Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile in situ reductive synthesis of both nitrogen deficient and protonated g-C3N4 nanosheets for the synergistic enhancement of visible-light H2 evolution†
Weisong
Li
ab,
Zheng
Guo
a,
Litong
Jiang
bc,
Lei
Zhong
a,
Guoning
Li
a,
Jiajun
Zhang
a,
Kai
Fan
a,
Sergio
Gonzalez-Cortes
 a,
Kuijuan
Jin
c,
Chunjian
Xu
a,
Kuijuan
Jin
c,
Chunjian
Xu
 *a,
Tiancun
Xiao
*b and
Peter P.
Edwards
*a,
Tiancun
Xiao
*b and
Peter P.
Edwards
 *b
*b
aSchool of Chemical Engineering & Technology, State Key Laboratory of Chemical Engineering, Tianjin University, Tianjin 300350, China. E-mail: cjxu@tju.edu.cn
bInorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK. E-mail: xiao.tiancun@chem.ox.ac.uk; peter.edwards@chem.ox.ac.uk
cInstitute of Physics, Chinese Academy of Sciences, Beijing 100190, China
First published on 3rd February 2020
Abstract
A new strategy is reported here to synthesize both nitrogen deficient and protonated graphitic carbon nitride (g-C3N4) nanosheets by the conjoint use of NH4Cl as a dynamic gas template together with hypophosphorous acid (H3PO2) as a doping agent. The NH4Cl treatment allows for the scalable production of protonated g-C3N4 nanosheets. With the corresponding co-addition of H3PO2, nitrogen vacancies, accompanied by both additional protons and interstitially-doped phosphorus, are introduced into the g-C3N4 framework, and the electronic bandgap of g-C3N4 nanosheets as well as their optical properties and hydrogen-production performance can be precisely tuned by careful adjustment of the H3PO2 treatment. This conjoint approach thereby results in improved visible-light absorption, enhanced charge-carrier separation and a high H2 evolution rate of 881.7 μmol h−1 achieved over the H3PO2 doped g-C3N4 nanosheets with a corresponding apparent quantum yield (AQY) of 40.4% (at 420 nm). We illustrate that the synergistic H3PO2 doping modifies the layered g-C3N4 materials by introducing nitrogen vacancies as well as protonating them, leading to significant photocatalytic H2 evolution enhancements, while the g-C3N4 materials doped with phosphoric acid (H3PO4) are simply protonated further, revealing the varied doping effects of phosphorus having different (but accessible) valence states.
Introduction
Graphitic carbon nitride (g-C3N4), a prototypical metal-free semiconductor, has been intensively studied owing to its excellent photocatalytic applications in H2 production, environment remediation, CO2 reduction and photosynthesis.1–5 Bulk g-C3N4 with high thermal, chemical and photocatalytic stabilities can be readily synthesized via direct thermal polymerization of nitrogen-rich precursors. However, the visible-light photocatalytic efficiency of such directly polymerized g-C3N4 material is far from satisfactory stemming from its limited visible-light absorption range, low surface area, confined active sites, and rapid charge-carrier recombination. Thus, various techniques including morphology engineering,6,7 constructing Z-scheme heterostructure or unique surface bonding states,8–10 doping with both nonmetal and metal elements,11,12 introducing elemental defects or vacancies and protonation have been applied in order to improve the photocatalytic performance of g-C3N4.13–15 Inspired by the two-dimensional graphene-like nanosheet of the material, efforts have also been made to transform bulk g-C3N4 into porous structures composing thin nanosheets which help deliver superior photocatalytic activities.7Recently, it has been demonstrated that the photocatalytic activity of g-C3N4 under visible-light irradiation can be significantly enhanced by introducing nitrogen defects into the basic g-C3N4 “melon” structure as well as doping with compounds of phosphorus.2,12 To date, nitrogen deficient g-C3N4 was synthesized through alkali-assisted polymerization, hydrogen reduction, high-temperature thermal polymerization and hydrothermal routes.2,13,16,17 Unlike other methods-which routinely suffer from control difficulties-the so-called alkali-assisted polymerization achieves a highly effective control of both the type and the abundance of the introduced nitrogen defects leading to enhanced photocatalytic H2 evolution.2 However, this alkali-assisted approach, using KOH, NaOH or Ba(OH)2 is unlikely to work in the “bottom-up” strategy which utilizes NH4Cl as a so-called dynamic gas template for scalable production of g-C3N4 nanosheets, due to the non-coexistence of NH4Cl and alkali metal hydroxides.7 Moreover, it has been shown that the alkali-assisted process progressively decreases the layer stacking distance and reduces the specific surface area of g-C3N4 nanosheets when excessive KOH is added.2 This suggests the over-addition of alkali metal hydroxides would cause a severe decrease in photocatalytic activity because of the unfavorable crystal morphology changes.
Phosphorus, has emerged as a likely contender to be introduced into the g-C3N4 matrix to achieve enhanced photocatalytic performance. In most of the prior work,15,18–20 pentavalent phosphorus such as H3PO4, (NH4)3PO4, BmimPF6 ionic liquid, Na2HPO4, and CO(NH2)2·H3PO4 figures strongly as the phosphorus doping agents. To the best of our knowledge, there is little work reported on the synthesis of phosphorus-doped g-C3N4 using phosphorus compounds having lower valence states and the photocatalytic ability of such low-valent phosphorus doped g-C3N4 has hardly been studied.4
Here, we present a one-step, in situ reduction method utilizing the strategy of using NH4Cl as dynamic gas template and H3PO2 doping for the synthesis of nitrogen deficient g-C3N4 nanosheets. The photocatalytic activity of g-C3N4 was greatly enhanced with the formation of both protonated and porous nanosheets by using NH4Cl as dynamic gas template. With the co-addition of H3PO4 as dopant, g-C3N4 nanosheets were further protonated and delivered superior photocatalytic activities. However, besides protonation, the g-C3N4 nanosheets doped by the lower-valent phosphorus compound H3PO2 also led to a reduced electronic bandgap in the material and enhanced visible light absorption. The differences observed between the H3PO2 and H3PO4 doped g-C3N4 nanosheets promotes a discussion of using dopants with phosphorus at different valence states. It demonstrates the possibility of using low-valent phosphorus compounds to both synthesize and modify g-C3N4 in one single step, thereby offering a comprehensive enhanced and efficient visible-light photocatalytic performance. Thus, the preparation strategy reported here represents an effective approach to optimize the morphology, chemical composition, optical response and resulting activity of g-C3N4 photocatalysts.
