largely predetermined by steric and electronic properties of the linker.12 By contrast, remarkably few studies have investigated ways to dynamically control the kinetics of self-immolation using external signals after removal of the trigger. Recently, de Alaniz and co-workers reported the first example of reversible ‘pausing’ of a self-immolative depolymerisation reaction in response to temperature changes.13 This work highlights an important advantage of dynamically responsive self-immolation: the ability to switch the cascade between active and paused states ensures that payload delivery proceeds only under specific environmental conditions and slows or ceases entirely if those conditions suddenly change. Considering the paucity of reported examples, there are unexplored opportunities for dynamically controlling self-immolation kinetics using transient or fluctuating signals.
It is well known that self-immolative linkers containing basic residues are sensitive to changes in acidity.12 This feature is exploited during the synthesis of cyclisation–elimination self-immolative polymers14 and, most recently, for tuning the initial release kinetics of a self-immolative H2S delivery agent.15 However, to the best of our knowledge there are no studies that use transient changes in acidity to dynamically switch a self-immolative linker between active and dormant states following trigger removal. Herein we investigate triazole-based self-immolative linkers that can be reversibly paused or slowed and restarted throughout their self-immolation sequences upon addition of acid and base to the reaction medium. The pausing mechanism relies on the formation of a metastable intermediate during linker degradation that eliminates under mild basic conditions but remains stable in more acidic conditions. By alternating between acidic and basic conditions, the cascade can be switched rapidly between active and paused states to control the payload release rate even after the trigger group is removed.
Results and discussion
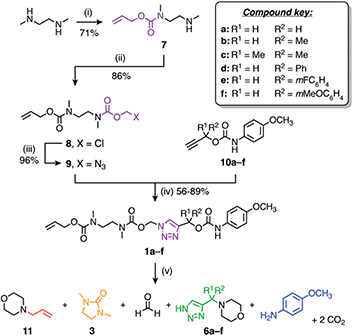
Our linker system is comprised of a diamine-derived cyclisation spacer14 connected in series to a triazole 1,4-elimination spacer (Scheme 1).16 Incorporating a diamine-derived spacer next to the triazole ring permits the use of carbamate trigger groups,17 which drastically expands the scope of ester triggers reported previously.18 Removal of the trigger group from 1 under basic conditions exposes a secondary amine nucleophile (2) that undergoes a cyclisation–elimination reaction to afford a cyclic urea (3) and a short-lived triazolyl-1-methanol species. Rapid elimination of formaldehyde affords a metastable 1H-triazole intermediate (4). Under basic conditions, triazole 4 undergoes 1,4-elimination via a triazolide anion to release the payload molecule, carbon dioxide and triazafulvene 5, which is rapidly converted to 6via a Michael addition. However, addition of acid to the medium hinders deprotonation of 1H-triazole 4, thereby pausing or slowing (depending on the position of equilibrium) self-immolation. Addition of a suitable base reactivates the cascade, which proceeds to completion along the same kinetics profile as before acidification.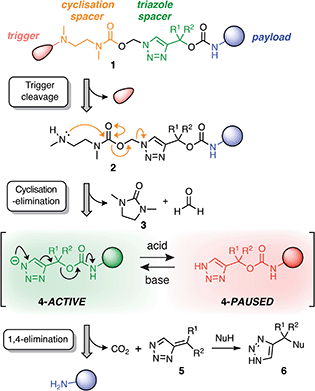 | ||
| Scheme 1 Overview of the self-immolation sequence for hybrid diamine–triazole linkers reported herein. Trigger removal from 1 leads to the formation of 1H-triazole 4, which can be reversibly switched between active and paused states to gate the release of the payload molecule. ‘Nu’ denotes a generic nucleophile. R-groups are specified in Scheme 2. | ||
The self-immolative linker is formed by a copper(I)-catalysed azide–alkyne cycloaddition (CuAAC) reaction between azide 9 and a propargylic carbamate-protected payload molecule (10a–f) (Scheme 2). To standardise the initial trigger activation step, the model systems studied herein feature an allyl carbamate trigger group that is cleaved rapidly in the presence of palladium(0) under basic conditions. Allyl-protected azide 9 was synthesised in ∼60% yield over three steps starting with the treatment of 1,2-dimethylethylenediamine with allyl phenyl carbonate to furnish mono-protected amine 7 in good yield. Subsequent treatment with chloromethyl chloroformate gave 8, followed by azidation with sodium azide in DMF to afford azide 9.
