Open Access Article
Open Access ArticleMulti-color tunable circularly polarized luminescence in one single AIE system†
Hongxing
Shang‡
a,
Zeyang
Ding‡
a,
Yue
Shen
a,
Bing
Yang
 a,
Minghua
Liu
a,
Minghua
Liu
 *b and
Shimei
Jiang
*b and
Shimei
Jiang
 *a
*a
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: smjiang@jlu.edu.cn
bCAS Key Laboratory of Colloid, Interface and Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: liumh@iccas.ac.cn
First published on 15th January 2020
Abstract
Circularly polarized luminescence (CPL) materials with a large luminescence dissymmetry factor (glum) and multi-color properties are very attractive. While multi-color tunable CPL can be realized by different organic dyes, the challenge of realizing both a higher glum and multiple colors using a single component remains. Here, we design an aggregation-induced emission (AIE) fluorophore, which is a pyridine functionalized cyanostilbene attached to a chiral unit, and realize multi-color tunable CPL with a high glum. The compound can self-assemble into a nanohelix and form both gel and xerogel films, exhibiting blue CPL with large glum values of −3.0 × 10−2 and −1.7 × 10−2, respectively. With the assistance of pyridine protonation, the xerogel films exhibit red-shifted CPL signals from 480 nm to 530 nm, covering from blue via green and yellow to orange. Additionally, the glum remains constant during the process. This work paves a simple and convenient way to construct multi-color tunable CPL materials using a single molecule.
Introduction
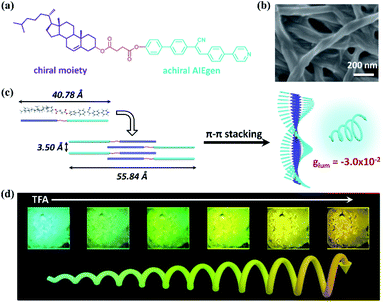
Circularly polarized luminescence (CPL), a sensitive tool to reflect the chiral information in the excited state, has received widespread attention in recent years on account of its widespread potential applications in the storage of information, chemical sensors and stereoscopic displays.1 These applications require CPL materials which exhibit a large luminescence dissymmetry factor (glum) and multi-color tunable properties in aggregates. To amplify the glum, methods like supramolecular self-assembly, energy transfer and doping of liquid crystals are utilized.2 To realize multi-color CPL, a typical approach is to use several chiral compounds emitting different colors.3 But tedious and complicated organic syntheses are usually needed in these processes. It remains a challenge to combine multi-color CPL with a large glum in one fluorescent dye.To achieve this goal, we design a single molecule system and realize both higher glum and color controllable CPL in aggregates. The molecule consists of a pyridine functionalized π-structure (cyanostilbene), a chiral cholesterol group and a linking group (ester bond) and is called Chol-CN-Py (Fig. 1a). Firstly, the achiral cyanostilbene is a well-known aggregation-induced emission (AIE) fluorophore, which contributes to strong emission in the condensed state.4 Besides, the negligible chirality of cholesterol in the monomer state can be amplified and transferred to the achiral fluorophore upon aggregation, leading to anticipated aggregation induced circular dichroism (AICD) and aggregation induced circularly polarized luminescence (AICPL) properties.5 Secondly, pyridine is a well-known electronegative agent capable of protonation, which allows the change of energy levels in the protonated state. Correspondingly, possible tunable emission as well as CPL would be realized in a single molecule system.6
It has been found that Chol-CN-Py shows great gelation ability and forms an obvious nanohelix, which allows intense blue emission and CPL in both the gel and xerogel film state. Remarkably, the glum of the gel and xerogel film are up to −3.0 × 10−2 and −1.7 × 10−2, respectively. Under the effect of trifluoroacetic acid (TFA), the xerogel film exhibits rapid and sensitive red-shifted emission modulation owing to the equal and smooth spatial distribution and large surface area of the xerogel film.