Experimental
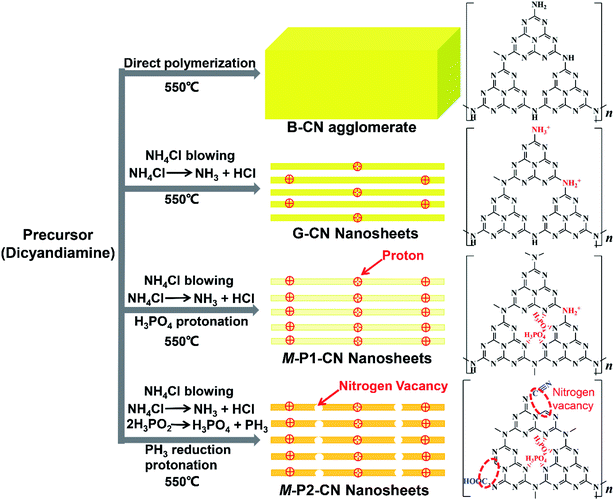
The standard bulk g-C3N4 reference (B-CN) was prepared by the direct pyrolysis polymerization of dicyandiamide. Preliminary, 4 g dicyandiamide powder was placed in a 50 mL alumina crucible with cover and then was calcined in static air at 550 °C for 4.5 hours with a ramping rate of 2.3 °C min−1. The g-C3N4 nanosheets were prepared by the modified “bottom-up” strategy with NH4Cl as dynamic gas template,7 and the as prepared g-C3N4 nanosheets without and with addition of phosphorus dopants were labeled as G-CN and M-Pn-CN, respectively. Here, M represents the phosphorus dopant usage (weight percentage of the added phosphorus element to the dicyandiamide precursor, ranging from 0.2 to 3.2) and Pn (n = 1 or 2) meant that the corresponding dopant was H3PO4 and H3PO2, respectively. Firstly, 4 g dicyandiamide and 20 g NH4Cl were premixed with 5 mL solution containing the designed amount of dopant (for G-CN, the addition amount of dopants was zero). For example, in the preparation of 0.8-Pn-CN samples, the addition amounts of 85 wt% H3PO4 and 50 wt% H3PO2 were 119.0 and 136.3 mg, respectively. Then, the powder and solution mixture were stirred to form homogeneous slurry. Finally, the slurry was calcined with the same procedures in B-CN preparation. All the as prepared samples were collected and stored at room temperature in normal atmosphere.Full experimental details including reagents, characterization, transient photocurrent measurement, RTK-Solar visible-light H2 evolution system (Fig. S1†) and AQY measurement can be found in the ESI.†
Results and discussion
Morphology
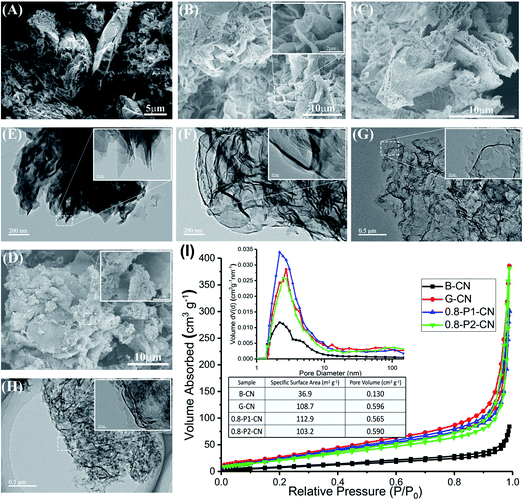
Fig. 1A–D show representative g-C3N4 morphologies characterized by field emission electron scanning microscope (SEM). Compared with the B-CN powder which shows a large agglomerate appearance (Fig. 1A), the g-C3N4 synthesized using the NH4Cl-assisted “bottom-up” strategy shows characteristics of thin nanosheets with crinkly structures (Fig. 1B–D). It can be seen that G-CN is mainly consisted of micrometer scale petals-like nanosheets with relatively smooth surfaces and edges (Fig. 1B). The 0.8-P1-CN nanosheets (Fig. 1C) remain a flower-like structure and part of the nanosheets become much smaller with serrated edges, which could be ascribed to the reaction of g-C3N4 nanosheets with H3PO4.18 In contrast, few flower-like structures are found in the H3PO2 doped powder (Fig. 1D) and even smaller nanosheets can be observed with some large nanosheets surrounded. Further to transmission electron microscope (TEM) images, the dense stacking structure of bulk B-CN is illustrated in Fig. 1E, while the as prepared g-C3N4 nanosheets using NH4Cl as dynamic gas template (Fig. 1F–H) present porous structures. In the N2 physisorption measurements, the specific surface areas, pore volumes and pore size distributions of the g-C3N4 samples were obtained (Fig. 1I). All the tested g-C3N4 samples possess well-defined mesopores. The specific areas and pore volumes of g-C3N4 nanosheets are nearly 3 and 4.5 times enlarged than those of B-CN, respectively. Thus, the porous g-C3N4 nanosheets assemblages appeared to us to provide more active sites for an enhanced photocatalytic performance.Structure characterization
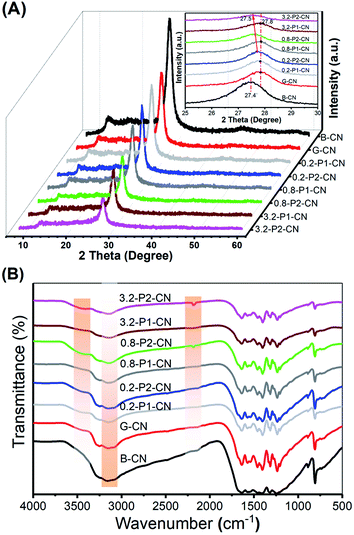
In Fig. 2A we present the X-ray diffraction (XRD) patterns of the g-C3N4 samples. All samples show the two characteristic diffraction peaks at around 13.0° and 27.4°, which were assigned to the in-plane (100) and interlayer-stacking (002) crystal planes of g-C3N4, respectively.2 Compared with the peaks in B-CN, diffraction peaks of g-C3N4 nanosheets samples (G-CN and M-Pn-CN) became weaker and the peak intensities of the phosphorus doped g-C3N4 nanosheets were continuously weakened with increasing addition of phosphorus dopants. Notably, all H3PO2 doped g-C3N4 samples shows lower peak intensities compared to the corresponding H3PO4 doped g-C3N4 nanosheets at the same amount of phosphorus usage, which indicates H3PO2 could more easily cause the loss of ordered structures within g-C3N4 framework.2 The magnified XRD patterns (inset in Fig. 2A) depict that the (002) diffraction peak of G-CN nanosheets shifted to 27.8°, suggesting a smaller gallery distance between the basic layers in the nanosheets. Earlier pioneering work6,21 demonstrated that so-called planarizing the potentially undulated layers in g-C3N4 would result in denser stacking, thereby resulting in shifts in the characteristic (002) diffraction peaks to higher 2θ angles. Thus, the (002) diffraction peaks shifts in XRD patterns also indicate the formation of planarized g-C3N4 nanosheets as also demonstrated in our SEM studies. Interestingly, the (002) peaks of the H3PO2 doped g-C3N4 nanosheets continuously shifted back to around 27.5° with increasing dopant addition, while those of the H3PO4 doped samples were nearly centered at the same position with G-CN (ESI, Fig. S2†). This (002) peak shift in the H3PO2 doped nanosheets should be attributed to the increased disorder in the g-C3N4 in-planar matrix and consequently resulted in larger layer distance compared with G-CN and H3PO4 doped g-C3N4 nanosheets.1,2 Thus, this interesting observation suggests that this H3PO2 doping protocol could help to relieve the layer stacking problems with remaining nanosheets structures.In the Fourier Transform Infrared (FTIR) spectroscopy (Fig. 2B), the typical peaks of g-C3N4 at 810 cm−1 and in the range of 900–1800 cm−1 were observed among all the tested g-C3N4 samples, which originated from the heptazine ring out-of-plane bending and the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N heterorings in the “melon” framework.2 Compared with the pristine B-CN, three distinct changes (highlighted by the orange shaded regions) occurred with varying dopants and their usages. One was the progressive loss of peak intensities in the phosphorus doped nanosheets located between 3000 and 3300 cm−1 which were attributed to the N–H stretching vibrations. Another difference observed at 2184 cm−1 as a new peak appeared in H3PO2 doped g-C3N4 nanosheets representing the stretching vibration mode of cyano groups (–C
N heterorings in the “melon” framework.2 Compared with the pristine B-CN, three distinct changes (highlighted by the orange shaded regions) occurred with varying dopants and their usages. One was the progressive loss of peak intensities in the phosphorus doped nanosheets located between 3000 and 3300 cm−1 which were attributed to the N–H stretching vibrations. Another difference observed at 2184 cm−1 as a new peak appeared in H3PO2 doped g-C3N4 nanosheets representing the stretching vibration mode of cyano groups (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N).2,22,23 Moreover, a weak and broad peak centered at around 3460 cm−1 was developed in the g-C3N4 nanosheets samples and this peak could be assigned to the stretching vibration mode of hydroxyl groups (–OH).24,25 It is noteworthy that the hydroxyl group stretching vibration peaks in the H3PO2 doped nanosheets are relatively stronger than those in the H3PO4 doped samples, suggesting more exposure of hydroxyl groups in the H3PO2 doped g-C3N4 nanosheets, which will be further evidenced by the after-mentioned X-ray photoelectron spectroscopy analysis.
