Video-based instruction on safety rules in the chemistry laboratory: its effect on student achievement
Bülent
Pekdağ

Balıkesir University, Necatibey Education Faculty, Department of Secondary Mathematics and Science Education, Chemistry Education, 10100 Balıkesir, Turkey. E-mail: pekdag@balikesir.edu.tr
First published on 21st May 2020
Abstract
This study explores the effect of video-based instruction on the safety rules in the chemistry laboratory on student achievement. The sample for the study comprised 61 ninth grade students enrolled in two different classes at a public high school. The students in the class designated as the experimental group (N = 32) were given video-based instruction on the safety rules required to be applied in the chemistry laboratory, while the other class that was designated as the control group (N = 29) was taught the same topic, but with traditional instruction. The experimental and control groups were randomly selected, and the same teacher instructed both groups. The students in both groups were administered an academic achievement test on safety rules made up of six open-ended questions as a pretest and as a posttest. The collection of the data took 3 weeks. The pretest was administered in the first week, the posttest in the third week. The data were subjected to content analysis, which was performed as qualitative analysis. It was found as a result of the analysis that the students exposed to video-based instruction showed greater improvement in their academic achievement compared to the students given traditional instruction. The results of the study provide helpful information for chemistry teachers and researchers in the field of chemistry education.
Introduction
Students are given the opportunity to enter the laboratory and perform experiments as part of their science courses (Akpullukcu and Cavas, 2017). A typical laboratory however contains dangers that are related to extreme temperatures or explosions due to high or low pressure, moving parts (e.g., centrifuges and vacuum pumps), and high voltage. At the same time, laboratories contain hazardous chemicals that can act as carcinogens, irritants, corrosives, allergens and sensitizers, asphyxiants, explosives, toxins, and flammables (Hill et al., 2019). The presence of chemical substances and experimental conditions are the inherent elements of laboratory work (Álvarez-Chávez et al., 2019). Safety precautions must be considered when engaged in laboratory activities (Karataş, 2016). When safety risks are taken lightly, this can increase the possibility of unexpected injury, property damage as well as experiment failure (Álvarez-Chávez et al., 2019).Without safety measures, the laboratory setting has the potential of causing small and sometimes even severe accidents (NRC, 2011). Learning the safety rules to be applied in the laboratory greatly contributes to preventing laboratory accidents (Walters et al., 2017). In order to prevent students’ exposure to possible laboratory accidents, we must teach them and make sure that they fully understand the rules of the laboratory because this is a matter that concerns human life. We need an effective teaching approach toward this end. It is our belief that video-based instruction, which is the subject of this study, can be such a teaching approach.
Laboratory accidents and their causes
It is reported that accidents frequently occur in science laboratories both in Turkey and around the world. Akpullukcu and Cavas (2017) note that in 2010, an explosion occurred during an experiment being carried out in the science laboratory of a private school, which ended up with a teacher and five students being injured. Similar accidents causing both students and teachers injuries happened across the country in different times.According to a study in Iowa State University, 33.3% out of 1497 laboratory accidents involved students (Simmons et al., 2017). Stuart and Toreki (2014) reported that between 2010–2013, 10% of the 5381 hazardous incidents that had been recorded took place in the laboratory setting. Based on the observation made by Chemical Safety Board (CSB) on 120 laboratory accidents (explosions, fires and chemical emission) occurring over the years 2001–2011, such accidents were responsible for a range of injuries, -from minor to severe- and death as well as extensive material damage (CSB, 2011).
In their review of local and national news, Aydoğdu and Yardımcı (2013) deduced that accidents involved test tubes, spirits, steel tubes, diffusion of chemicals, rising gas pressure and breaking mercury tubes. Accidents were mainly caused by (i) lack of sufficient chemical-related knowledge; (ii) failure of intervention in chemical spillage; (iii) inadequate knowledge of responsive actions; (iv) supervision-related issues in experiments; and (v) carelessness during experiment.
In the university context, more than half of the students experience at least one accident in the laboratory, and failure to handle the equipment properly is the leading cause (Álvarez-Chávez et al., 2016). In similar studies, where compliance of students with laboratory safety rules was observed in terms of following the rules, it was found that majority of students made numerous errors in obeying safety rules due to reasons such as lack of knowledge, training and diligence (Su and Hsu, 2008; Alaimo et al., 2010; Adane and Abeje, 2012; Karataş, 2016).
Laboratory safety education
The accidents that students are involved in have pointed to the vital importance of laboratory safety education. Many laboratory accidents have revealed evidence of the deficiency in safety education (Hill, 2016). It has been concluded that reducing the number of laboratory accidents depends on the implementation of more education and training on laboratory safety (Walters et al., 2017).In the last 25 years, there have been different approaches to the matter of laboratory safety education (Table 1).
| Authors | Themes |
|---|---|
| Hill and Greco (1995) | Skits (safety is no laughing matter) |
| Helser (1999a) | Lab safety “scavenger hunt” |
| Helser (1999b) | Safety wordsearch (wordsearch puzzles) |
| Moody and Freeman (1999) | Chemical safety seminar |
| Gublo (2003) | Laboratory safety trivia game |
| Wright (2005) | Introducing safety topics using a student-centered approach |
| Matson et al. (2007) | Laboratory safety videos |
| Alaimo et al. (2010) | Establishing class-wide safety teams |
| McGarry et al. (2013) | Student involvement in improving the culture of safety in academic laboratories |
| Wedlich et al. (2013) | Incorporating professional safety standards into the laboratory experience |
| Carr and Carr (2016) | Watching a short and humorous video of a character named Mr Bean, who visits a chemistry laboratory and commits several safety violations |
| Staehle et al. (2016) | An approach to enhance the safety culture of an academic chemistry research laboratory |
| Huston et al. (2018) | Development of a course in chemical laboratory safety through an academic/industrial collaboration |
| Kumasaki et al. (2018) | Using a graphic novel, Manga, to effectively teach chemical safety to students |
| Schenk et al. (2018) | Identifying the scope of safety issues and challenges to safety management |
| Hill et al. (2019) | Introduction to laboratory safety: an active-learning endeavor |
| Tsokou et al. (2019) | Measuring and reducing chemical spills by students |
The activities shown in Table 1 aimed at raising students’ awareness about the natural risks and hazards of the laboratory. The students were additionally urged to understand the importance of following safety rules.
Matson et al. (2007) prepared a 17 minute laboratory safety video for the general chemistry class in an effort to reduce the students’ laboratory anxiety. The authors reported that the video was effective in teaching the students about laboratory safety applications. The students also found the video entertaining. A short and funny video was presented to students in another study about a character by the name of Mr Bean who visits a chemistry laboratory and commits several safety violations. The students were able to correctly identify the important laboratory safety violations shown in the video. Most of the students said they found the video more entertaining and easier to remember than having to read a textbook summarizing safety applications or listening to a lecture in the laboratory on laboratory safety rules. One of the students said that the video allowed the actual visualization of possible safety violations in the laboratory. Another student stated that the video demonstrated the consequences of not complying with safety rules. Additionally, most of the students expressed the view that they had expanded their knowledge of laboratory safety. In short, the students said that the video was beneficial in that it helped them to visualize safety issues in the laboratory (Carr and Carr, 2016). The later mentioned studies focus on training laboratory safety in a general chemistry course and the use of pre-existing multimedia as a teaching tool for laboratory safety, respectively. On the other hand, the present study aimed at teaching the laboratory safety rules for the secondary level students by using videos that were prepared in-house by the author.