Moreover, in the xerogel film state, the chiral structures and chirality transfer are maintained during the protonation process. Thus, through controlling the degree of protonation, the xerogel films exhibit red-shifted CPL signals from 480 nm to 530 nm, covering the multi-color circularly polarized emission from blue via green and yellow to orange color, with a constant handedness and glum (Fig. 1). To the best of our knowledge, this is the first multi-color tunable CPL material using a single molecule.
Results and discussion
Chol-CN-Py was synthesized by a series of Knoevenagel, Suzuki and esterification reactions as a yellow solid (Scheme S1†). The experimental details and corresponding characterization data are given in the ESI (Fig. S1–S5†). Besides, with the assistance of the synergy of van der Waals interactions and π–π interactions offered by cholesterol and the cyanostilbene group, Chol-CN-Py could form a stable gel in DMSO through a normal heating-cooling procedure (Table S1†).7 The gel was also characterized by rheology experiments. The higher storage moduli (G′) and the relatively low loss moduli (G′′) indicated the gel formation as well as its better mechanical properties (Fig. S6†).8Further characterization was performed to investigate the structure of the gel, which displayed entangled winding 3D networks consisted of left-handed nanohelix fibers, as shown in the scanning electron microscopy (SEM) images, indicating a kind of chiral expression in the microstructures (Fig. 1b and S7†).9 Although multiple chiral centers existed in cholesterol moiety, only left-handed nanohelices were observed even in various concentrations, suggesting stable chiral self-assembly behaviors. In order to understand the packing modes, X-ray diffraction (XRD) was employed (Fig. S8†). The XRD pattern of xerogel showed four strong reflection peaks at 2θ = 1.54°, 3.12°, 4.71° and 6.32° in the small angle region. Based on Bragg's equation, their corresponding d-spacing values were calculated to be 55.84 Å, 28.10 Å, 18.74 Å and 13.97 Å, respectively, following the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3
1/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/4, indicating a layered packing structure with an interlayer distance of 55.84 Å.10 In the wide angle region, an obvious diffraction peak with a d-spacing of 3.50 Å was observed, which could be assigned to the π–π stacking distance between the cyanostilbene units in the packing structures.11 Considering that the length of the Chol-CN-Py molecule was only 40.78 Å, the interlayer distances of the layers were longer than the length of the molecule, but shorter than twice that. Thereby, Chol-CN-Py self-assembled into a bilayer structure through the π–π interactions.12 The bilayer structure served as the basic unit and then twisted into helical nanofibers. Fibers were entangled with each other to form the chiral supramolecular gel finally. A schematic illustration is shown in Fig. 1c.
1/4, indicating a layered packing structure with an interlayer distance of 55.84 Å.10 In the wide angle region, an obvious diffraction peak with a d-spacing of 3.50 Å was observed, which could be assigned to the π–π stacking distance between the cyanostilbene units in the packing structures.11 Considering that the length of the Chol-CN-Py molecule was only 40.78 Å, the interlayer distances of the layers were longer than the length of the molecule, but shorter than twice that. Thereby, Chol-CN-Py self-assembled into a bilayer structure through the π–π interactions.12 The bilayer structure served as the basic unit and then twisted into helical nanofibers. Fibers were entangled with each other to form the chiral supramolecular gel finally. A schematic illustration is shown in Fig. 1c.