N).2,22,23 Moreover, a weak and broad peak centered at around 3460 cm−1 was developed in the g-C3N4 nanosheets samples and this peak could be assigned to the stretching vibration mode of hydroxyl groups (–OH).24,25 It is noteworthy that the hydroxyl group stretching vibration peaks in the H3PO2 doped nanosheets are relatively stronger than those in the H3PO4 doped samples, suggesting more exposure of hydroxyl groups in the H3PO2 doped g-C3N4 nanosheets, which will be further evidenced by the after-mentioned X-ray photoelectron spectroscopy analysis.
The FTIR results reveal that the addition of H3PO2 and H3PO4 in g-C3N4 nanosheets synthesis could significantly decrease N–H concentration and cyano groups can be very effectively introduced by using H3PO2. The introduction of oxygen containing groups like hydroxyl group should be attributed to the high temperature (550 °C) synthesis and the atmospheric sample storage. Notably, recent research results claimed that the photocatalytic activity of g-C3N4 can be greatly enhanced by introducing cyano groups into the melon framework.2,24 Thus, the H3PO2 doped g-C3N4 nanosheets are expected to possess superior photocatalytic activities as would be demonstrated in the H2 production test.
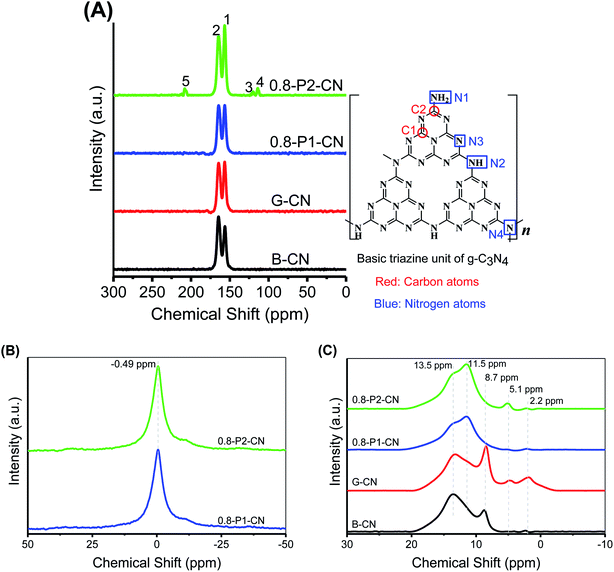
Besides XRD and FTIR studies, the chemical structures of the g-C3N4 samples were further characterized by solid-state 13C, 31P and 1H magic angle spinning (MAS) NMR spectra. Fig. 3A presents the 13C NMR spectra, in which all samples show two strong peaks at 156.3 and 164.6 ppm corresponding to the chemical shifts of C3N (1) and C2N–NHx (2) in the corrugated g-C3N4 melon networks, respectively.26–28 Compared with B-CN, the C3N (1) peak in the g-C3N4 nanosheets powders was intensified, which indicates the loss of NHx groups, in accordance with the FTIR results. Moreover, G-CN and 0.8-P1-CN were found very similar, suggesting that the nonoxidative H3PO4 would not strongly disintegrate the g-C3N4 heptazine units. However, the C3N (1) peak was further intensified in the 0.8-P2-CN sample and three new peaks (peak 3, 4 and 5) emerged. These new peaks with a chemical shift of 119.9, 112.3 and 208.1 ppm could be assigned to the carbon atoms in cyano groups, sp2 hybridized carbon atoms and the carbon atoms in the carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) containing species, respectively.2,28–30
O) containing species, respectively.2,28–30
 | ||
| Fig. 3 (A) Solid-state NMR spectra of 13C and basic g-C3N4 triazine unit structure. (B and C) Solid-state 31P and 1H NMR spectrums, respectively. | ||
No obvious differences are observed on the 31P NMR spectra (Fig. 3B) of 0.8-P2-CN and 0.8-P1-CN nanosheets. Both samples show single sharp peak centered at −0.49 ppm which corresponds to the P–N coordinate bonds with phosphorus atom being connected to two adjacent pyridinic N atoms from two separated g-C3N4 triazine units,31 indicating that the phosphorus atoms sourced from H3PO2 and H3PO4 were in the same final state and interstitially doped in the g-C3N4 matrices.32 As the synthesis was conducted in the high temperature air, these doped phosphorus atoms would most likely be in pentavalent oxidation state and absorb moisture from air and eventually transform into the form of phosphoric acid. In terms of the reasons for the differences between the H3PO4 and H3PO2 doped g-C3N4 nanosheets, a detailed discussion will be presented later.
Considering NH4Cl and H3PO2 would generate HCl and H3PO4 which were commonly used for g-C3N4 protonation,14,18 the as prepared g-C3N4 could be protonated. As seen in Fig. 3C, the five peaks at 2.2, 5.1, 8.7, 11.5 and 13.5 ppm in the 1H NMR spectra were assigned to the protons in aliphatic groups, residual water, NHx groups, the hydrogen bonds in acid groups (such as carboxylic acid and phosphoric acid) and g-C3N4 framework,33–35 respectively. Thus, the intensification of the 8.7 or 11.5 ppm peak can be regarded as evidence for the protonation of g-C3N4 nanosheets. Compared with B-CN, the 8.7 ppm peak in G-CN was obviously strengthened because of the formation of hydrogen bonds between the proton and nitrogen atoms in the g-C3N4 framework. Despite of the weakened peaks at 8.7 ppm over the phosphorus doped nanosheets, the intensified peaks at 11.5 ppm in 0.8-P1-CN and 0.8-P2-CN should be attributed to the protonation with protons connected with phosphoric acid groups.
The measured zeta potentials (ESI, Fig. S3†) reveal that the surface charge properties were significantly changed in the g-C3N4 nanosheets. The zeta potentials of G-CN, 0.8-P1-CN and 0.8-P2-CN were increased from −16.3 mV (B-CN) to −6.1, −3.1 and −4.5 mV, respectively, and these zeta potential changes further confirm the protonation of the g-C3N4 nanosheets.15 Besides the positive potential on the dispersibility, electronic bandgap structure and surface area of g-C3N4, one of the other important advantages of protonation is to induce higher ionic conductivity to the g-C3N4 framework and then enable the acceleration of charge carrier migration, which would benefit for better photocatalytic performances.14,18,36 Thus, higher H2 evolution ability could be expected for the protonated g-C3N4 nanosheets.
In addition, combining with the newly emerged carbonyl group peak in the 13C spectra and the enhanced acid groups hydrogen bonds 1H NMR signal in 0.8-P2-CN nanosheets, it is reasonable to propose that carboxyl groups (–COOH) were introduced into the H3PO2 doped nanosheets, thereby resulting in a stronger 11.5 ppm peak in 0.8-P2-CN than that of 0.8-P1-CN, and this peak intensification is in agreement with the observation that the H3PO2 doped g-C3N4 nanosheets showed stronger hydroxyl group (–OH) peak intensities in FTIR spectra.