Videos
Educational videos can be traced back to 1957, when a series of lessons were first filmed (Slabaugh and Hatch, 1958). Earlier educational videos, which were relatively long, were in the form of lectures and documentaries on the chemical processes used in industry. With time, lengthy videos soon gave way to short films of usually 5–10 minutes, limited to only a single area of content, requiring the presentation of specific knowledge (Pekdağ and Le Maréchal, 2010; Brame, 2016; Kulgemeyer, 2018). This way, videos began to be produced on a specific laboratory technique or as a demonstration of a particular experiment, which led to relatively cheaper and versatile products (Pekdağ, 2017).Being regarded as successful at eliminating understanding and conceptualization difficulties, misconceptions, and motivation issues, videos are considered an alternative and effective teaching tool (Pekdağ, 2010; Box et al., 2017). The results of recent studies provide evidence in support of these aspects (Burewicz and Miranowicz, 2006; Mayo et al., 2009; Lichter, 2012; Nadelson et al., 2015; Carr and Carr, 2016; Schmidt-McCormack et al., 2017).
The use of video in school setting has several benefits such as (i) the convenience of creating visualizations of scientific knowledge, (ii) economic benefits, (iii) psychological benefits (motivation, pleasure of learning), and (iv) cognitive benefits (learning more and better, memorizing, remembering) (Pekdağ, 2017).
Videos are described as the active image of reality (Pekdağ and Le Maréchal, 2007a) and are favoured due to their capacity to introduce scientific processes in motion, eliminating difficulties of following the progression of rapidly occurring scientific phenomena and demonstrating hard-to-describe scientific events (Pekdağ, 2017). In addition, videos provide opportunities to explain particularly difficult subjects (Pekdağ, 2010). Videos contain visual, dynamic, spatial and veridical representations of information (Kumar, 2010).
At the same time, videos are user-friendly in that they can be paused at any time, interpreted and restarted, allowing students to learn at their own pace (Schwan and Riempp, 2004; Chen and Cowie, 2014; Jordan et al., 2016; Box et al., 2017) and this control is an important element in learning (Pekdağ and Le Maréchal, 2010).
Problems and limitations likely to be dictated by time, finance and hazard related issues or difficulties with procuring expensive chemicals and preparing extensively for an experiment can simply be overcome by the introduction of videos (Pekdağ, 2010; Chen and Cowie, 2014).
Using videos as teaching materials in a certain subject draws the interest of students and whets their curiosity, raises their motivation, allows them to maintain their focus on the subject; additionally, it makes learning more entertaining (Harwood and McMahon, 1997; Matson et al., 2007; Lichter, 2012; Dorfman et al., 2019). In other words, videos help students to mentally visualize a scientific concept, facilitating learning (Pekdağ and Le Maréchal, 2010). The use of video in the learning setting contributes to the creation of high-quality mental models and the development of cognitive abilities such as interpretation, critical thinking and problem-solving (Pekdağ, 2017). Moreover, videos contribute to keeping scientific knowledge and important points stored in the memory to be recalled (Pekdağ and Le Maréchal, 2007b), and they enable students to understand scientific processes or mechanisms (Jordan et al., 2016; Dorfman et al., 2019). Students find it easier to understand the subject and learn more and better (Escalada and Zollman, 1997; Harwood and McMahon, 1997; Mayo et al., 2009; Lau, 2020). Video-oriented instruction increases student knowledge, leading to a positive impact on the accumulation of knowledge and to meaningful and enhanced learning (Zahn et al., 2004; Lichter, 2012; Nadelson et al., 2015; Carr and Carr, 2016; Box et al., 2017).
However, the technological tools such as videos should be designed in such a way as to prevent unnecessary cognitive overload for students. It is important to ease the load placed on students’ visual and auditory memory by reducing the amount of knowledge to be learned. This must be done because cognitive overload has an impact on learning (Winberg and Berg, 2007). Cognitive load theory suggests that visual and auditory memory capacities are limited. In other words, both visual memory and auditory memory have a finite capacity to process, encode and store information (Sweller, 1988; Chandler and Sweller, 1991; Baddeley, 1992). Presenting too many elements or events that are to be treated in the visual or auditory memory causes overload in students.
Studies on the use of video in chemistry education
Harwood and McMahon (1997) explored the effect of a video on the achievement and attitudes of first-year high school students taking a general chemistry course. The results of the study showed that the video had a positive impact on the achievement of the students in chemistry and on their attitudes toward the course. On the other hand, Velázquez-Marcano et al. (2004) provided an informative video for students to watch in the chemistry laboratory about substances. It was observed that the students exhibited an improvement in their conceptual understanding after watching the video. In connection, Burewicz and Miranowicz (2006) reported that video-supported instruction reduced the number of mistakes students made during an experiment and shortened experimentation time.Additionally, Lichter (2012) asked students in a general chemistry course to create and upload a video to YouTube that could be used to learn the rules of solubility. The video assignment improved learning of the rules and promoted interest in chemistry in a majority of the students involved in the activity. Students who produced videos performed significantly better than the rest of their classmates in terms of remembering the rules of solubility. Remarkably, students who did not make videos but watched them did better than students who did not produce or watch videos.
Moreover, Teo et al. (2014) started out classes in the chemistry laboratory by making students watch videos. It was seen that the students watching the videos understood the theoretical knowledge better and were better at coping with complex practical procedures. It was observed that students were less anxious in the laboratory and that their work productivity increased. Nadelson et al. (2015), on the other hand, developed a series of instructional videos designed to teach students the basic techniques and concepts in the chemistry laboratory. The students watching the videos showed an improvement in their procedural and content knowledge as compared to the students who did not watch the videos. Also, students who had watched the videos completed their experiments in a shorter period of time.
Similarly, Jordan et al. (2016) used videos to teach organic chemistry laboratory techniques and procedures. They found at the end of their study that the videos had effectively prepared the students for the laboratory. Moreover, the students were able to correctly respond to questions about the laboratory procedure. Ultimately, students watching the video were able to be more independent in the lab and perform better than students who had received a teaching assistant's instruction alone. In align with this, Box et al. (2017) found that the group of students watching a video completed the organic chemistry laboratory activity faster than the students who did not watch the video. At the same time, it was seen that the students watching the video better understood the experimental technique. The video presentation had made a major positive impact on the number of questions the students answered correctly. In the follow-up survey, it was noted that the students found the video beneficial in completing the laboratory activity. It was ultimately noted that the instructional videos had a positive effect on laboratory preparation and learning outcomes.