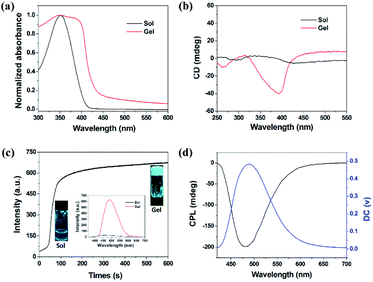
In view of the observed chiral nanohelix as well as the existence of a chiral center in the cholesterol moiety, the chirality at the ground state was probed by UV-vis absorption and CD spectra. Upon gel formation, the absorption peak exhibited a red shift in comparison to the solution, revealing that Chol-CN-Py adopted a J-aggregation arrangement during the self-assembly process (Fig. 2a).13 As far as the CD spectra were concerned, the initial solution showed no signals, while in the gel state, a weak positive Cotton effect at approximately 265 nm and a strong negative Cotton effect at 393 nm were observed (Fig. 2b). Since the cholesterol-containing unit was CD silent at any wavelength longer than 350 nm, the strong Cotton effect was attributed to the chirality transfer from the cholesterol pendants to the cyanostilbene moiety upon gel formation, expressing aggregation induced circular dichroism (AICD) properties.14
Upon gel formation, obvious fluorescence characteristics appeared as well. In Fig. 2c, the solution containing Chol-CN-Py exhibited a very weak emission at 455 nm, while the emission peak red-shifted to 485 nm together with enhanced intensity in the gel state. Correspondingly, a gel with brilliant blue emission was observed. Similarly, in tetrahydrofuran (THF), consistent emission enhanced curves were observed with the addition of water (more than 60% water fraction). As for the absorption spectra, level-off tails in the visible region proved the formation of aggregates (Fig. S9†). The dynamic AIE behaviors could also be reflected during the cooling gelation process utilizing the time-dependent fluorescence spectra. The initial hot solution of Chol-CN-Py was almost non-emissive. Under cooling, the emission intensity increased rapidly and then remained steady as observed by monitoring the intensity variation at 485 nm, indicating that the non-emissive solution completely transformed into a gel with blue emission. In addition, various photophysical data of the gel were also measured and are listed in Fig. S10† and Table 1. It is observed that the fluorescence quantum efficiency (φF) of the gel is up to 0.61, implying a favorable application in fluorescent materials.
| λ em (nm) | τ av (ns) | τ i (ns) | φ F | k r (ns−1) | k nr (ns−1) | |
|---|---|---|---|---|---|---|
| a λ em: maximum emission wavelength; φF: fluorescence quantum yield determined using a calibrated integrating sphere; average lifetime: τav = (A1τ12 + A2τ22 + A3τ32)/(A1τ1 + A2τ2 + A3τ3); radiative transition rate constant: kr = φF/τav; non-radiative transition rate constant: knr = (1 − φF)/τav. | ||||||
| Gel | 485 | 13.87 | 2.41 (0.03), 8.76 (0.34), 18.28 (0.63) | 61% | 0.0443 | 0.0278 |
| Protonated sol | 535 | 2.30 | 1.92 (0.99), 9.69 (0.01) | 16% | 0.0690 | 0.3658 |
| Film | 485 | 13.16 | 1.16 (0.23), 4.80 (0.48), 17.57 (0.29) | 22% | 0.0164 | 0.0595 |
| Protonated film | 550 | 17.18 | 2.17 (0.14), 8.68 (0.46), 21.63 (0.40) | 32% | 0.0189 | 0.0393 |
According to the CD and fluorescence spectra results, the gel was not only chiral but also emissive. Thus, CPL, a unique property to evaluate the excited-state supramolecular chirality of a gel, is worth expecting in the gel state (Fig. 2d). Similar to the results of the CD spectra, no CPL signals were detected in solution. Interestingly, the gel exhibited strong right-handed CPL at 480 nm. Therefore, both ground-state and excited-state supramolecular chirality were amplified upon gel formation. Furthermore, the magnitude of CPL could be evaluated using glum, which is defined as glum = 2(IL − IR)/(IL + IR), where IL and IR refer to the intensity of left- and right-handed CPL, respectively.1e,15 The calculated value of glum of the CPL signals was about −3.0 × 10−2 (Fig. S11†), which was relatively large in comparison to the ones reported for most self-assembled materials.16
Owing to the gelation caused chirality transfer and amplification, we have obtained a Chol-CN-Py gel with blue CPL and a large glum. We know that CPL is sensitive to chirality and emission. If the chirality is constant, the emission regulation mechanism probably takes effect in the realization of versatile CPL. Pyridine is a functional group which can capture protons upon acid stimulus. After protonation, the enhanced electron withdrawing ability of pyridine can lead to lowered energy levels as well as red-shifted emissions.6 TFA (pKa = 0.23) with super acid can exhibit an easy and sensitive protonation effect with the pyridyl group. Thus, combining the emission modulation strategy and excited-state chirality transfer concept, we develop a multi-color CPL system by protonation.