Chemical compositions
The g-C3N4 compositions obtained from organic elemental analysis (OEA) and X-ray photoelectron spectroscopy (XPS) can be found in Table 1 (ESI, XPS spectra in Fig. S4†). As can be seen in Table 1, the increased H/C ratios in g-C3N4 nanosheets can also be regarded as evidences for protonation.15 The N/C atomic ratio of B-CN obtained from OEA and XPS were 1.467 and 1.318, respectively. According to XPS results, the N/C ratio of G-CN slightly decreased from 1.318 to 1.283 when B-CN was transformed into G-CN, and this was ascribed to higher degree polymerization with more NH3 release during the formation of nanosheet structures.7 Although the N/C ratio of H3PO4 doped nanosheets dropped to about 1.249, it was still very close to that of G-CN. However, the N/C ratio of H3PO2 doped g-C3N4 nanosheets fell to 0.904 as a result of the intensive loss of nitrogen caused by the addition of H3PO2. Meanwhile, the O/C ratio of the H3PO2 doped g-C3N4 nanosheets drastically increased to 0.279. Thus, it was most likely that the H3PO2 induced the opening of g-C3N4 heptazine rings and caused nitrogen vacancies. The exposed defective edges would then react with oxygen to form oxygen containing groups such as carboxyl groups etc.24| Sample | OEA | XPS | ||||
|---|---|---|---|---|---|---|
| N/C | O/C | H/C | N/C | O/C | P/C | |
| a OEA and XPS represent the results obtained from organic elemental analysis and X-ray photoelectron spectroscopy, respectively. | ||||||
| B-CN | 1.467 | 0.007 | 0.023 | 1.318 | 0.038 | 0 |
| G-CN | 1.421 | 0.018 | 0.027 | 1.283 | 0.064 | 0 |
| 0.2-P1-CN | 1.389 | 0.047 | 0.029 | 1.287 | 0.079 | 0.018 |
| 0.8-P1-CN | 1.381 | 0.064 | 0.031 | 1.266 | 0.105 | 0.038 |
| 3.2-P1-CN | 1.365 | 0.073 | 0.032 | 1.249 | 0.112 | 0.043 |
| 0.2-P2-CN | 1.293 | 0.091 | 0.030 | 1.204 | 0.127 | 0.010 |
| 0.8-P2-CN | 1.117 | 0.135 | 0.035 | 1.037 | 0.223 | 0.017 |
| 3.2-P2-CN | 1.002 | 0.162 | 0.037 | 0.904 | 0.279 | 0.024 |
In accordance with the fact that H3PO2 will decompose to release both PH3 and H3PO4,4 the differences highlighted between the H3PO2 and H3PO4 doped g-C3N4 nanosheets provides strong evidence that the introduction of nitrogen vacancies can be attributed to the strongly reductive PH3 rather than H3PO4. In the reduction synthesis of nitrogen defective g-C3N4,16 the energy changes for removing a lattice nitrogen atom located at N1, N2, N3 and N4 (referring to Fig. 3A) and terminating the dangling bonds of C atoms with H atoms using H2 as reduction agent were 0.65, 0.83, 1.40 and 2.39 eV, respectively,16 and the H2 reduction caused homogeneous sp2 hybridized nitrogen atoms losses on the g-C3N4 heptazine rings. Changing H2 to PH3, we calculated the corresponding energy changes and the four energy changes decrease to 0.61, 0.75, 1.28 and 2.27 eV (ESI†), respectively, indicating the stronger reduction agent PH3 would be more favorable for opening heptazine rings and result in significant nitrogen atoms in situ reduction removal. Thus, it was reasonable to propose that intensive nitrogen vacancies could be introduced into the H3PO2 doped g-C3N4 nanosheets because of that the strong reducing agent PH3, produced via H3PO2 decomposition.
The narrow scan C 1s, N 1s, P 2p and O 1s XPS spectra were collected and deconvoluted into their components (Fig. 4A–D). The four peaks in C 1s spectra are located at 284.8, 286.2, 288.1 and 289 eV, corresponding to the adventitious hydrocarbons (C–C or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), C–NHx (x = 1, 2), N–C
C), C–NHx (x = 1, 2), N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N coordination and carboxyl groups, respectively.2,37,38 It is obvious that the 288.1 eV signal lost intensity, while the 286.2 eV peak is significantly intensified in the 0.8-P2-CN powder. As cyano group has similar C 1s binding energy to C-NHx,2 the intensified 286.2 eV signal together with the weakened N–C
N coordination and carboxyl groups, respectively.2,37,38 It is obvious that the 288.1 eV signal lost intensity, while the 286.2 eV peak is significantly intensified in the 0.8-P2-CN powder. As cyano group has similar C 1s binding energy to C-NHx,2 the intensified 286.2 eV signal together with the weakened N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N coordination peak could be further evidence for the heptazine rings opening and the formation of cyano groups in the H3PO2 doped nanosheets. Besides, the enhanced 289 eV peak in 0.8-P2-CN confirms the considerable introduction of carboxyl groups into H3PO2 doped g-C3N4 nanosheets. The three peaks centered at 398.6, 400 and 401 eV in the N 1s XPS spectra are assigned to the sp2-hybridized (N2C), sp3-hybridized (N3C) nitrogen atoms and NHx groups, respectively.2 For the H3PO2 doped nanosheets, the drastically weakened NHx peak and shift of N3C to lower binding energy also indicate the NHx loss and formation of cyano groups, which are in agreement with the results obtained by FTIR and NMR spectra.
N coordination peak could be further evidence for the heptazine rings opening and the formation of cyano groups in the H3PO2 doped nanosheets. Besides, the enhanced 289 eV peak in 0.8-P2-CN confirms the considerable introduction of carboxyl groups into H3PO2 doped g-C3N4 nanosheets. The three peaks centered at 398.6, 400 and 401 eV in the N 1s XPS spectra are assigned to the sp2-hybridized (N2C), sp3-hybridized (N3C) nitrogen atoms and NHx groups, respectively.2 For the H3PO2 doped nanosheets, the drastically weakened NHx peak and shift of N3C to lower binding energy also indicate the NHx loss and formation of cyano groups, which are in agreement with the results obtained by FTIR and NMR spectra.
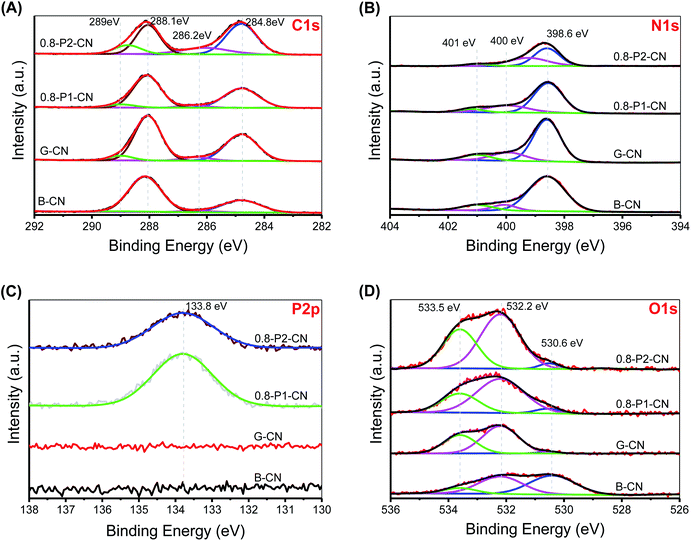 | ||
| Fig. 4 (A) C 1s XPS narrow scan. (B) N 1s XPS narrow scan. (C) P 2p XPS narrow scan. (D) O 1s XPS narrow scan. | ||
Both the P 2p XPS spectra of the H3PO4 and H3PO2 doped nanosheets show one single pentavalent phosphorus peak centered at 133.8 eV, indicating that no P–C bonds formed.18 Moreover, the elemental mapping conducted on scanning transmission microscope (ESI, Fig. S5†) reveal that phosphorus is distributed evenly in both 0.8-P1-CN and 0.8-P2-CN with an actual content of around 0.5 wt%. The parallel intensification of the 533.5 and 532.2 eV peaks representing –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups39–41 in the 0.8-P2-CN O 1s spectra are consistent with the hypothesis that carboxyl groups was introduced into the H3PO2 doped g-C3N4 nanosheets. According to the summarized XPS data (ESI, Table S1†), the N2C/C ratio in the g-C3N4 nanosheets remarkably dropped from 0.917 to 0.568 and the corresponding O/C ratio increased from 0.062 to 0.217 with the addition of H3PO2, these results also suggest the introduction of nitrogen defects and oxygen species into the H3PO2 doped g-C3N4 nanosheets.