Last but not least, Schmidt-McCormack et al. (2017) determined that videos had a positive effect on students’ ability to successfully complete their experiments. Lau (2020) reported that the use of videos on the subject of titration enhanced student achievement.
To sum, aforementioned studies reveal many positive contributions of the use of video in chemistry lessons and laboratories from providing meaningful learning to saving time during the experimentation, from gaining positive attitude towards the course to improving understanding of chemical phenomena to reducing the mistakes during the experimentation.
Purpose of the study
Studies on the subject of the laboratory are fundamentally concerned with materials, methods and techniques. Unfortunately, it can be seen that the matter of laboratory safety has been treated at only a limited level (Gublo, 2003; Alaimo et al., 2010; Wedlich et al., 2013; Hill et al., 2019). This is despite the fact that the subject of laboratory safety is of vital importance in the context of preventing laboratory accidents. While safety considerations are an issue for everyone exposed to potentially hazardous substances, persons who routinely work with chemicals, that is, students and teachers working in laboratories, are particularly at risk (Walters et al., 2017). Because of this, instruction to be provided to students on laboratory safety is of crucial importance (Álvarez-Chávez et al., 2019).Laboratory safety has gathered more attention recently because of some serious accidents that have occurred (Huston et al. 2018). It has been due to this that chemistry education programs now include the topic of laboratory safety (Agustian and Seery, 2017). Science teachers should be informed about the potential dangers of the laboratory and how to ensure safety during the course of an experiment. Teachers should be familiar with and know how to use laboratory equipment as well as how to use and store each chemical. Teachers need to transfer the knowledge they have gained from their chemistry education program to their students. According to the program of instruction, high school students are required to know about such topics as the use of chemicals, labeling, protecting the eyes and face, electrical safety, the use of glass materials, utilizing hot containers and fire control (Ministry of National Education/MEB, 2018). Because the matter of laboratory safety is such a vital issue, students must fully understand the rules of laboratory safety and comply with these rules when they are performing experiments.
Instructing students about laboratory safety is an important step in preparing them for the laboratory setting (Walters et al., 2017). However, laboratory safety education is generally considered boring and uninteresting (Carr and Carr, 2016). Because of this, methods now used in laboratory safety instruction are different from the traditional manner of instruction implemented in the past (See section on Laboratory safety education). One of these methods is the method of video-based instruction. Unfortunately, there are only a few studies to be found on such an important topic as laboratory safety instruction and its effect on student achievement (Matson et al., 2007; Carr and Carr, 2016). The results of these studies however are promising. It is because of this that the present study aimed to explore the subject of video-based instruction on safety in the chemistry laboratory.
Toward this objective, answers were sought to the following research question:
What is the effect of video-based instruction on the improvement in student's understanding regarding chemistry laboratory safety rules compared to traditional instruction?
Method
Study context
The safety rules required to be applied in the chemistry laboratory is a topic that is taught in the 9th grade chemistry course. The ninth grade chemistry course contains the following topics: (i) The Science of Chemistry (Unit 1), (ii) The Atom and the Periodic System (Unit 2), (iii) Interaction between Chemicals (Unit 3), (iv) States of Matter (Unit 4) and (v) Nature and Chemistry (Unit 5). The subject of complying with safety rules in the chemistry laboratory is treated in Unit 1, Section 4 of the syllabus (Occupational Health and Safety). The time allotted to teaching this topic in the 9th grade syllabus is 1 class hour. The topic is taught in the fall semester. The learning outcomes specified in the syllabus are: “9.1.4.1. Students explain the safety rules that must be followed in chemistry laboratories” (MEB, 2018).Producing a video
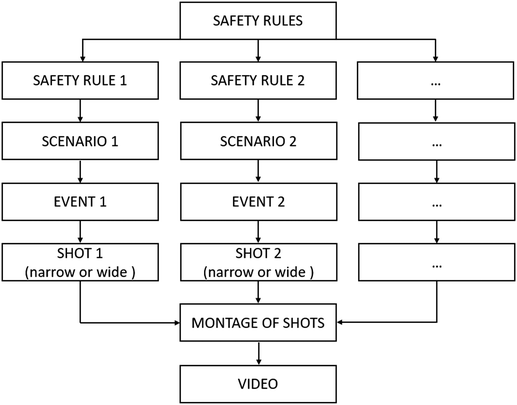
The person producing the instructional video used in the present study is the author, who has 20 years of experience in producing videos on chemistry experiments. The persons with roles in the video were students in their last year in the chemistry education department. The video was shot in a chemistry laboratory in the university. Professional equipment was used in the production of the video.A safety rule required to be applied in the chemistry laboratory was chosen and a scenario was drawn up. Considering the scenario the event to take place was decided. The style of video shot (narrow or wide shot) was determined and the scenario was then staged. For the actual production of the video the shots were montaged. The end product was a 2′ 30′′ video (see Appendix 1). Fig. 1 shows in details the steps of production of a video.
An example of a video image produced using a narrow shot style was given in Table 2.
The video image given in Table 2 demonstrates the result of violating a laboratory safety rule.
The safety rules that were in the focus of the study were provided in Appendix 2.
Research design
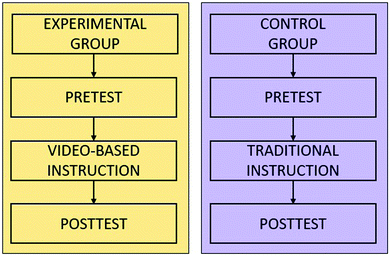
A pre- and posttest control group quasi-experimental research design was used in this study. One experimental group and one control group were selected, and each treatment (video-based instruction and traditional instruction) was randomly assigned. Schematically, the research design can be presented as follows (Fig. 2):Participants
Before beginning the research study, the researcher applied to the administration of the school where the treatment is going to be implemented by submitting the documents including the timeline and content of the study. For ethical purposes, the school administration and the local directorate were submitted required paperwork and written permissions to conclude such research work were obtained. These procedures are regarded as sufficient to carry out experimental research in schools. After obtaining the necessary permissions, the participants were informed about the aim of the work to be conducted. Participants were also informed that their anonymity and confidentiality will be respected and they will be free to discontinue participation at any time, in case they accepted to participate. All participants stated their voluntariness.The sample for the study comprised 61 ninth grade students enrolled in two different classes at a public high school located in western Turkey. The students in the class designated as the experimental group (N = 32) were given video-based instruction on the safety rules that are required in the chemistry laboratory, while the other class, designated as the control group (N = 29) was taught the same topic, but with traditional instruction. The experimental and control groups were randomly selected.