Russell and co-workers established that the rate of 1,4-elimination across a triazole ring is sensitive to substituents at the triazole α-methine position, enabling tuning of the payload release rate.18 To ascertain if this sensitivity is preserved in our system, we synthesised a series of α-substituted propargylic carbamates (10a–f) carrying a p-anisidine model payload. Model compounds 1a–f were subsequently prepared by treating alkynes 10a–f with azide 9 under copper(I) catalysis in DMF at 50 °C (Scheme 2). NMR and MS analyses confirmed successful formation of 1a–f, and FTIR confirmed loss of the alkyne and azide stretching frequencies following CuAAC coupling (ESI, Section S5†).
Detailed analyses of the self-immolation kinetics of model compounds 1a–1f were performed in DMSO-d6 to elucidate the self-immolation mechanism in a non-nucleophilic solvent. Reactions were monitored by in situ1H NMR spectroscopy at 60 °C (ESI, Section S6†).19 Linkers were activated by adding a suspension of Pd(PPh3)4 in DMSO-d6 to a solution of the model compound (10–30 mM) containing excess morpholine (50 equiv.) to render all base-mediated reaction steps pseudo-first-order. Control experiments confirmed that the model compounds were stable towards base in the absence of Pd(PPh3)4 (ESI, Fig. S70†).
For each model compound, the three stages of the self-immolation cascade were distinguishable by NMR, allowing calculation of pseudo-first-order rate constants (kobs) for each step (Scheme 3; ESI, Section S6.6†). Rate constants for trigger removal and cyclisation–elimination were consistent between compounds, proceeding on the order of 10−2 s−1 and 10−3 s−1, respectively. For both stages, elimination products (urea 3 and amine 11, Scheme 2) were identified by comparison with reference 1H NMR spectra (Section S6.4†). By contrast, collapse of 1H-triazole 4via 1,4-elimination varied widely between the different compounds, ranging from minutes (1c–f) and hours (1b) up to several days (1a) to achieve maximum (ca. 90%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 payload release (Fig. 1). As anticipated, the rate of 1,4-elimination was influenced by the triazole α-methine substituent to a degree, whereby rates increased with the degree of substitution, and thus stability, of triazafulvene 5. However, we did not observe appreciable differences between 1,4-elimination rates of 1c–f due to competition between the cyclisation and 1,4-elimination steps for these compounds (Fig. 4b; ESI, Section S6.6 for details†).21 Nonetheless, rate differences between 1a, 1b and 1c–f illustrate payload delivery across a wide range of release lifetimes.
20 payload release (Fig. 1). As anticipated, the rate of 1,4-elimination was influenced by the triazole α-methine substituent to a degree, whereby rates increased with the degree of substitution, and thus stability, of triazafulvene 5. However, we did not observe appreciable differences between 1,4-elimination rates of 1c–f due to competition between the cyclisation and 1,4-elimination steps for these compounds (Fig. 4b; ESI, Section S6.6 for details†).21 Nonetheless, rate differences between 1a, 1b and 1c–f illustrate payload delivery across a wide range of release lifetimes.