O groups39–41 in the 0.8-P2-CN O 1s spectra are consistent with the hypothesis that carboxyl groups was introduced into the H3PO2 doped g-C3N4 nanosheets. According to the summarized XPS data (ESI, Table S1†), the N2C/C ratio in the g-C3N4 nanosheets remarkably dropped from 0.917 to 0.568 and the corresponding O/C ratio increased from 0.062 to 0.217 with the addition of H3PO2, these results also suggest the introduction of nitrogen defects and oxygen species into the H3PO2 doped g-C3N4 nanosheets.
Proposed schematic structures
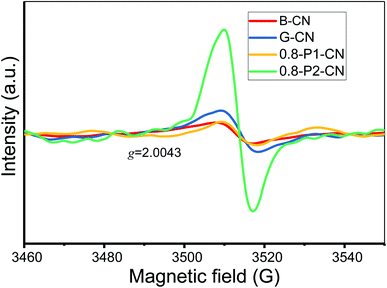
Further to our XPS and OEA analysis, electron paramagnetic resonance (EPR) experiments were conducted to provide fingerprint evidence for probing the surface nitrogen vacancies introduced to the g-C3N4 nanosheets. Several different samples having closely similar weights were examined to ensure a meaningful comparison of the peak intensities (areas). As shown in Fig. 5, all samples display a single Lorentzian line with an electronic g value of 2.0043 in the magnetic field from 3460 to 3560 G, which represents the unpaired electrons of sp2 hybrid carbon atoms in π-conjugated aromatic rings.42,43 Thus the formation of two-coordinated nitrogen vacancies in the heptazine rings of g-C3N4 would donate unpaired electrons to the sp2-carbon atoms. Therefore, compared with the negligible EPR signals of B-CN, G-CN and 0.8-P1-CN, the significantly enhanced EPR intensity in 0.8-P2-CN nanosheets adds weight to the idea of the opening of heptazine rings and the formation of two-coordinated nitrogen vacancies.Thus, we believe that combining the various techniques including FTIR, NMR, zeta potential, XPS and EPR analysis, the controllable generation of nitrogen vacancies, protonation and newly introduced functional groups in g-C3N4 framework are soundly confirmed. From all this information, the proposed schematic molecule structures and evolution processes of the g-C3N4 samples can now be advanced (Fig. 6).
In brief, compared with the directly polymerized bulk g-C3N4 agglomerate, protonated g-C3N4 nanosheets could be readily fabricated with the help of dynamic gas template NH4Cl. With co-addition of H3PO4 as dopant, the g-C3N4 nanosheets could be further protonated with phosphorus atoms interstitially doped into the frameworks. However, apart from the additional protonation, the addition of the reducing dopant H3PO2 could also induce significant nitrogen loss to generate nitrogen vacancies and variously introduce, cyano and carboxyl functional groups, into the g-C3N4 nanosheets.
Optical properties
The optical properties and light harvesting abilities of g-C3N4 samples were significantly modified by the specific addition of NH4Cl and phosphorus dopants. The obvious changes were well observed as the yellow dense B-CN powder was transformed into porous and fluffy states having different colours (Fig. 7A). Compared with B-CN and G-CN, the colours of the M-P1-CN nanosheets became lighter with the increasing addition of H3PO4, whereas those of the H3PO2 doped nanosheets turned from dark yellow to brown. The absorption edge of G-CN shifted to a lower wavelength as compared with B-CN and it continuously shifted to the lower direction with increasing H3PO4 addition. The blueshifts in the G-CN and H3PO4 doped nanosheets were attributed to the enhanced protonation.5,14 Unlike H3PO4, a progressive redshift was achieved by increasing H3PO2 usage, which indicates the bandgap structures of the H3PO2 doped nanosheets could be easily tuned by adjusting the amount of H3PO2.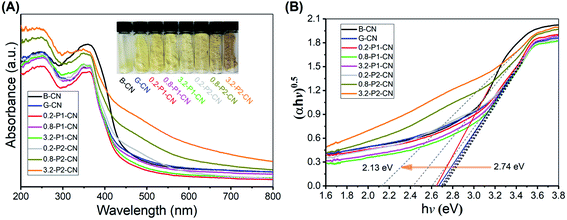 | ||
| Fig. 7 (A) UV-Vis DRS spectra and. (B) Plots of transformed Kubelka–Munk function versus proton energy for a variety of g-C3N4 samples. | ||
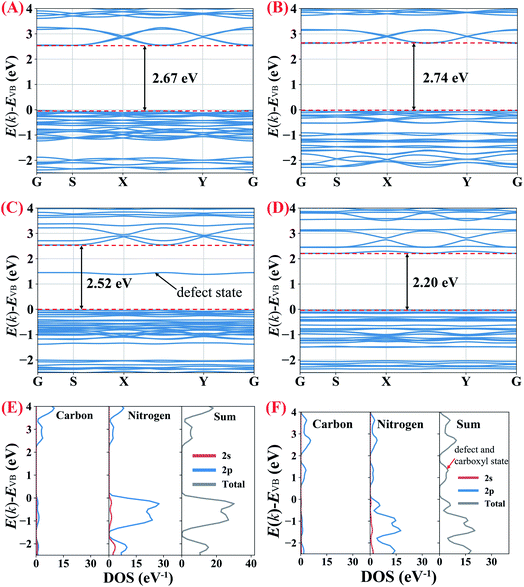
According to the transformed Kubelka–Munk function determined electronic bandgaps shown in Fig. 7B, the bandgaps of H3PO4 doped g-C3N4 nanosheets were slightly increased to a narrow range of 2.70–2.74 eV from 2.67 eV (G-CN), while that of the H3PO2 doped nanosheets can be significantly narrowed to 2.13 eV. The narrowed bandgaps reveal the enhanced visible light harvesting ability of the nitrogen deficient g-C3N4 nanosheets which were doped by H3PO2.
To understand the influence of protonation (including both the protons which connect with nitrogen atoms and the interstitially doped phosphoric acid groups), nitrogen vacancies and carboxyl groups (–COOH) on the bandgaps of the g-C3N4 samples, partial density of states (PDOS) and density-functional theory (DFT) calculations were performed (ESI for details, Fig. S6†).
As shown in Fig. 8A and B, the calculated bandgap for B-CN is 2.67 eV which becomes slightly enhanced to 2.74 eV in the protonated g-C3N4 nanosheets, indicating that the protonation (no matter induced by NH4Cl gas template or interstitially doped phosphorus) does not significantly impact on the magnitude of bandgap. To clarify the effects of the nitrogen vacancies and carboxyl groups, g-C3N4 unit cells only containing nitrogen vacancies or carboxyl groups were built, respectively. The calculations in Fig. 8C and D show that the nitrogen vacancies and the carboxyl groups decrease the bandgap to 2.52 and 2.20 eV, respectively. The defect energy level observed in the g-C3N4 only containing nitrogen vacancies, composing of both C 2p and N 2p orbitals, is about 1.5 eV above valence band (VB). The narrowed bandgap of the g-C3N4 containing nitrogen vacancies and carboxyl groups agrees with the trend observed in the UV-Vis DRS analysis above. As the PDOS seen in Fig. 8E, the conduction band (CB) of B-CN is composed of C 2p and N 2p orbits, while C 2p and N 2p orbits mainly contribute to valence band; this result is consistent with previous work.2,44 According to Fig. 8F, the significantly narrowed bandgap width of the H3PO2 doped g-C3N4 nanosheets is due primarily to a lowering of CB minimum by about 0.4 eV with the emergence of defect and carboxyl states. These results therefor confirm that the coexistence of nitrogen vacancies and carboxyl groups would decrease the width of g-C3N4 bandgap. As confirmed in previous work,2 the coexistence of cyano groups and nitrogen vacancies would also lower the CB minimum and result in narrower electronic energy bandgaps. By analogy, the coexistence of cyano groups, carboxyl groups and nitrogen vacancies would synergistically decrease the bandgap width of the as prepared g-C3N4 nanosheets which were doped by H3PO2.