Instrument
An academic achievement test (AAT) composed of 6 open-ended questions was used as a data collection instrument (see Appendix 3). The instrument was named as an academic achievement test to imply that the improvement in student's understanding regarding laboratory safety rules was measured. The AAT was created in the light of the student textbook (Avcı, 2018) and the learning outcomes specified in the chemistry education syllabus (MEB, 2018). The opinions of 3 experts were enlisted in the development of the test, to provide the face and content validity, and the necessary revisions were made on the basis of these opinions. A pilot study was carried out prior to the actual procedure. In the pilot study, the time in which the students were able to answer the questions on the AAT as well as the comprehensibility, content and readability of the questions were considered. According to the feedbacks obtained from the students, needed revisions were made to give the test its final form.Procedure
Before beginning with the instruction on the safety rules required to be applied in chemical laboratory, the experimental and control groups were administered the academic achievement test as a pretest in order to determine the extent of knowledge the students had about this subject. Subsequently, the same chemistry teacher with 18 years of professional experience instructed both groups about the topic. The subject was taught to the experimental group in the form of video-based instruction. On the other hand, the traditional “talk-and-chalk” type of method of teacher-centered instruction and the usual 9th grade chemistry textbook (Avcı, 2018) were used in teaching the control group the same subject. After the instruction, both groups were administered an academic achievement test as a posttest. The timeline for the study procedures can be seen in Table 3.| Time | Procedure |
|---|---|
| First lesson | Application of the AAT as a pretest to both groups |
| Second lesson | Instruction to both groups about the topic of “required safety rules to be applied in chemistry laboratories.” |
| Third lesson | Application of the AAT as posttest to both groups |
The topic of the required safety rules to be applied in chemistry laboratories was treated in both groups in October of the fall semester of the 2018–2019 academic year. The time allotted to the teaching of the topic was 1 class hour.
The factors that were considered to minimise the bias on the control between experimental group and control group were given in Appendix 4. As for the elements to control the validity and reliability issues of the study, certain factors were taken into consideration as the following: selection and history of the participants, confounding variables of the research, instrumentation issues, design contamination, testing effect, and matters of morality.
Video-based instruction
The lesson began with the presentation of a video is the experimental group. As they watched, the students studied every event that took place in the video. The teacher took care to call the students’ attention to that was happening in the video and then asked the class for their opinions. Each event was reviewed in class discussions. The teacher explained each safety rule that had been violated in each case. Then the teacher explained the safety rules with a PowerPoint presentation.The lesson content and time frame in the experimental group is provided in Table 4.
| Lesson content | Time |
|---|---|
| 1. Video presentation | 2′ 30′′ |
| 2. Examining each event taking place in the video | 30 min |
| 3. PowerPoint presentation (sum up) | 7′ 30′′ |
| Total | 40 min |
Traditional instruction
The lesson began with introducing the subject by the lecturer. Teacher-centered instruction continued by leaving students to watch the PowerPoint presentation including the content to be learned. At the end, discussion including active participation of the students was incorporated.The lesson content and time frame in the control group is provided in Table 5.
| Lesson content | Time (min) |
|---|---|
| 1. Introducing the subject | 2 |
| 2. PowerPoint presentation (lecturing) | 30 |
| 3. Discussion followed by summing up | 8 |
| Total | 40 |
Data analysis
In this study, an iterative process was used to describe and interpret the data obtained from the students’ written responses to the questionnaire containing 6 open-ended questions. The percentages of the students’ responses for each category were calculated. Later, the findings were presented in terms of frequencies and percentages.The data were collected from the written responses of the control group students and the experimental group students to the open-ended questions. The data gathered from both groups of students were analysed qualitatively with a view to responding to the research question. The responses of each student were carefully studied. All of the student responses were encoded in this study using numbers, with students being represented by letters. The encoding for the control group students was implemented as CS1, CS2, CS3, etc. while that of the experimental group students was in the form of ES1, ES2, ES3, etc. In the pretest, encoding was in the form of ES1-PrT or CS1-PrT, while in the posttest, the encoding was on the basis of ES1-PoT or CS1-PoT. The responses of each student were read separately several times. Similar responses were combined, and general category descriptions were constructed. As a result, in data analysis, each participant was treated as a separate case. Each open-ended question was used to generate a summary of each participant's views. This process was repeated for all open-ended questions. After this initial round of analysis, the summaries were searched for categories. These categories were checked against confirmatory or contradictory evidence in the data and were modified accordingly. Several rounds of category generation, confirmation, and modification were conducted to satisfactorily reduce and organize the data. These categories were employed to generate a profile of the participants’ views.
Limitations of the study
The short duration of the study (a week's education) can be considered as a limitation. Another limitation of the study was that the results cannot be generalized since the sample was restricted to only two classes of the 9th grade in one public high school. In this study, to measure retention of the safety rules a follow-up test after 1 month or more was not administered, but could be suggested to be conducted in the future.Results
This section expounds on the effect of video-based instruction about the required rules of safety in chemistry laboratories on student academic achievement as compared to traditional instruction.There were a rubric associated with each question separately. The rubric included correct and wrong statements categories and the score likely to be gained for the response was over 100 points. The students’ responses including scientifically correct statements with respect to the safety rules were categorized as correct. The students’ responses were categorized as incorrect, in case they confuse the rules, present knowledge gleaned from everyday life and unscientific statements. The scores obtained (by each student) for each question were averaged.
The results of the analysis of the responses given to the 6 open-ended questions in the data collection instrument are presented in Table 6.
| Questions | Control group | Experimental group | ||
|---|---|---|---|---|
Pretest (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ) ) |
Posttest (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ) ) |
Pretest (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ) ) |
Posttest (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ) ) |
|
| 1. General safety rules | 17 | 65 | 20 | 77 |
| 2. Glass materials | 25 | 63 | 22 | 85 |
| 3. Electrical devices | 25 | 70 | 27 | 89 |
| 4. Chemicals | 16 | 65 | 18 | 78 |
| 5. Hot containers | 22 | 68 | 20 | 88 |
| 6. Open flame | 20 | 60 | 15 | 82 |
| AAT Mean | 21 | 65 | 20 | 83 |
As can be seen in Table 6, both groups exhibited an increase in the academic achievement scores for each question following the instruction. In the comparison of the academic achievement posttest mean scores of both groups, it was determined that the academic achievement score of the experimental group was higher than that of the control group (control group: ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) = 65; experimental group:
= 65; experimental group: ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) = 83).
= 83).
To test the improvement in the scores from pre-test to post-test paired-samples t test analysis was performed. For the experimental group the improvement was statistically significant with t(31) = 43.99, p = 0.00. Similarly, in the control group the difference between pre- and post-test mean scores was statistically significant with t(28) = 25.85, p = 0.00.
To determine whether the difference between the posttest mean scores of the groups is significant, statistical analyses were conducted. Significance level for AAT was accepted as 0.05 and the results were evaluated on this basis. The assumptions for the parametric statistical analyses were checked for the sample using the Kolmogorov-Smirnov test and the results showed that the academic achievement scores [z(61) = 0.955, p = 0.321] exhibited normal distribution. Following this, an independent-samples t-test analysis was conducted.
The results of the analysis indicates that there is a statistically significant difference between the posttest mean scores of the experimental and control groups, t(59) = 12.51, p = 0.00. This result suggests that the students in the experimental group scored (M = 83.00, SD = 7.08) significantly higher on the AAT than the students in the control group (M = 65.00, SD = 3.83), meaning they had developed better understanding of the safety rules required to be applied in chemical laboratory.