TFA triggered protonation of Chol-CN-Py was characterized by 1H NMR spectroscopic measurements first (Fig. S12†). With the addition of TFA, the proton peak near the pyridine nitrogen atom exhibited an obvious shift variation from 8.72 ppm to 9.02 ppm, indicating typical protonation of the pyridine unit, which further influenced the optical properties of Chol-CN-Py.17 Upon TFA stimulus, gradual red-shifted absorption peaks (330–348 nm) were observed (Fig. S13†). Remarkably, in the fluorescence spectra, the initial peak with a weak blue emission red-shifted to a new one with a yellow emission (Δλ = 85 nm) accompanied by progressively enhanced intensity (Fig. S14†). It should be observed that the variations in 1H NMR and optical features could be recovered with the assistance of triethylamine (TEA) caused deprotonation.
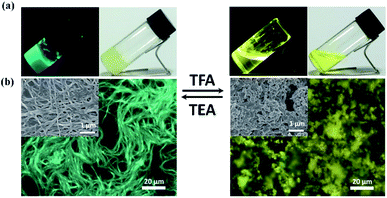
The process could also occur in the gel sate, which realized reversible gel–sol transitions accompanied with blue-yellow emission variations upon TFA/TEA stimuli. As shown in the fluorescence microscope and SEM images (Fig. 3), blue fibers changed to macrocrystals with yellow emission, implying the loss of the self-assembly ability after protonation. As far as the fluorescence spectra were concerned, when increasing the amount of TFA we also found obvious gradually red-shifted emission peaks (485–542 nm) (Fig. S15†). The final protonated solution showed relatively lower φF and shorter fluorescence lifetime in comparison with the initial gel (Fig. S16† and Table 1). The reformation of the gel and recovery of emission were realized upon TEA stimulus and the reversible variations could be repeated many times. In addition, on account of the loss of helical structures, the chiral information couldn't exist, which was reflected in the CD spectra. Upon TFA/TEA stimuli, reversible on-off CD signal transformations were detected, verifying the further change of chiral nanostructures (Fig. S17†).
To explain the optical variations, theoretical calculations for Chol-CN-Py and protonated Chol-CN-Py were employed with density functional theory (DFT) (Fig. 4). Although the HOMOs and LUMOs of Chol-CN-Py and protonated Chol-CN-Py were all localized over the cyanostilbene part, the LUMO of protonated Chol-CN-Py possessed nearer electron distribution to pyridyl hydrogen, leading to a greater decrease of LUMOs (from −2.42 eV to −3.12 eV) in comparison with HOMOs (from −5.92 eV to −6.09 eV).18 Correspondingly, a narrower energy gap of the protonated state was reached, demonstrating the shifts of the absorption and emission to longer wavelength upon protonation.19 In addition, the absorption spectra of both states were also calculated using time-dissolved DFT (TD-DFT) (Fig. S18†). The calculated absorption wavelengths (S0–S1 transition) for Chol-CN-Py and protonated Chol-CN-Py were assigned as 342.9 nm and 367.7 nm, respectively. The almost 25 nm difference was in accordance with the experimental results (Δλ = 18 nm). The decreased energy gaps (from 3.62 eV to 3.37 eV) were similar to the results of HOMO and LUMO energy levels as well, implying red-shifted absorption upon protonation.