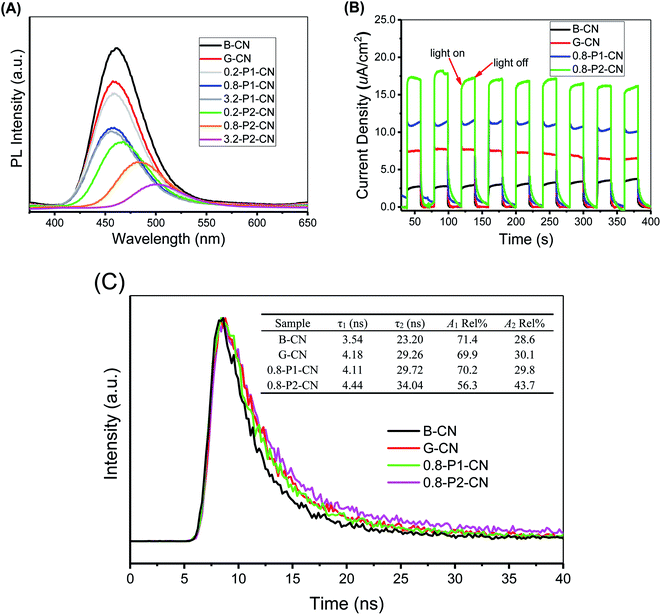
As the photoluminescence (PL) spectra illustrated in Fig. 9A, B-CN shows an intense fluorescence signal at around 460 nm under visible-light irradiation and this signal in G-CN is significantly decreased. Because the PL spectra can be interpreted as the radiative recombination of surface trapping states, thus the weakened PL intensity could indicate the enhanced separation of photo-excited electrons and holes.45,46 By adding H3PO4, the peak is further weakened. It is noted that much lower peak intensities are found over H3PO2 doped g-C3N4 nanosheets and the peak continuously shifts to around 510 nm with the increasing addition of H3PO2. The PL peak red-shift in the H3PO2 doped g-C3N4 nanosheets is associated with the decreased bandgap energy of the samples and consistent with the trend as seen in UV-Vis DRS.46 As illustrated in Fig. 9B, all samples exhibit sensitive photocurrent responses during the visible-light on/off irradiation. The photocurrent density values for the four representative samples (B-CN, G-CN, 0.8-P1-CN and 0.8-P2-CN) are ≈2.5, 7.5, 11.1 and 17.0 μA cm−2, respectively.
To investigate the dynamic electron immigration process, time-resolved fluorescence spectra were collected. As seen in Fig. 9C, the lifetime of the photo-induced charge carriers in the H3PO2 doped g-C3N4 nanosheets were significantly prolonged compared with B-CN, G-CN and H3PO4 doped nanosheets sample, revealing the accelerated charge transfer performance.46
Both photoluminescence and photocurrent tests indicate that the g-C3N4 nanosheets possess much better performance in the critical process of photo-excited charge carrier separation and visible-light response than the corresponding directly polymerized B-CN. Regarding G-CN and H3PO4 doped g-C3N4 nanosheets, the enhanced separation of charge carriers could be attributed to their large specific surface areas and protonation.7,14 Besides, the interstitially doped phosphorus atoms in the H3PO4 doped g-C3N4 nanosheets would likely facilitate optimization of the π-conjugated heptazine rings to improve the carrier mobility and offer a new channel for carrier migration,18,32 thereby offering enhanced charge carriers separation. Besides the interstitial phosphorus doping, the nitrogen vacancies in the H3PO2 doped g-C3N4 nanosheets would also induce unpaired sp2-carbon atoms within the π-conjugated heptazine rings. These electron defected sp2-carbon atoms and newly introduced electron-withdrawing groups (cyano and carboxyl groups) can redistribute the π-electrons and result in improved visible light absorption and photo-excited charge carriers separation.4,47–49 Thus, a confluence of the synergistic effect of protonation, interstitial phosphorus doping, nitrogen vacancies and newly introduced functional groups in the H3PO2 doped g-C3N4 nanosheets was achieved and expected to offer a comprehensive enhancement of visible-light photocatalysis. We now turn to these experiments.
Photocatalytic H2 evolution performances
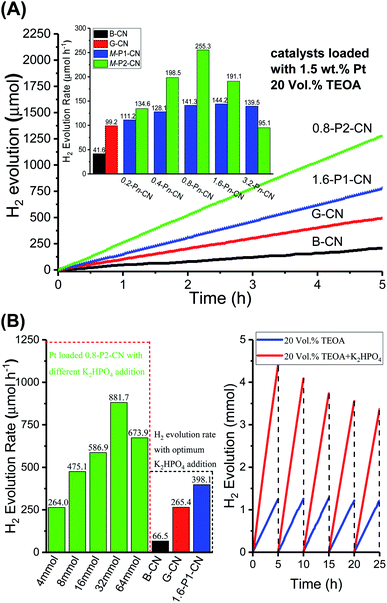
The photocatalytic performances of the g-C3N4 samples were evaluated by measuring the visible-light (λ ≥ 400 nm) H2 evolution in 20 vol% triethanolamine (TEOA) aqueous solution with 1.5 wt% of Pt loading. As shown in Fig. 10A, the g-C3N4 nanosheets exhibited much higher photocatalytic activities than the standard reference B-CN. The H2 generation rate of G-CN reached 99.1 μmol h−1, whilst only 41.6 μmol h−1 was obtained over B-CN. Importantly, the H2 evolution rate of g-C3N4 nanosheets was significantly improved with the doping of H3PO4 and H3PO2, respectively. The H3PO4 doped g-C3N4 nanosheets exhibited a H2 evolution rate of 144.2 μmol h−1 by the 1.6-P1-CN sample. Interestingly, 0.8-P1-CN and 3.2-P1-CN gave a very similar H2 evolution rate as 1.6-P1-CN, which could be ascribed to that the limited visible light absorption of the H3PO4 protonated g-C3N4 nanosheets confines the highest H2 evolution rate when a certain level of H3PO4 addition was reached. For the H3PO2 doped nanosheets, 0.8-P2-CN showed the highest H2 evolution rate of 255.3 μmol h−1 which was 6.14, 2.58 and 1.77 times of that over B-CN, G-CN and 1.6-P1-CN, respectively. The bandgap structures determined by the valence band XPS spectra and UV-Vis DRS results indicate that the narrowed bandgap of the H3PO2 doped g-C3N4 nanosheets originates from the conduction band decrease (ESI, Fig. S7 and Table S2†). Thus, the excessively lowered reduction driving force for H2 evolution may result in lower H2 evolution activity even though the visible light harvesting ability of g-C3N4 was progressively intensified by increasing H3PO2 usage. These findings could explain why the highest H2 generation performance was achieved on the Pt loaded 0.8-P2-CN.Because our RTK-Solar H2 evolution system was used for the first time for photocatalytic H2 evolution tests, its accuracy was verified in two steps. First, the evolved gas was confirmed to be only H2 with the help of Hiden HPR 20 gas chromatograph/mass spectrometer system (ESI, Fig. S8†). Second, H2 evolution experiments over 1.5 wt% Pt loaded B-CN, G-CN, 1.6-P1-CN and 0.8-P2-CN in 20 vol% TEOA solution were further conducted under the same conditions as those in the RTK-Solar system. With the quartz reactor being connected to a gas circulation system, the generated H2 amount was quantified by a calibrated gas chromatography equipped TCD detector (Clarus 580, PerkinElmer, helium as carrier gas) for every 1 hour. The gas chromatography determined H2 evolution rates over the 1.5 wt% Pt loaded B-CN, G-CN, 1.6-P1-CN and 0.8-P2-CN were 42.8, 106.6, 140.1 and 261.6 μmol h−1, respectively (ESI, Fig. S9 and Table S3†). The ratios of the H2 evolution rates determined by gas chromatography method and the RTK-Solar system over the four representative g-C3N4 samples were located in a very narrow range of 0.972 to 1.076, confirming the reliability of RTK-Solar system for measuring the amount of generated H2. The photocatalytic H2 evolution results reveal the significantly enhanced g-C3N4 photocatalytic performance over the nitrogen deficient and protonated g-C3N4 nanosheets produced by this facile in situ reductive synthesis strategy.