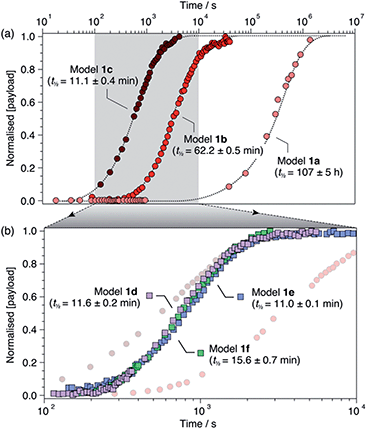 | ||
| Fig. 1 Normalised payload release profiles for (a) 1a–c and (b) 1d–f (1b and 1c shown in background) in DMSO-d6 at 60 °C with 50 equiv. morpholine. Release half-lives are shown in parentheses and were calculated from fitting analyses (ESI, Section S6†). Dotted lines are included as visual guides. | ||
For all models except 1c22 we observed transient formation of 1H-triazole intermediate 4 (identified by a 1H signal around 7.7 ppm), which formed at the same rate as the cyclisation products and was consumed at the same rate as payload release (ESI, Fig. S76†). The triazolide anion, which has been detected in strongly basic media,18 was not directly observed under the mildly basic conditions provided by morpholine. This is consistent with the pKa difference between morpholinium (∼9.2 in DMSO23) and 1H-1,2,3,-triazole (∼13.9 in DMSO24), which suggests that intermediate 4 exists predominantly in its 1H-triazole form during the cascade with only a small fraction dissociated at any time. Despite limited dissociation, 4 undergoes efficient 1,4-elimination due to irreversible loss of CO2, leading to efficient release of the anisidine payload.
Having established that models 1a–f undergo successful self-immolation in DMSO-d6, we sought to evaluate their behaviour in the presence of water. In situ1H NMR kinetics using models 1a, 1b and 1d (chosen to cover the slow, medium and fast kinetics regimes observed in DMSO-d6) were performed in DMSO-d6/D2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) at 60 °C. Poor solubility of the compounds in neat D2O precluded higher aqueous fractions. Control experiments showed that no background hydrolysis occurred in DMSO-d6/D2O (8
2, v/v) at 60 °C. Poor solubility of the compounds in neat D2O precluded higher aqueous fractions. Control experiments showed that no background hydrolysis occurred in DMSO-d6/D2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) containing morpholine (50 equiv.), confirming the excellent stability of all three compounds (ESI, Section 7.1†). Pd(PPh3)4 successfully initiated the cascades following brief induction periods of 1–3 min reflecting delayed trigger removal (ESI, Section 7.2†), most likely due to reduced solubility of Pd(PPh3)4 in the presence of water. Following the induction periods, self-immolation proceeded smoothly for all compounds. Interestingly, pseudo-first-order rate constants for trigger removal and cyclisation–elimination were similar to those recorded in DMSO-d6. However, 1,4-elimination was four-fold (1d), 11-fold (1b) and 30-fold (1a) faster in the presence of D2O, which we attribute to rapid nucleophilic attack of triazafulvene 5 by the solvent (ESI, Table S3†). A similar trend in payload release rates was observed, with half-lives of 3.45 ± 0.06 h (1a), 5.85 ± 0.14 min (1b) and 2.94 ± 0.07 min (1d).
2, v/v) containing morpholine (50 equiv.), confirming the excellent stability of all three compounds (ESI, Section 7.1†). Pd(PPh3)4 successfully initiated the cascades following brief induction periods of 1–3 min reflecting delayed trigger removal (ESI, Section 7.2†), most likely due to reduced solubility of Pd(PPh3)4 in the presence of water. Following the induction periods, self-immolation proceeded smoothly for all compounds. Interestingly, pseudo-first-order rate constants for trigger removal and cyclisation–elimination were similar to those recorded in DMSO-d6. However, 1,4-elimination was four-fold (1d), 11-fold (1b) and 30-fold (1a) faster in the presence of D2O, which we attribute to rapid nucleophilic attack of triazafulvene 5 by the solvent (ESI, Table S3†). A similar trend in payload release rates was observed, with half-lives of 3.45 ± 0.06 h (1a), 5.85 ± 0.14 min (1b) and 2.94 ± 0.07 min (1d).