Examples of the responses of the student groups to the pretest and posttest questions about safety rules are provided in Table 7.
| Questions | Groups | Pretest | Posttest |
|---|---|---|---|
| 1. General safety rules | Control | Performing experiments without being dressed appropriately is absolutely prohibited. | Wearing a smock is required. |
| Experimental | Our outfit must be appropriate for the laboratory. | We must use personal protective clothing and equipment such as a smock, goggles and gloves when we’re working in the laboratory. | |
| 2. Glass materials | Control | Depending upon the chemical, we need to consider the thickness of the glass. | We need to be careful that glass materials don’t break. |
| Experimental | We have to be careful since glass materials can break. | Broken glass materials should not be used. | |
| 3. Electrical devices | Control | Water should not be spilled on electric cables. | We shouldn’t touch anything with wet hands. |
| Experimental | We need to connect electric cables correctly. | We shouldn’t touch electric cables and sockets with wet hands. | |
| 4. Chemicals | Control | We need to combine wastes. | We need to separate chemicals. |
| Experimental | We need to stay away from irritant agents. | Unlabeled chemicals should not be used. | |
| 5. Hot containers | Control | Touching hot containers should be avoided. | We should not pick up hot containers with our naked hands. |
| Experimental | We must keep hot containers away from our bodies. | We need to wear gloves to pick up hot containers. | |
| 6. Open flame | Control | We should not come close to an open flame. | Chemicals should not come in contact with an open flame. |
| Experimental | We should keep away from an open flame. | We should fill up a test tube with only 1/3 of water when heating. |
It can be seen that prior to the instruction, the responses to the pretest of the students in both the control and experimental groups were very far from accurately stating the safety rules. On the other hand, following the instruction, the responses in both groups on the posttest were expressed in a manner that expressed the required safety rules. It was however seen that compared with the control group, the students in the experimental group provided much better worded, more correct/accurate and scientific answers on the posttest.
The distribution of the wrong responses the student groups gave in the posttest, by category, are provided in Table 8.
| Categories | Control group | Experimental group | ||
|---|---|---|---|---|
| f | (%) | f | (%) | |
| f: the number of wrong answers given by students. | ||||
| Confusing the rules | 7 | 16 | 14 | 56 |
| Knowledge gleaned from everyday life | 10 | 23 | 10 | 40 |
| Unscientific answers | 26 | 61 | 1 | 4 |
| Total | 43 | 100 | 25 | 100 |
An examination of the students’ responses shows that the wrong answers of the students in both the control and experimental groups can be divided into 3 categories (Table 8). It was found however that the wrong responses given by the students in the control group on the posttest were more concentrated in the category of unscientific answers (26 out of 43 wrong answers, 61%). On the other hand, the posttest wrong answers of the students in the experimental group were more concentrated in the category of confusing the rules (14 out of 25 wrong answers, 56%).
Examples of the wrong answers of the student groups to the posttest questions about safety rules are provided in Table 9.
| Categories | Groups | Posttest |
|---|---|---|
| Confusing the rules | Control | Things like slippers should not be worn. |
| Experimental | Chemicals should not be sniffed. | |
| Knowledge gleaned from everyday life | Control | Water should not be spilled on electric cables. |
| We should not burn a flame randomly anywhere. | ||
| Experimental | Electrical devices should not be touched with a screwdriver. | |
| Broken glass materials should not be touched. | ||
| Unscientific answers | Control | We need to come to the laboratory with our stomachs full. |
| Experimental | We need to carry hot containers with a cart. |
Discussion
In order to successfully ensure that students grasp the learning objectives of the instruction, scientific content must be presented to the class in an interesting and meaningful manner (Feng and Tuan, 2005). It was toward this aim and to enhance their academic achievement that students were provided in this study with video-based instruction on the subject of required safety rules to be applied in the chemistry laboratory.The study was conducted in order to understand the effect of video-based instruction on the improvement in student's understanding regarding chemistry laboratory safety rules compared to traditional instruction. The scores of both the experimental and control students on the AAT administered as a pretest before the instruction on safety rules in the chemistry laboratory were considerably low. It was also seen that the students’ pretest responses were quite far from expressing the safety rules, meaning that the answers were not very much related to the safety rules at all and students left most of the answers blank. This was because students are first initiated to the safety rules of the laboratory when they are in 9th grade. The topic of laboratory safety instruction is not treated in the science program at the middle school level.
It was observed that after the instruction on laboratory safety rules, the students in both the experimental and control groups displayed an increase in their posttest AAT scores. The increase however was much greater in the experimental group. At the same time, it was seen that compared with the control group, the students in the experimental group provided much better worded, more correct/accurate and scientific answers on the posttest. This outcome indicates that as compared with traditional instruction, video-based instruction on the subject of safety rules to be applied in the chemistry laboratory is more beneficial. In other words, it was observed that video-based instruction has a greater and more positive effect on student's understanding than traditional instruction.
Additionally, video-based instruction freed up more time for active learning, therefore the combination of the active learning component lasting 30 minutes and video-based instruction increased student learning as opposed to 30 minute-long passive lecture and traditional instruction.
The results of our study are consistent with the few studies in the literature that have demonstrated that video-based instruction is successful in teaching the rules of safety in the chemistry laboratory (Matson et al., 2007; Carr and Carr, 2016).
On the other hand, it was seen that the wrong answers of the students in the control group on the posttest mostly fell into the category of unscientific responses. The wrong answers of the students in the experimental group on the posttest mostly fell in the category of confusing the rules. This finding indicates that some students being taught with traditional instruction have difficulty responding in exact scientific terms. It was observed that when there was too much scientific knowledge to be learned, some of the students in the experimental group experienced mental overload and demonstrated confusion in citing the rules. It is understood that video-base instruction is effective in teaching laboratory safety rule but that when the video contains too much information or events, this cause mental overload in some students (Winberg and Berg, 2007), however infrequently this might be.
Traditional instruction about chemistry laboratory safely involves teacher-centered approaches. Lessons on the basic aspects of laboratory safety are taught using the lecturing method and the textbooks. Students in these classes are expected to absorb as many rules as possible. This teacher-centered approach represents a missed opportunity to effectively teach one of the most vital topics in the science of chemistry, the subject of laboratory safety (Carr and Carr, 2016). It is possible after all to prevent or reduce the number of accidents by making use of video-based instruction on laboratory safety.
To conclude, it was determined in this study that the use of video in instruction heightened achievement (Lau, 2020) and enhanced students’ scientific knowledge (Lichter, 2012; Teo et al., 2014; Nadelson et al., 2015). In the light of the positive effect of video-based instruction on learning outcomes (Box et al., 2017), it can be said that this method has the potential of being an effective method of instruction in laboratory safety education.