As proposed by Ye,1 using the nature-inspired strategy of adding K2HPO4 to TEOA solution, the H2 evolution performance of g-C3N4 could be significantly enhanced. In Ye's important work,1 it was shown that the added HPO42− can function both as a proton-pump in natural photosynthesis (to facilitate proton transport in the reaction solution) and also act as a mediator to give rise to a new proton-reduction pathway. The use of HPO42− instead of H2O provides the necessary protons to react with photo-generated electrons to produce H2 and PO43− on the surface of Pt co-catalyst at the very beginning. Following the H2 evolution from HPO42−, the resulting PO43− entity immediately combined with H+ from H2O to regenerate HPO42− and finally complete the proton-reduction cycle.
In other words, the added K2HPO4 would not be consumed during the photocatalytic H2 evolution. In addition, K2HPO4 would also promote the oxidation of TEOA. The synergy of enhanced proton reduction and improved photooxidation of TEOA boosted the visible-light photocatalytic H2 evolution over the Pt loaded g-C3N4 catalysts. Thus, enhanced H2 evolution over the Pt loaded 0.8-P2-CN could also be expected by adding K2HPO4 to the TEOA solution.
As illustrated in Fig. 10B, with the optimum addition of K2HPO4 (32 mmol) to the 20 vol% TEOA solution, the H2 evolution of the Pt loaded 0.8-P2-CN was drastically enhanced to a rate of 881.7 μmol h−1, and impressive H2 generation could be observed (ESI, Video S1†). Similarly, the H2 generation rates of the Pt loaded B-CN, G-CN and 1.6-P1-CN were also promoted by the optimum TEOA/K2HPO4 solution, but the 0.8-P2-CN with 1.5 wt% Pt loading showed the highest increase among others. Though, G-CN and 1.6-P1-CN nanosheets possess well improved charge carrier separation abilities through protonation and phosphorus interstitial doping, and their H2 evolution rates could greatly enhanced by adding K2HPO4 to facilitate both proton reduction and the photooxidation of TEOA. However, their limited visible light absorption abilities with a wide bandgap of around 2.7 eV will confine the further improvement of visible-light H2 evolution.
In contrast, the 0.8-P2-CN nanosheets could harvest much more visible light to generate photo-excited electrons and holes with a significantly narrowed bandgap of 2.42 eV (ESI, Fig. S7†). Thus, together with the enhanced charge carrier separation, proton reduction and photooxidation of TEOA, the significantly intensified visible-light absorption ability could help the Pt loaded 0.8-P2-CN nanosheets to boost visible-light H2 evolution rate to an extremely high level.
In our 25 hours long-term H2 evolution experiments, it was found that the Pt loaded 0.8-P2-CN generated nearly the same amount of H2 for each 5 hours period in the TEOA solution, while the H2 generated in the last 5 hours period could still remain a 76.5% amount of that in the first 5 hours for the optimized TEOA/K2HPO4 mixture solution. As stated above, the K2HPO4 would not be consumed during the photocatalytic H2 evolution. The gradual decrease of the long-term H2 evolution rate in the TEOA/K2HPO4 solution should be attributed to the significant consumption of TEOA during the photocatalytic H2 evolution process. Thus, both the long-term H2 evolution experiments conducted in TEOA and TEOA/K2HPO4 solutions indicated the excellent photocatalytic stability of the H3PO2 doped nanosheets. The FTIR, elemental analysis conducted using STEM and XPS narrow scan results of the fresh and used 0.8-P2-CN suggest that there are no obvious changes which may induce adverse effect on the photocatalytic performances, confirming the stability of the as-prepared g-C3N4 nanosheets (ESI, Fig. S10–S12†).
In addition, the spent 0.8-P2-CN catalyst still showed observable H2 generation ability (ESI, Video S2†). The 0.8-P2-CN with 1.5 wt% Pt loading achieved an AQY of 10.7% and 40.4% at 420 nm in the TEOA and TEOA/K2HPO4 (ESI, Fig. S13†), respectively, indicating the extremely efficient visible-light photocatalytic H2 evolution over the H3PO2 doped g-C3N4 nanosheets compared with the previously reported work (Table S4†). The AQY measurement results at other wavelengths including 400, 450, 500, 550 and 600 nm were also supplemented in the ESI (Fig. S14 and Table S5†).
Conclusions
Nitrogen deficient and protonated g-C3N4 nanosheets were successfully synthesized through a one-step, in situ reduction process combining both a NH4Cl-assisted strategy and H3PO2 doping. It has been demonstrated that the protonated g-C3N4 nanosheets have superb photocatalytic activities and can be robustly fabricated by using NH4Cl as a dynamic gas template. Compared with the g-C3N4 nanosheets which were simply further protonated by interstitial H3PO4 doping, the electronic bandgaps of the nitrogen deficient g-C3N4 nanosheets can be easily controlled by adjusting the added H3PO2. The enhanced protonation and reduced electronic bandgaps of H3PO2 doped g-C3N4 nanosheets can not only readily harvest visible light but also serve to separate the excited state charge carriers more effectively, thereby offering extremely efficient H2 production under visible-light irradiation.With the addition of K2HPO4 to TEOA solution, the H2 evolution rate over the 1.5 wt% Pt loaded H3PO2 doped g-C3N4 nanosheets could be boosted to 881.7 μmol h−1 with an apparent quantum yield of 40.4% at 420 nm. In addition, this comprehensive investigation on different phosphorus compounds for g-C3N4 nanosheets modification indicates that H3PO2 is a promising dopant to synergistically modify the g-C3N4 photocatalysts in a single step. We believe this work will provide guidance for the facile synthesis of nitrogen defective and protonated g-C3N4-based materials for further synergistic enhancements of visible-light photocatalysts performance.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from State Key Laboratory of Chemical Engineering (Tianjin University) and EPSRC. We appreciate the valuable suggestions provided by Dr Xiangyu Jie (Chemistry department, University of Oxford) for manuscript preparation. Weisong Li and Litong Jiang also gratefully thank the China Scholarship Council (CSC) for scholarships.Notes and references
- G. Liu, T. Wang, H. Zhang, X. Meng, D. Hao, K. Chang, P. Li, T. Kako and J. Ye, Angew. Chem., 2015, 127, 13561 CrossRef PubMed.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L. Wu, C. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- L. Ge, C. Han and J. Liu, Appl. Catal., B, 2011, 108, 100 CrossRef.
- X. Liu, P. Wang, H. Zhai, Q. Zhang, B. Huang, Z. Wang, Y. Liu, Y. Dai, X. Qin and X. Zhang, Appl. Catal., B, 2018, 232, 521 CrossRef CAS.
- G. Zhang, S. Zang, L. Lin, Z. A. Lan, G. Li and X. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 2287 CrossRef CAS PubMed.
- P. Niu, L. Zhang, G. Liu and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 4763 CrossRef CAS.
- X. Lu, K. Xu, P. Chen, K. Jia, S. Liu and C. Wu, J. Mater. Chem. A, 2014, 2, 18924 RSC.
- C. Li, S. Yu, H. Dong, C. Liu, H. Wu, H. Che and G. Chen, Appl. Catal., B, 2018, 238, 284–293 CrossRef CAS.
- C. Li, Y. Du, D. Wang, S. Yin, W. Tu, Z. Chen, M. Kraft, G. Chen and R. Xu, Adv. Funct. Mater., 2017, 27, 1604328 CrossRef.
- C. Li, Y. Xu, W. Tu, G. Chen and R. Xu, Green Chem., 2017, 19, 882–899 RSC.
- Z. Li, C. Kong and G. Lu, J. Phys. Chem. C, 2015, 120, 56 CrossRef.
- Y. P. Zhu, T. Z. Ren and Z. Y. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 16850 CrossRef CAS PubMed.
- P. Niu, G. Liu and H. M. Cheng, J. Phys. Chem. C, 2012, 116, 11013 CrossRef CAS.
- Y. Zhang, A. Thomas, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2008, 131, 50–51 CrossRef PubMed.
- M. Wu, T. Ding, J. Cai, Y. Wang, H. Xian, H. Zhang, Y. Tian, T. Zhang and X. Li, ACS Sustainable Chem. Eng., 2018, 6, 8167 CrossRef CAS.
- P. Niu, L. C. Yin, Y. Q. Yang, G. Liu and H. M. Cheng, Adv. Mater., 2014, 26, 8046 CrossRef CAS PubMed.
- Z. Hong, B. Shen, Y. Chen, B. Lin and B. Gao, J. Mater. Chem. A, 2013, 1, 11754 RSC.
- L. Shi, K. Chang, H. Zhang, X. Hai, L. Yang, T. Wang and J. Ye, Small, 2016, 12, 4431 CrossRef CAS PubMed.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294 CrossRef CAS PubMed.
- J. Yuan, Q. Gao, X. Li, Y. Liu, Y. Fang, S. Yang, F. Peng and X. Zhou, RSC Adv., 2014, 4, 52332 RSC.
- M. Groenewolt and M. Antonietti, Adv. Mater., 2005, 17, 1789 CrossRef CAS.
- Y. Cui, Z. Ding, X. Fu and X. Wang, Angew. Chem., 2012, 124, 11984 CrossRef.
- W. Lei, D. Portehault, R. Dimova and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 7121 CrossRef CAS PubMed.
- J. Fu, B. Zhu, C. Jiang, B. Cheng, W. You and J. Yu, Small, 2017, 13, 1603938 CrossRef PubMed.
- G. Dong, Z. Ai and L. Zhang, RSC Adv., 2014, 4, 5553 RSC.
- J. Sehnert, K. Baerwinkel and J. Senker, J. Phys. Chem. B, 2007, 111, 10671 CrossRef CAS PubMed.
- B. Jürgens, E. Irran, J. Senker, P. Kroll, H. Müller and S. Wolfgan, J. Am. Chem. Soc., 2003, 125, 10288 CrossRef PubMed.
- V. W. H. Lau, I. Moudrakovski, T. Botari, S. Weinberger, M. B. Mesch, V. Duppel, J. Senker, V. Blum and B. V. Lotsch, Nat. Commun., 2016, 7, 12165 CrossRef CAS PubMed.
- Y. Fu, J. Zhu, C. Hu, X. Wu and X. Wang, Nanoscale, 2014, 6, 12555 RSC.
- S. J. Makowski, D. Gunzelmann, J. Senker and W. Schnick, Z. Anorg. Allg. Chem., 2009, 635, 2434 CrossRef CAS.
- M. Zhu, S. Kim, L. Mao, M. Fujitsuka, J. Zhang, X. Wang and T. Majima, J. Am. Chem. Soc., 2017, 139, 13234 CrossRef CAS PubMed.
- X. Ma, Y. Lv, J. Xu, Y. Liu, R. Zhang and Y. Zhu, J. Phys. Chem. C, 2012, 116, 23485 CrossRef CAS.
- G. R. Goward, M. F. Schuster, D. Sebastiani, I. Schnell and H. W. Spiess, J. Phys. Chem. B, 2002, 106, 9322 CrossRef CAS.
- T. Emmler, S. Gieschler, H. H. Limbach and G. Buntkowsky, J. Mol. Struct., 2004, 700, 29 CrossRef CAS.
- Y. Chen, B. Lin, H. Wang, Y. Yang, H. Zhu, W. Yu and J. M. Basset, Chem. Eng. J., 2016, 286, 339 CrossRef CAS.
- C. Ye, J. X. Li, Z. J. Li, X. B. Li, X. B. Fan, L. P. Zhang, B. Chen, C. H. Tung and L. Z. Wu, ACS Catal., 2015, 5, 6973–6979 CrossRef CAS.
- M. Rong, Z. Cai, L. Xie, C. Lin, X. Song, F. Luo, Y. Wang and X. Chen, Chem.–Eur. J., 2016, 22, 9387 CrossRef CAS.
- H. Gao, S. Yan, J. Wang, Y. A. Huang, P. Wang, Z. Li and Z. Zou, Phys. Chem. Chem. Phys., 2013, 15, 18077 RSC.
- K. B. Yatsimirskii, V. V. Nemoskalenko, V. G. Aleshin, Y. I. Bratushko and E. P. Moiseenko, Chem. Phys. Lett., 1977, 52, 481 CrossRef CAS.
- M. A. Mohamed, M. F. M. Zain, L. J. Minggu, M. B. Kassim, N. A. S. Amin, W. N. W. Salleh, M. N. I. Salehmin, M. F. M. Nasir and Z. A. M. Hir, Appl. Catal., B, 2018, 236, 265 CrossRef CAS.
- J. Liu, T. Zhang, Z. Wang, G. Dawson and W. Chen, J. Mater. Chem., 2011, 21, 14398 RSC.
- G. Zhang, M. Zhang, X. Ye, X. Qiu, S. Lin and X. Wang, Adv. Mater., 2014, 26, 805 CrossRef CAS PubMed.
- W. Tu, Y. Xu, J. Wang, B. Zhang, T. Zhou, S. Yin, S. Wu, C. Li, Y. Huang, Y. Zhou, Z. Zou, J. Robertson, M. Kraft and R. Xu, ACS Sustainable Chem. Eng., 2017, 5, 7260 CrossRef CAS.
- G. Dong, K. Zhao and L. Zhang, Chem. Commun., 2012, 49, 6178 RSC.
- J. Qin and H. Zeng, Appl. Catal., B, 2017, 209, 161–173 CrossRef CAS.
- F. Wang, Y. Wang, Y. Feng, Y. Zeng, Z. Xie, Q. Zhang, Y. Su, P. Chen, Y. Liu, K. Yao, W. Lv and G. Liu, Appl. Catal., B, 2018, 221, 510–520 CrossRef CAS.
- Y. Zhao, M. Shalom and M. Antonietti, Appl. Catal., B, 2017, 207, 311 CrossRef CAS.
- J. Zhang, M. Zhang, R. Sun and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145 CrossRef CAS PubMed.
- G. Liu, G. Zhao, W. Zhou, Y. Liu, H. Pang, H. Zhang, D. Hao, X. Meng, P. Li, T. Kako and J. Ye, Adv. Funct. Mater., 2016, 26, 6822 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Sample characterization, H2 evolution equipment, supplementary XRD, XPS and elemental mapping data, photocatalytic H2 evolution experimental results. See DOI: 10.1039/c9sc05060d |
| This journal is © The Royal Society of Chemistry 2020 |