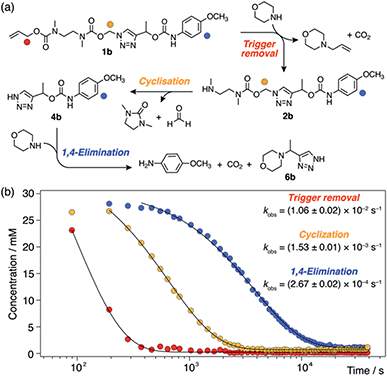
Observation of 1H-triazole 4 as a key intermediate in the self-immolation cascade led us to hypothesise that acidifying the system would hinder deprotonation of 4, thereby interrupting the 1,4-elimination step and slowing or even pausing payload release mid-cascade. To establish proof-of-concept of switchable self-immolation, we employed model 1b due to its moderately fast 1,4-elimination rate and its relatively long-lived 1H-triazole intermediate (Scheme 4). Palladium-activated self-immolation of 1b with excess morpholine proceeded with rapid trigger removal followed by cyclisation–elimination. After completion of the cyclisation step (∼35 min), trifluoroacetic acid (TFA, 5 equiv.) was added, which immediately halted payload release and stabilised the concentrations of all species, confirming that the system had entered a paused state (Scheme 4b). Crucially, the concentration of intermediate 6b remained stable at ∼9 mM for least 45 min, confirming that 1,4-elimination effectively ceases in the absence of base. 1H NMR analysis indicated that the 1H-triazole form of 4b was the main dormant species, rather than its protonated triazolium congener (ESI, Section S8†), which is consistent with the poor basicity of 1,2,3-triazoles.25
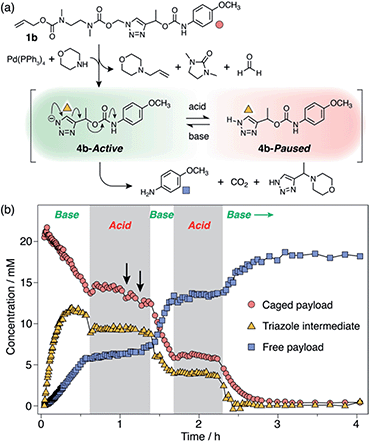
Attempts to reactivate self-immolation by the addition of sub-stoichiometric Cs2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 (2 × 2 equiv., arrows in Scheme 4b) produced only slight changes in the self-immolation rate. Only upon complete neutralization of the acid (6 equiv. total Cs2CO3) did the cascade return to its active state. In contrast to morpholine, Cs2CO3 facilitated more complete deprotonation of 4b leading to formation of the triazolide anion, as evidenced in the 1H NMR spectra by the up-field shift of the aromatic triazole peak from ∼7.78 ppm to ∼7.73 ppm (ESI, Fig. S96†).
26 (2 × 2 equiv., arrows in Scheme 4b) produced only slight changes in the self-immolation rate. Only upon complete neutralization of the acid (6 equiv. total Cs2CO3) did the cascade return to its active state. In contrast to morpholine, Cs2CO3 facilitated more complete deprotonation of 4b leading to formation of the triazolide anion, as evidenced in the 1H NMR spectra by the up-field shift of the aromatic triazole peak from ∼7.78 ppm to ∼7.73 ppm (ESI, Fig. S96†).
To ascertain if multiple switching was possible, a second aliquot of TFA (8 equiv.) was added after a further 30 min of self-immolation. Once again, the system entered its paused state, characterised by a plateau in the kinetics profiles of key species that lasted at least 55 min, until the addition of further Cs2CO3 (9.5 equiv.) returned the system to its active state (Scheme 4b). Upon completion of the cascade, the system reached a comparable degree of payload release to the non-switched experiments, indicating that switching between protonated and deprotonated states occurred cleanly and without appreciable deleterious side reactions.
Dynamic switching experiments were also conducted on models 1a and 1e in DMSO-d6 to study the degree of rate control in the ‘slow’ and ‘fast’ kinetics regimes. In both cases, addition of TFA (5 equiv.) to the activated linker systems drastically altered their self-immolation kinetics. Model 1a exhibited a paused phase similar to 1b that remained stable over ∼50 h (ESI, Fig. S94†). By contrast, 1e slowed drastically but did not pause completely (ESI, Fig. S98†). This can be attributed to the instability of 1H-triazole intermediate 4e, which undergoes rapid 1,4-elimination due to the stabilizing influence of its aromatic α-methine substituent. In both cases, neutralization of the acid with Cs2CO3 returned both systems to their original active phases to engender complete payload delivery. These results confirm that the rate of payload release can be influenced under all three kinetics regimes, with stable pausing achieved for 1a and 1b, and significant rate modulation without pausing for 1e.