Implications
The present study offers useful knowledge about video-based instruction in the teaching of chemistry laboratory safety rules. At the same time, the fact that there are very few studies in the literature on video-based instruction points to the significance of this study. When the positive effect of video-based instruction on student's understanding is considered, it can be said that such a teaching approach can make a contribution, however small, to eliminating the learning difficulties (Alaimo et al., 2010; Adane and Abeje, 2012; Karataş, 2016) students have in learning the safety rules to be applied in the chemistry laboratory.Educational information to be transmitted should be designed so that students’ cognitive systems do not have to function on overload. Scientific knowledge is learned in more depth when students are not on overload in terms of their visual and/or auditory memory (Mayer and Moreno, 2002). Also, in designing educational videos, the principles set down in the Cognitive Theory of Multimedia Learning (Mayer, 2001, 2003; Mayer and Moreno, 2002) should be considered.
In summary, video-based instruction appears to be an effective approach to teaching chemistry. Teachers can use this video-based approach in their classes to ensure that students improve their understanding of concepts in chemistry. The results of the study may serve as an encouragement to teachers so that they might use this teaching approach in their classes. At the same time, the video-based instruction used in this study likely provides a useful example for teachers and for teachers’ education. New long-term research (e.g., covering one academic year) conducted in the future may add to the results about the effect of video-based instruction on learning and academic achievement, and the emotional aspects (e.g., motivation, interest) of learning.
Ethical approval
This research project was approved by the Balikesir Directorate of National Education (24-9-18).Conflicts of interest
There are no conflicts to declare.Appendix 1 video images concerning laboratory safety rules
Appendix 2
(1) General safety rules to be applied when working in chemistry laboratoriesRule 1: Learn where to call in case of emergencies.
Rule 2: Do not wear sandals or open footwear.
Rule 3: Never play around.
Rule 4: Do not eat or drink anything.
Rule 5: Work under supervision of your teacher and only do the experiments asked from you.
Rule 6: Wear protective equipment such as a smock, goggles and gloves.
Rule 7: Carefully read the steps necessary for the experiment before the experiment starts.
Rule 8: Mind the information and attention statements regarding safety.
(2) Safety rules to be applied in chemistry laboratories when working with glass materials
Rule 1: Never use broken glass materials.
Rule 2: Never use cheap glass materials.
Rule 3: Always use clean glass materials.
Rule 4: Clean and put back glass materials after use.
(3) Safety rules to be applied in chemistry laboratories when working with electrical devices
Rule 1: Never touch cords, outlets or electrical devices with wet hands.
Rule 2: Do not leave unused electrical devices plugged.
Rule 3: If you notice the electrical device unusually heat up during the experiment, notify your teacher about it.
(4) Safety rules to be applied in chemistry laboratories when working with chemicals
Rule 1: Learn about safety measures and dangers of chemicals to be used.
Rule 2: Never use a chemical that has not been labeled.
Rule 3: Do not put your hands, hair, pencils or any chemicals in your mouth.
Rule 4: Never sniff chemicals.
Rule 5: Do not rub your eyes if they come in contact with chemicals.
Rule 6: Wash the eyes or skin with plenty of water if they come in contact with chemicals.
Rule 7: Do not pour out any solution into the waste basket or sink.
Rule 8: Create a separate container for leftover chemicals.
Rule 9: When you want to dilute acid, add acid to water, do not pour water into acid.
(5) Safety rules to be applied in chemistry laboratories when working with hot containers
Rule 1: Handle with care and use gloves.
Rule 2: Handle the test tube with tongs when heating.
(6) Safety rules to be applied in chemistry laboratories when working with open flame
Rule 1: Do not leave devices such as Bunsen burners or gas burners unattended.
Rule 2: Fill up a test tube with only 1/3 of water when heating.
Appendix 3
Academic Achievement Test (AAT)(1) What are the required general safety rules to be applied when working in chemistry laboratories?
(2) What are the required safety rules to be applied in chemistry laboratories when working with glass materials?
(3) What are the required safety rules to be applied in chemistry laboratories when working with electrical devices?
(4) What are the required safety rules to be applied in chemistry laboratories when working with chemicals?
(5) What are the required safety rules to be applied in chemistry laboratories when working with hot containers?
(6) What are the required safety rules to be applied in chemistry laboratories when working with open flame?
Appendix 4
Validity and reliability of the study
The factors explained below were considered to minimise bias.Selection: The two groups that constituted the study sample were randomly assigned as experimental and control groups. The scores of the experimental and control group students on the High School Entrance Exam were similar. Moreover, the lack of a difference between the pretest results of the two groups was another indication of that similarity. Since only one middle school science curriculum was implemented across the country, the previous science knowledge of the experimental and control groups was expected to be similar.
History: This threat can be defined as the specific events occurring between the first and second measurement in addition to the experimental variable (Campbell and Stanley, 1966). Therefore, over the duration of the study, care was taken to ensure that the students in the control and experimental groups were not made aware of each other.
Confounding variable: The same teacher instructed both the experimental and the control groups on the safety rules to be applied in chemistry laboratories. This teacher had 18 years of experience in teaching chemistry. Additionally, the instructor was knowledgeable and experienced in video-based teaching. The teacher kept class lesson plans on hand in order to avoid any mixup with the type of method to be used with the control and experimental groups.
Instrumentation: This threat is defined as changes in the calibration of a measuring instrument or changes in the observers or scorers used that may produce changes in the obtained measurements (Campbell and Stanley, 1966). To control this threat, the precautions that were taken were as follows: (1) The content of the AAT was developed in line with the instruction given to the experimental and control groups. Expert opinions were obtained in developing this tool and the necessary revisions were made in keeping with the opinions provided. Later, the instrument was subjected to pilot studies. (2) The data were collected with the AAT instrument that had already been tested for validity and reliability to ensure that objective planning could be made with this instrument. (3) Before the administration of the instrument, students both in the experimental and control groups were informed as to how to answer the AAT. (4) In order to enhance the validity and reliability of the data analysis, firstly, the researcher and two external chemistry education researchers cooperatively analyzed seven student papers. Then, fifteen randomly-selected papers out of 61 (around 25% of the sample) were independently analyzed by these three researchers, and the ratings were compared. Inter-rater agreement was found to be 79%, which was considered to be high (Cohen et al., 2007). The researchers sorted out differences by reviewing the students’ responses. The discrepancies were resolved through discussions among the researchers.
Design contamination: In the implementation of the AAT as a pretest and a posttest, the students of the experimental and control groups were given equal and adequate time. The pretest and posttest were each administered to the students over the course of one class hour (40 min). The pretest and the posttest were administered in the classroom environment to both groups independently of each other and during the chemistry class hour. Before the administration of the AAT and, the students of both the experimental and control groups were informed individually about (i) the significance of the research that was being conducted (student guidance) and (ii) were cautioned about not looking at each other's answers, and about the importance of responding without influencing each other when working on the measurement tool. In both groups, the chemistry teacher and the researcher of the study were present during the implementation of the AAT.
Testing effect: Because the pretest-posttest with control group design exposed both groups to a pretest, the test effect could be kept under control (Campbell and Stanley, 1966). To prevent the occurrence of the subject characteristics threat, the students in both groups were informed about the process of testing (i.e., pretest and posttest). It was therefore assumed that the students in both groups were responsive to the treatment in the same way. Additionally, the experimental group students were informed that the content of the topic was the same as the topic taught in the control group. The aim of treating two groups the same except for the administration of the treatment, without disrupting the natural circumstances of the environment or calling attention to the task, is to control the unusual behavior of participants. The threat posed by participant attitudes was thus kept under control.