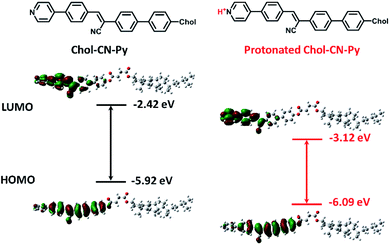 | ||
| Fig. 4 The molecular structures and frontier molecular orbitals (including HOMO and LUMO energy levels) of Chol-CN-Py and protonated Chol-CN-Py. | ||
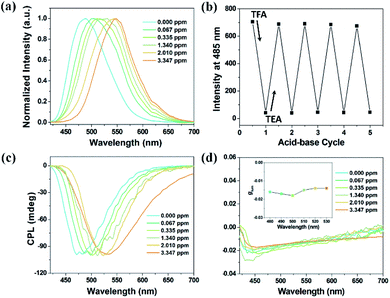
In consideration of the further application of CPL materials, films are necessary and thus xerogel films were fabricated. In particular, with the formation of the film, the aggregation state could be maintained and intense emission including CPL was expected. The hot solution containing Chol-CN-Py was painted onto a quartz plate to form the xerogel film to monitor the emission as well as CPL change. Upon exposure to different concentration TFA vapor, red-shifted emission peaks were observed (Fig. 5a). We also measured the detailed photophysical data for the initial and final states and found no obvious φF and fluorescence lifetime change, indicating outstanding stable optical properties in xerogel films during the protonation process (Fig. S19,†Table 1). It can be noted that good reversibility in the xerogel film state was also observed with alternative TEA/TFA stimuli (Fig. 5b). Additionally, the equal and smooth spatial distribution in xerogel films provided a large surface area, which made the emission modulation rapid and sensitive (Fig. S20 and S21†).20 Upon exposure to 0.067 ppm TFA, the xerogel film with blue emission turned into one with green emission in 10 s. With the increase of the amount of TFA (0.335, 1.340, 2.010, and 3.347 ppm), a series of red-shifted xerogel films including yellow and orange were also obtained (Fig. S22†).
Simultaneously, the chiral nanostructures of the xerogel films remained constant during the TFA caused protonation process, which was demonstrated by SEM images (Fig. S23†). The chirality transfer could occur due to the stable chiral structures, leading to anticipated CPL properties. The xerogel film exhibited right-handed CPL at 480 nm and the glum was about −1.7 × 10−2. Upon TFA stimulus, CPL signals red-shifted to 530 nm gradually, covering the multi-color circularly polarized emission from blue via green and yellow to orange color (Fig. 5c). In particular, the handedness and glum of CPL were almost unchanged throughout the whole course, indicating the excellent stability of the CPL xerogel films (Fig. 5d).
Conclusions
In conclusion, we developed a gel and xerogel film system which exhibited AIE as well as AICPL properties. In virtue of the gelation caused chirality transfer and amplification, both the gel and xerogel film exhibited blue CPL with a large glum (−3.0 × 10−2, −1.7 × 10−2). Due to the protonation of the pyridine group, reversible red-shifted emission together with gel–sol transition was realized upon TFA/TEA stimuli. In particular, in the xerogel film state, the chirality transfer and corresponding chiral structures were maintained upon protonation. In this case, by controlling the degree of protonation, the xerogel films exhibited red-shifted CPL signals from 480 nm to 530 nm, covering the multi-color circularly polarized emission from blue via green and yellow to orange. Meanwhile, the φF and glum remained constant, indicating the outstanding stability of the xerogel films. This work provides a new perspective to develop novel smart CPL tunable materials in one single system.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51673082 and 21374036).Notes and references
- (a) J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1–16 CrossRef CAS; (b) H. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, K. Nobusawa, H. Tsumatori and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9266–9269 CrossRef CAS PubMed; (c) R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673–7686 RSC; (d) S. T. Wu, Z. W. Cai, Q. Y. Ye, C. H. Weng, X. H. Huang, X. L. Hu, C. C. Huang and N. F. Zhuang, Angew. Chem., Int. Ed., 2014, 53, 12860–12864 CrossRef CAS PubMed; (e) J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef; (f) F. Song, Z. Xu, Q. Zhang, Z. Zhao, H. Zhang, W. Zhao, Z. Qiu, C. Qi, H. Zhang, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam, Z. Zhao, A. Qin, D. Ma and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1800051 CrossRef; (g) K. Takaishi, M. Yasui and T. Ema, J. Am. Chem. Soc., 2018, 140, 5334–5338 CrossRef CAS PubMed; (h) J. Ouyang and J. Crassous, Coord. Chem. Rev., 2018, 376, 533–547 CrossRef CAS.