Finally, pH switching experiments were performed in DMSO-d6/D2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) using models 1a and 1b27 to demonstrate that the pausing mechanism could operate successfully in aqueous solvent mixtures. Switching was achieved by sequential additions of aqueous HCl and NaOH (20–30 equiv. each) to the reaction mixtures. Similar to their behaviour in neat DMSO, both model systems could be switched cleanly between active and paused phases multiple times during their cascade sequences (ESI, Section S9†). In both cases, stable paused phases were observed and final payload release efficiencies of >90% were achieved upon completion of the cascades, thus demonstrating that pH-mediated switching performs well in aqueous mixtures.
2, v/v) using models 1a and 1b27 to demonstrate that the pausing mechanism could operate successfully in aqueous solvent mixtures. Switching was achieved by sequential additions of aqueous HCl and NaOH (20–30 equiv. each) to the reaction mixtures. Similar to their behaviour in neat DMSO, both model systems could be switched cleanly between active and paused phases multiple times during their cascade sequences (ESI, Section S9†). In both cases, stable paused phases were observed and final payload release efficiencies of >90% were achieved upon completion of the cascades, thus demonstrating that pH-mediated switching performs well in aqueous mixtures.
Conclusions
In conclusion, we have demonstrated a mechanism for dynamically pausing and restarting an activated self-immolation cascade in response to pH changes. The pausing mechanism exploits the formation of a metastable 1H-triazole intermediate, whose deprotonation dictates the overall payload release profile. The system is remarkably stable under acidic conditions, allowing repeated switching in both organic and aqueous-organic solvent mixtures. The ability to dynamically regulate self-immolation using external signals has high relevance to ‘smart’ payload release systems featuring stimulus-responsive kinetics.28 Our findings thus highlight a new application of self-immolative triazoles, which have found remarkably little use in small molecule linker designs and are yet to feature in self-immolative polymers (SIPs). We believe the modularity of this system offers broad scope for developing new stimuli-responsive controlled-release systems, especially in materials that operate under mildly basic conditions such as paints, resins and surface coatings.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. A. R. acknowledges support from the Australian Research Council Discovery Early-Career Researcher Award scheme (DE190100797), the EU Horizon 2020 Marie Skłodowska-Curie Fellowship (705475), and the DVCR Office at The University of Sydney. B. S. P. acknowledges the Green Chemicals Beacon Research Fellowship from the University of Nottingham. T. N. D. received funding under the EPSRC Doctoral Training Partnership (EP/R513052/1). M. M. S. acknowledges funding from the Swedish Research Council (VR 4-478/2016). Prof. Jonathan Nitschke is thanked for hosting D. A. R. and B. S. P. at the University of Cambridge, and Dr Christopher Wood, Dr Andrew Danos and Dr Toby Hudson are thanked for helpful discussions. Raw data are available at DOI: 10.5281/zenodo.3699304.Notes and references
- (a) A. Albert, Nature, 1958, 182, 421–422 CrossRef CAS PubMed; (b) J. Li, J. Yu, J. Zhao, J. Wang, S. Zheng, S. Lin, L. Chen, M. Yang, S. Jia, X. Zhang and P. R. Chen, Nat. Chem., 2014, 6, 352–361 CrossRef CAS PubMed; (c) Y. Li, G. Liu, X. Wang, J. Hu and S. Liu, Angew. Chem., Int. Ed., 2016, 55, 1760–1764 CrossRef CAS PubMed; (d) X. Ji, Z. Pan, B. Yu, L. K. De La Cruz, Y. Zheng, B. Ke and B. Wang, Chem. Soc. Rev., 2019, 48, 1077–1094 RSC.