Mortality: Data obtained from students who had only participated in one of the tests were not taken into the assessment (this pertained to only a total of two students from the control group).
Acknowledgements
This research was supported by Balikesir University and the Balikesir Directorate of National Education.References
- Adane L. and Abeje A., (2012), Assessment of familiarity and understanding of chemical hazard warning signs among university students majoring chemistry and biology: a case study at Jimma University, Southwestern Ethiopia, World Appl. Sci. J., 16(2), 290–299.
- Agustian H. Y. and Seery M. K., (2017), Reasserting the role of pre-laboratory activities in chemistry education: a proposed framework for their design, Chem. Educ. Res. Pract., 18(4), 518–532.
- Akpullukcu S. and Cavas B., (2017), The development of laboratory safety questionnaire for middle school science teachers, Sci. Educ. Int., 28(3), 224–231.
- Alaimo P. J., Langenhan J. M., Tanner M. J. and Ferrenberg S. M., (2010), Safety teams: an approach to engage students in laboratory safety, J. Chem. Educ., 87(8), 856–861.
- Álvarez-Chávez C. R., Ruiz-Talavera R., Muños-Osuna F., Marín Ramírez L., Zavala-Reyna A., Pérez-Ríos R. and Arce-Corrales M., (2016), Laboratory incidents in the University of Sonora: Student's perspective, Proceedings of the 252nd American Chemical Society National Meeting, Philadelphia, PA, USA.
- Álvarez-Chávez C. R., Marín L. S., Perez-Gamez K., Portell M., Velazquez L. and Munoz-Osuna F., (2019), Assessing college students’ risk perceptions of hazards in chemistry laboratories, J. Chem. Educ., 96(10), 2120–2131.
- Avcı B. A., (2018), Ortaöğretim kimya 9. sınıf ders kitabı [High school 9th grade chemistry textbook], Ankara: Sonuç Yayınları.
- Aydoğdu C. and Yardımcı E., (2013). Accidents occured in elementary science laboratories and teachers’ behaviour manners toward these accidents, Hacettepe Univ. J. Educ., 44, 52–60.
- Baddeley A., (1992), Working memory, Science, 255, 556–559.
- Box M. C., Dunnagan C. L., Hirsh L. A. S., Cherry C. R., Christianson K. A., Gibson R. J., Wolfe M. I. and Gallardo-Williams M. T., (2017), Qualitative and quantitative evaluation of three types of student-generated videos as instructional support in organic chemistry laboratories, J. Chem. Educ., 94(2), 164–170.
- Brame C. J., (2016), Effective educational videos: principles and guidelines for maximizing student learning from video content, CBE – Life Sci. Educ., 15(4), 1–6.
- Burewicz A. and Miranowicz N., (2006), Effectiveness of multimedia laboratory instruction, Chem. Educ. Res. Pract., 7(1), 1–12.
- Campbell D. T. and Stanley J. C., (1966), Experimental and quasi-experimental designs for research, Boston: Houghton Mifflin Company.
- Carr J. M. and Carr J. M., (2016), What can students learn about lab safety from Mr Bean? J. Coll. Sci. Teach., 45(6), 32–35.
- Chandler P. and Sweller J., (1991), Cognitive load theory and the format of instruction, Cognit. Instr., 8, 293–332.
- Chen J. and Cowie B., (2014), Scientists talking to students through videos. Int. J. Sci. Math. Educ., 12(2), 445–465.
- Cohen L., Manion L. and Morrison K., (2007), Research methods in education, 6th edn, New York: Routledge.
- CSB [Chemical Safety Board], (2011), “Experimenting with danger” focuses on CSB case study on Texas Tech university accident, Washington, DC, USA: Laboratory deaths at UCLA and Dartmouth, Chemical Safety Board.
- Dorfman B.-S., Terrill B., Patterson K., Yarden A. and Blonder R., (2019), Teachers personalize videos and animations of biochemical processes: results from a professional development workshop, Chem. Educ. Res. Pract., 20(4), 772–786.
- Escalada L. T. and Zollman D. A., (1997), An investigation on the effects of using interactive digital video in a physics classroom on student learning and attitudes, J. Res. Sci. Teach., 34(5), 467–489.
- Feng S.-L. and Tuan H.-L., (2005), Using ARCS model to promote 11th graders’ motivation and achievement in learning about acids and bases, Int. J. Sci. Math. Educ., 3(3), 463–484.
- Gublo K. I., (2003), A laboratory safety trivia game, J. Chem. Educ., 80(4), 425.
- Harwood W. S. and McMahon M. M., (1997), Effects of integrated video media on student achievement and attitudes in high school chemistry, J. Res. Sci. Teach., 34(6), 617–631.
- Helser T. L., (1999a), A lab safety “scavenger hunt”. J. Chem. Educ., 76(1), 68.
- Helser T. L., (1999b), Safety wordsearch, J. Chem. Educ., 76(4), 495.
- Hill P. S. and Greco T. G., (1995), Safety is no laughing matter, J. Chem. Educ., 72(12), 1126–1127.
- Hill R. H., (2016), Undergraduates need a safety education! J. Chem. Educ., 93(9), 1495–1498.
- Hill D. J., Williams O. F., Mizzy D. P., Triumph T. F., Brennan C. R., Mason D. C. and Lawrence D. D., (2019), Introduction to laboratory safety for graduate students: an active- learning endeavor, J. Chem. Educ., 96(4), 652–659.
- Huston E. M., Milligan J. A., Powell J. R., Smith A. M., Neal D., Duval K. M., DiNardo M. A., Stoddard C., Bell P. A., Berning A. W., Wipf P. and Bandik G. C., (2018), Development of an undergraduate course in chemical laboratory safety through an academic/industrial collaboration, J. Chem. Educ., 95(4), 577–583.
- Jordan J. T., Box M. C., Eguren K. E., Parker T. A., Saraldi-Gallardo V. M., Wolfe M. I. and Gallardo-Williams M. T., (2016), Effectiveness of student-generated video as a teaching tool for an instrumental technique in the organic chemistry laboratory, J. Chem. Educ., 93(1), 141–145.
- Karataş F. Ö., (2016), Pre-service chemistry teachers’ competencies in the laboratory: a cross-grade study in solution preparation, Chem. Educ. Res. Pract., 17(1), 100–110.
- Kulgemeyer C., (2018), A framework of effective science explanation videos informed by criteria for instructional explanations, Res. Sci. Educ. DOI:10.1007/s11165-018-9787-7.
- Kumar D. D., (2010), Approaches to interactive video anchors in problem-based science learning, J. Sci. Educ. Technol., 19(1), 13–19.
- Kumasaki M., Shoji T., Wu T.-C., Soontarapa K., Arai M., Mizutani T., Okada K., Shimizu Y. and Sugano Y., (2018), Presenting safety topics using a graphic novel, Manga, to effectively teach chemical safety to students in Japan, Taiwan, and Thailand, J. Chem. Educ., 95(4), 584–592.