- (a) B. A. San Jose, J. Yan and K. Akagi, Angew. Chem., Int. Ed., 2014, 53, 10641–10644 CrossRef CAS PubMed; (b) R. Sethy, J. Kumar, R. Metivier, M. Louis, K. Nakatani, N. M. T. Mecheri, A. Subhakumari, K. G. Thomas, T. Kawai and T. Nakashima, Angew. Chem., Int. Ed., 2017, 56, 15053–15057 CrossRef CAS PubMed; (c) Y. Wang, X. Li, F. Li, W. Y. Sun, C. Zhu and Y. Cheng, Chem. Commun., 2017, 53, 7505–7508 RSC; (d) D. Yang, P. Duan, L. Zhang and M. Liu, Nat. Commun., 2017, 8, 15727 CrossRef CAS PubMed; (e) X. Li, Q. Li, Y. Wang, Y. Quan, D. Chen and Y. Cheng, Chem.–Eur. J., 2018, 24, 12607–12612 CrossRef CAS PubMed; (f) Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2019, 1900110 CrossRef PubMed; (g) X. Yang, J. Han, Y. Wang and P. Duan, Chem. Sci., 2019, 10, 172–178 RSC.
- (a) T. Goto, Y. Okazaki, M. Ueki, Y. Kuwahara, M. Takafuji, R. Oda and H. Ihara, Angew. Chem., Int. Ed., 2017, 56, 2989–2993 CrossRef CAS PubMed; (b) J. Han, J. You, X. Li, P. Duan and M. Liu, Adv. Mater., 2017, 29, 1606503 CrossRef PubMed; (c) H. Nishimura, K. Tanaka, Y. Morisaki, Y. Chujo, A. Wakamiya and Y. Murata, J. Org. Chem., 2017, 82, 5242–5249 CrossRef CAS PubMed; (d) M. Li, C. Zhang, L. Fang, L. Shi, Z. Tang, H. Y. Lu and C. F. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 8225–8230 CrossRef CAS PubMed; (e) N. Hellou, M. Srebro-Hooper, L. Favereau, F. Zinna, E. Caytan, L. Toupet, V. Dorcet, M. Jean, N. Vanthuyne, J. A. G. Williams, L. D. Bari, J. Autschbach and J. Crassous, Angew. Chem., Int. Ed., 2017, 56, 8236–8239 CrossRef CAS PubMed; (f) K. Dhbaibi, L. Favereau, M. Srebro-Hooper, M. Jean, N. Vanthuyne, F. Zinna, B. Jamoussi, L. D. Bari, J. Autschbach and J. Crassous, Chem. Sci., 2018, 9, 735–742 RSC.