- (a) J. Yan, S. Lee, A. Zhang and J. Yoon, Chem. Soc. Rev., 2018, 47, 6900–6916 RSC; (b) S. Gnaim and D. Shabat, Acc. Chem. Res., 2019, 52, 2806–2817 CrossRef CAS PubMed.
- (a) Q. Shao and B. Xing, Chem. Soc. Rev., 2010, 39, 2835–2846 RSC; (b) T. Slanina, P. Shrestha, E. Palao, D. Kand, J. A. Peterson, A. S. Dutton, N. Rubinstein, R. Weinstain, A. H. Winter and P. Klan, J. Am. Chem. Soc., 2017, 139, 15168–15175 CrossRef CAS PubMed; (c) D. Kand, L. Pizarro, I. Angel, A. Avni, D. Friedmann-Morvinski and R. Weinstain, Angew. Chem., Int. Ed., 2019, 58, 4659–4663 CrossRef CAS PubMed.
- M. E. Roth, O. Green, S. Gnaim and D. Shabat, Chem. Rev., 2016, 116, 1309–1352 CrossRef CAS PubMed.
- P. L. Carl, P. K. Chakravarty and J. A. Katzenellenbogen, J. Med. Chem., 1981, 24, 479–480 CrossRef CAS PubMed.
- S. Gnaim and D. Shabat, J. Am. Chem. Soc., 2017, 139, 10002–10008 CrossRef CAS PubMed.
- (a) F. M. de Groot, W. J. Loos, R. Koekkoek, L. W. van Berkom, G. F. Busscher, A. E. Seelen, C. Albrecht, P. de Bruijn and H. W. Scheeren, J. Org. Chem., 2001, 66, 8815–8830 CrossRef CAS PubMed; (b) A. Gopin, N. Pessah, M. Shamis, C. Rader and D. Shabat, Angew. Chem., Int. Ed., 2003, 42, 327–332 CrossRef CAS PubMed.
- C. A. Blencowe, A. T. Russell, F. Greco, W. Hayes and D. W. Thornthwaite, Polym. Chem., 2011, 2, 773–790 RSC.
- (a) K. M. Schmid, L. Jensen and S. T. Phillips, J. Org. Chem., 2012, 77, 4363–4374 CrossRef CAS PubMed; (b) Q. E. A. Sirianni, A. Rabiee Kenaree and E. R. Gillies, Macromolecules, 2018, 52, 262–270 CrossRef; (c) A. L. Acton, F. Leroux, A. Feula, K. Melia, M. R. Sambrook, W. Hayes and A. T. Russell, Chem. Commun., 2019, 55, 5219–5222 RSC; (d) A. Dal Corso, V. Borlandelli, C. Corno, P. Perego, L. Belvisi, L. Pignataro and C. Gennari, Angew. Chem., Int. Ed., 2020, 59, 4176–4181 CrossRef CAS PubMed.
- (a) A. Sagi, R. Weinstain, N. Karton and D. Shabat, J. Am. Chem. Soc., 2008, 130, 5434–5435 CrossRef CAS PubMed; (b) B. Fan, J. F. Trant, A. D. Wong and E. R. Gillies, J. Am. Chem. Soc., 2014, 136, 10116–10123 CrossRef CAS PubMed; (c) C. E. Diesendruck, G. I. Peterson, H. J. Kulik, J. A. Kaitz, B. D. Mar, P. A. May, S. R. White, T. J. Martinez, A. J. Boydston and J. S. Moore, Nat. Chem., 2014, 6, 623–628 CrossRef CAS PubMed; (d) G. Liu, G. Zhang, J. Hu, X. Wang, M. Zhu and S. Liu, J. Am. Chem. Soc., 2015, 137, 11645–11655 CrossRef CAS PubMed; (e) K. Yeung, H. Kim, H. Mohapatra and S. T. Phillips, J. Am. Chem. Soc., 2015, 137, 5324–5327 CrossRef CAS PubMed; (f) A. Rabiee Kenaree and E. R. Gillies, Macromolecules, 2018, 51, 5501–5510 CrossRef CAS.