- Lau P. N., (2020), Enhancing formative and self-assessment with video playback to improve critique skills in a titration laboratory, Chem. Educ. Res. Pract., 21(1), 178–188.
- Lichter J., (2012), Using YouTube as a platform for teaching and learning solubility rules, J. Chem. Educ., 89(9), 1133–1137.
- Matson M. L., Fitzgerald J. P. and Lin S., (2007), Creating customized, relevant, and engaging laboratory safety videos, J. Chem. Educ., 84(10), 1727–1728.
- Mayer R. E., (2001), Multimedia learning, Cambridge, UK: Cambridge University Press.
- Mayer R. E., (2003), The promise of multimedia learning: using the same instructional design methods across different media, Lear. Instr., 13(2), 125–139.
- Mayer R. E. and Moreno R., (2002), Aids to computer-based multimedia learning, Lear. Instr., 12(1), 107–119.
- Mayo A., Sharma M. D. and Muller D. A., (2009), Qualitative differences between learning environments using video in small groups and whole class discussions: a preliminary study in physics, Res. Sci. Educ., 39(4), 477–493.
- McGarry K. A., Hurley K. R., Volp K. A., Hill I. M., Merritt B. A., Peterson K. L., Rudd P. A., Erickson N. C., Seiler L. A., Gupta P., Bates F. S. and Tolman W. B., (2013), Student involvement in improving the culture of safety in academic laboratories, J. Chem. Educ., 90(11), 1414–1417.
- MEB [Ministry of National Education], (2018), Ortaöğretim kimya dersi öğretim programı [High school chemistry curriculum], Ankara: MEB.
- Moody A. E and Freeman R. G., (1999), Chemical safety and scientific ethics in a sophomore chemistry seminar, J. Chem. Educ., 76(9), 1224–1225.
- Nadelson L. S., Scaggs J., Sheffield C. and McDougal O. M., (2015), Integration of video-based demonstrations to prepare students for the organic chemistry laboratory, J. Sci. Educ. Technol., 24(4), 476–483.
- NRC [National Research Council], (2011), Prudent practices in the laboratory: Handling and management of chemical hazards, Washington, DC: The National Academies Press.
- Pekdağ B., (2010), Kimya öğreniminde alternatif yollar: Animasyon, simülasyon, video ve multimedya ile öğrenme [Alternative methods in learning chemistry: Learning with animation, simulation, video and multimedia]. Türk Fen Eğitimi Dergisi [J. Turk. Sci. Educ.], 7(2), 79–110.
- Pekdağ B., (2017), Deney videoları ile kimya öğretimi [Chemistry teaching with experiment videos], in Ayas A. and Sözbilir M. (ed.), Kimya öğretimi [Chemistry Teaching], 2nd edn, Ankara: Pegem Akademi, pp. 591–616.
- Pekdağ B. and Le Maréchal J.-F., (2007a), Bilimsel filmlerin hazırlanması [Preparation of scientific movies], Necatibey Eğitim Fakültesi Elektronik Fen ve Matematik Eğitimi Dergisi [Necatibey Fac. Educ., Elect. J. Sci. Math. Educ.], 1(1), 57–84.
- Pekdağ B. and Le Maréchal J.-F., (2007b), Memorisation of information from scientific movies, in Pintó R. and Couso D. (ed.), Contributions from science education research, Dordrecht: Springer, pp. 199–210.
- Pekdağ B. and Le Maréchal J.-F., (2010), Movies in chemistry education, Asia-Pac. Forum Sci. Learn. Teach., 11(1), 1–19.
- Schenk L., Taher I. A. and Öberg M., (2018), Identifying the scope of safety issues and challenges to safety management in Swedish middle school and high school chemistry education, J. Chem. Educ., 95(7), 1132–1139.
- Schmidt-McCormack J. A., Muniz M. N., Keuter E. C., Shaw S. K. and Cole R. S., (2017), Design and implementation of instructional videos for upper-division undergraduate laboratory courses, Chem. Educ. Res. Pract., 18(4), 749–762.
- Schwan S. and Riempp R., (2004), The cognitive benefits of interactive videos: learning to tie nautical knots, Lear. Instr., 14(3), 293–305.
- Simmons H. E., Matos B. and Simpson S. A., (2017), Analysis of injury data to improve safety and training, J. Chem. Health Saf., 24(1), 21–28.
- Slabaugh W. H. and Hatch C. V., (1958), General chemistry via television, J. Chem. Educ., 35, 95–96.
- Staehle I. O., Chung T. S., Stopin A., Vadehra G. S., Hsieh S. I., Gibson J. H. and Garcia-Garibay M. A., (2016), An approach to enhance the safety culture of an academic chemistry research laboratory by addressing behavioral factors, J. Chem. Educ., 93(2), 217–222.
- Stuart R. and Toreki R., (2014), Learning opportunities in three years of hazmat headlines, J. Chem. Health Saf., 21(2), 2–8.
- Su T.-S. and Hsu I.-Y., (2008), Perception towards chemical labeling for college students in Taiwan using globally harmonized system, Saf. Sci., 46, 1385–1392.
- Sweller J., (1988), Cognitive load during problem solving: effects on learning, Cognit. Sci., 12(2), 257–285.
- Teo T. W., Tan K. C. D., Yan Y. K., Teo Y. C. and Yeo L. W., (2014), How flip teaching supports undergraduate chemistry laboratory learning, Chem. Educ. Res. Pract., 15(4), 550–567.
- Tsokou A. M., Howells A. and Stark M. S., (2019), Measuring and reducing chemical spills by students: a randomized controlled trial of providing feedback, J. Chem. Educ., 96(10), 2180–2187.
- Velázquez-Marcano A., Williamson V. M., Ashkenazi G., Tasker R. and Williamson K. C., (2004), The use of video demonstrations and particulate animation in general chemistry, J. Sci. Educ. Technol., 13(3), 315–323.
- Walters A. U. C., Lawrence W. and Jalsa N. K., (2017), Chemical laboratory safety awareness, attitudes and pratices of tertiary students, Saf. Sci., 96, 161–171.
- Wedlich R. C., Pires A., Fazzino L. and Fransen J. M., (2013), Good laboratory practice. Part 3. Implementing good laboratory practice in the analytical lab, J. Chem. Educ., 90(7), 862–865.
- Winberg T. M. and Berg C. A. R., (2007), Students’ cognitive focus during a chemistry laboratory exercise: effects of a computer-simulated prelab, J. Res. Sci. Teach., 44(8), 1108–1133.
- Wright S. M., (2005), Introducing safety topics using a student-centered approach, J. Chem. Educ., 82(10), 1519–1520.
- Zahn C., Barquero B. and Schwan S., (2004), Learning with hyperlinked videos – design criteria and efficient strategies for using audiovisual hypermedia, Lear. Instr., 14(3), 275–291.
| This journal is © The Royal Society of Chemistry 2020 |