- (a) B.-K. An, J. Gierschner and S. Y. Park, Acc. Chem. Res., 2012, 45, 544–554 CrossRef CAS; (b) M. Martinez-Abadia, R. Gimenez and M. B. Ros, Adv. Mater., 2018, 30, 1704161 CrossRef PubMed; (c) J. Seo, J. W. Chung, J. E. Kwon and S. Y. Park, Chem. Sci., 2014, 5, 4845–4850 RSC; (d) H.-J. Kim, D. R. Whang, J. Gierschner and S. Y. Park, Angew. Chem., Int. Ed., 2016, 55, 15915–15919 CrossRef CAS PubMed; (e) C. Y. Y. Yu, H. Xu, S. Ji, R. T. K. Kwok, J. W. Y. Lam, X. Li, S. Krishnan, D. Ding and B. Z. Tang, Adv. Mater., 2017, 29, 1606167 CrossRef PubMed; (f) G. Niu, X. Zheng, Z. Zhao, H. Zhang, J. Wang, X. He, Y. Chen, X. Shi, C. Ma, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, K. S. Wong, P. Wang and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 15111–15120 CrossRef CAS PubMed; (g) X. Jin, D. Yang, Y. Jiang, P. Duan and M. Liu, Chem. Commun., 2018, 54, 4513–4516 RSC.
- (a) J. Liu, H. Su, L. Meng, Y. Zhao, C. Deng, J. C. Y. Ng, P. Lu, M. Faisal, J. W. Y. Lam, X. Huang, H. Wu, K. S. Wong and B. Z. Tang, Chem. Sci., 2012, 3, 2737–2747 RSC; (b) H. Li, S. Xue, H. Su, B. Shen, Z. Cheng, J. W. Lam, K. S. Wong, H. Wu, B. S. Li and B. Z. Tang, Small, 2016, 12, 6593–6601 CrossRef CAS PubMed; (c) J. Roose, B. Z. Tang and K. S. Wong, Small, 2016, 12, 6495–6512 CrossRef CAS PubMed; (d) H. Li, B. S. Li and B. Z. Tang, Chem.–Asian J., 2019, 14, 674–688 CrossRef CAS PubMed; (e) H.-T. Feng, X. Gu, J. W. Y. Lam, Y.-S. Zheng and B. Z. Tang, J. Mater. Chem. C, 2018, 6, 8934–8940 RSC; (f) Y.-X. Yuan, J.-B. Xiong, J. Luo, M. Hu, H. Jiang, M. Liu and Y.-S. Zheng, J. Mater. Chem. C, 2019, 7, 8236–8243 RSC; (g) F. Song, Y. Cheng, Q. Liu, Z. Qiu, J. W. Y. Lam, L. Lin, F. Yang and B. Z. Tang, Mater. Chem. Front., 2019, 3, 1768–1778 RSC; (h) S. Zhang, J. Fan, Y. Wang, D. Li, X. Jia, Y. Yuan and Y. Cheng, Mater. Chem. Front., 2019, 3, 2066–2071 RSC.
- (a) M. Kondo, S. Miura, K. Okumoto, M. Hashimoto and N. Kawatsuki, Chem.–Asian J., 2014, 9, 3188–3195 CrossRef CAS PubMed; (b) S. Ma, J. Zhang, Y. Liu, J. Qian, B. Xu and W. Tian, J. Phys. Chem. Lett., 2017, 8, 3068–3072 CrossRef CAS PubMed; (c) Q. Sun, H. Wang, X. Xu, Y. Lu, S. Xue, H. Zhang and W. Yang, Dyes Pigm., 2018, 149, 407–414 CrossRef CAS; (d) J. Xiong, K. Wang, Z. Yao, B. Zou, J. Xu and X. H. Bu, ACS Appl. Mater. Interfaces, 2018, 10, 5819–5827 CrossRef CAS PubMed.
- (a) M. Liu, G. Ouyang, D. Niu and Y. Sang, Org. Chem. Front., 2018, 5, 2885–2900 RSC; (b) S. Datta and S. Bhattacharya, Soft Matter, 2015, 11, 1945–1953 RSC.