- (a) G. I. Peterson, M. B. Larsen and A. J. Boydston, Macromolecules, 2012, 45, 7317–7328 CrossRef CAS; (b) H. Mutlu and C. Barner-Kowollik, Polym. Chem., 2016, 7, 2272–2279 RSC; (c) S. Davies, B. L. Oliveira and G. J. L. Bernardes, Org. Biomol. Chem., 2019, 17, 5725–5730 RSC.
- A. Alouane, R. Labruere, T. Le Saux, F. Schmidt and L. Jullien, Angew. Chem., Int. Ed., 2015, 54, 7492–7509 CrossRef CAS PubMed.
- M. F. Nichol, K. D. Clark, N. D. Dolinski and J. R. de Alaniz, Polym. Chem., 2019, 10, 4914–4919 RSC.
- (a) M. A. Dewit, A. Beaton and E. R. Gillies, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3977–3985 CrossRef CAS; (b) E. K. Y. Chen, R. A. McBride and E. R. Gillies, Macromolecules, 2012, 45, 7364–7374 CrossRef CAS; (c) R. A. McBride and E. R. Gillies, Macromolecules, 2013, 46, 5157–5166 CrossRef CAS.
- A. K. Gilbert, Y. Zhao, C. E. Otteson and M. D. Pluth, J. Org. Chem., 2019, 84, 14469–14475 CrossRef CAS PubMed.
- (a) P. Bertrand and J. P. Gesson, J. Org. Chem., 2007, 72, 3596–3599 CrossRef CAS PubMed; (b) R. Delatouche, M. Mondon, A. Gil, G. Frapper, C. Bachmann and P. Bertrand, Tetrahedron, 2011, 67, 401–407 CrossRef CAS; (c) M. Mondon, R. Delatouche, C. Bachmann, G. Frapper, C. Len and P. Bertrand, Eur. J. Org. Chem., 2011, 2011, 2111–2119 CrossRef; (d) R. Delatouche, C. Bachmann, G. Frapper and P. Bertrand, Synthesis, 2012, 44, 1090–1094 CrossRef CAS.
- M. A. Dewit and E. R. Gillies, J. Am. Chem. Soc., 2009, 131, 18327–18334 CrossRef CAS PubMed.
- C. A. Blencowe, D. W. Thornthwaite, W. Hayes and A. T. Russell, Org. Biomol. Chem., 2015, 13, 8703–8707 RSC.
- A reaction temperature of 60 °C afforded conveniently measured reaction rates. Cascades proceeded at lower temperatures (e.g., 20 °C), albeit much slower.
- All model compounds achieved ∼90% free payload release, with ∼10% forming uncharacterised minor aromatic side-products (e.g., possibly Michael adducts of triazafulvene 5).
- Attempts to synthesise more rapidly cyclising carbonate spacers were complicated by their reduced stability under basic reaction conditions. Improving cyclisation rates remains the focus of ongoing work.
- For 6c, 1,4-elimination was so rapid that the putative 1H-triazole intermediate was consumed as soon as it formed (see ESI, Fig. S77†).
- M. R. Crampton and I. A. Robotham, J. Chem. Res., 1997, 22–23 RSC.
- F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456–463 CrossRef CAS.
- S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696–2718 CrossRef CAS PubMed.
- Addition of extra morpholine (up to 50 equiv.) failed to restart the cascade, presumably due to buffering effects. Cs2CO3 was therefore used to achieve complete neutralization of the TFA.
- Models in the fast kinetics regime (1c–f) were not studied as their extremely rapid cascades in DMSO-d6/D2O were complete within the time required to re-shim the NMR spectrometer after acid/base additions.
- G. Liu, X. Wang, J. Hu, G. Zhang and S. Liu, J. Am. Chem. Soc., 2014, 136, 7492–7497 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR and FTIR spectra, and detailed kinetics data. See DOI: 10.1039/d0sc00532k |
| This journal is © The Royal Society of Chemistry 2020 |