- (a) C. D. Jones and J. W. Steed, Chem. Soc. Rev., 2016, 45, 6546–6596 RSC; (b) A. Dawn and H. Kumari, Chem.–Eur. J., 2018, 24, 762–776 CrossRef CAS PubMed.
- (a) E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed; (b) L. Zhang, T. Wang, Z. Shen and M. Liu, Adv. Mater., 2016, 28, 1044–1059 CrossRef CAS PubMed.
- Y. Zhang, Y. Ma, M. Deng, H. Shang, C. Liang and S. Jiang, Soft Matter, 2015, 11, 5095–5100 RSC.
- (a) J. W. Liu, Y. Yang, C. F. Chen and J. T. Ma, Langmuir, 2010, 26, 9040–9044 CrossRef CAS PubMed; (b) P. Rajamalli and E. Prasad, Org. Lett., 2011, 13, 3714–3717 CrossRef CAS PubMed.
- (a) H. Chen, Y. Feng, G. J. Deng, Z. X. Liu, Y. M. He and Q. H. Fan, Chem.–Eur. J., 2015, 21, 11018–11028 CrossRef CAS PubMed; (b) D. Niu, L. Ji, G. Ouyang and M. Liu, Chem. Commun., 2018, 54, 1137–1140 RSC.
- (a) F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef PubMed; (b) B.-K. An, D.-S. Lee, J.-S. Lee, Y.-S. Park, H.-S. Song and S. Y. Park, J. Am. Chem. Soc., 2004, 126, 10232–10233 CrossRef CAS PubMed.
- (a) C. Yu, M. Xue, K. Liu, G. Wang and Y. Fang, Langmuir, 2014, 30, 1257–1265 CrossRef CAS PubMed; (b) H. Yu, Y. Lu, X. Chen, K. Liu and Y. Fang, Soft Matter, 2014, 10, 9159–9166 RSC.
- J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed.
- (a) S. Huo, P. Duan, T. Jiao, Q. Peng and M. Liu, Angew. Chem., Int. Ed., 2017, 56, 12174–12178 CrossRef CAS; (b) Y. Shi, P. Duan, S. Huo, Y. Li and M. Liu, Adv. Mater., 2018, 30, 1705011 CrossRef; (c) L. Ji, Y. Sang, G. Ouyang, D. Yang, P. Duan, Y. Jiang and M. Liu, Angew. Chem., Int. Ed., 2019, 58, 844–848 CrossRef CAS PubMed; (d) J. Han, D. Yang, X. Jin, Y. Jiang, M. Liu and P. Duan, Angew. Chem., Int. Ed., 2019, 58, 7013–7019 CrossRef CAS PubMed.
- (a) Z. Yang, W. Qin, J. W. Y. Lam, S. Chen, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Chem. Sci., 2013, 4, 3725–3730 RSC; (b) B. Pradhan, M. Gupta, S. K. Pal and A. S. Achalkumar, J. Mater. Chem. C, 2016, 4, 9669–9673 RSC.
- (a) M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09 Revision D.01, Gaussian Inc., Wallingford CT, 2009 Search PubMed; (b) W. Li, P.-P. Yang, L. Wang and H. Wang, J. Mater. Chem. C, 2015, 3, 3783–3789 RSC.
- (a) X. Chen, X. Y. Shen, E. Guan, Y. Liu, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2013, 49, 1503–1505 RSC; (b) S. Huang, Y. Wu, F. Zeng, L. Sun and S. Wu, J. Mater. Chem. C, 2016, 4, 10105–10110 RSC.
- (a) K. K. Kartha, S. S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834–4841 CrossRef CAS; (b) H. Wang, J. Zhao, G. Yang, F. Zhang, J. Sun and R. Lu, Org. Biomol. Chem., 2018, 16, 2114–2124 RSC; (c) Y. Ma, M. Cametti, Z. Džolić and S. Jiang, J. Mater. Chem. C, 2018, 6, 9232–9237 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc05643b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |