The intellectual demands of the intended chemistry curriculum in Czechia, Finland, and Turkey: a comparative analysis based on the revised Bloom's taxonomy†
Rıdvan
Elmas
 ab,
Martin
Rusek
ab,
Martin
Rusek
 *a,
Anssi
Lindell
*a,
Anssi
Lindell
 c,
Pasi
Nieminen
c,
Pasi
Nieminen
 c,
Koray
Kasapoğlu
c,
Koray
Kasapoğlu
 b and
Martin
Bílek
b and
Martin
Bílek
 a
a
aCharles University, Faculty of Education, Magdalény Rettigové 4, 116 39 Praha 1, Czech Republic. E-mail: martin.rusek@pedf.cuni.cz
bAfyon Kocatepe University, Faculty of Education, ANS Kampüsü, Gazlıgöl Yolu, 03200 AFYONKARAHİSAR, Turkey
cDepartment of Teacher Education, 40014 University of Jyväskylä, Jyväskylä, Finland
First published on 8th April 2020
Abstract
Understanding the intellectual demands of an intended curriculum is crucial as it defines the frames for teaching and learning processes and practice during lessons. In this study, upper-secondary school chemistry curricula contents in Czechia, Finland, and Turkey were analysed, and their objectives were compared using the Revised Bloom's Taxonomy (RBT). The intellectual demands were examined analysing the action verbs in the three curricula objectives based on their association with the intended cognitive process dimensions in the RBT. The Turkish upper-secondary chemistry curriculum was found to be more structured, detailed, and containing more objectives than the Czech and Finnish curricula. The domineering objectives in cognitive demands were understand (77.2%) and analyse and apply (both 7.1%). Conceptual items dominated (59.8%) with procedural items identified (29.1%). Also, there are five metacognitive items (3.9%). The Czech curriculum, compared to the Finnish and Turkish curricula, does not take modern trends in the field of chemistry into account. The cognitive demands in the Czech curriculum were skewed toward apply (40%) with understand and evaluate accordingly represented by 20%. Conceptual items dominate with a 53.3% of occurrence. In the Finnish curriculum, the cognitive demands were skewed toward apply (47.1%) with create (23.5%) and understand (17.6%). Procedural (35.3%) domains predominate, although metacognitive objectives represent a significant share (23.5%) too. These findings from the contents and intellectual demands of the curricula in each of the three countries have the potential to help teachers and other actors in education design the interventions and assessments implemented in the classes. Comparing the distribution of intellectual demands between the countries provides an international reference for educational reforms in hand in many countries.
Introduction
Curriculum documents present a rationale, general guidelines, and a plan about goals, values, learning objectives, skills, content, teaching methods, and assessment as envisioned by curriculum developers. The intended (written) curriculum is always the first to be blamed when the educational outcomes do not meet the needs of society. The inconsonance derives from the fact that curriculum reform processes are compromises among political, social, and economic ambitions (Elmas et al., 2014; van den Akker, 2013). Additionally, most curriculum reforms are not based on precise research evidence or school system outcomes (Schildkamp and Kuiper, 2010; van den Akker, 2013). Monitoring the three main phases of the curriculum and progress in each step is a critical outcome for any curriculum reform. These three crucial phases are intended, implemented, and attained curricula (cf.Thijs and van den Akker, 2009). There are alternative theoretical frameworks that help us understand how curriculum reforms are understood and implemented via a multi-level perspective, such as variation theory (Bussey et al., 2013). Despite the variety of frameworks, researchers prefer to use the general theoretical structure of the three curriculum levels. The intended curriculum represents the ideal underlying vision and philosophy; the implemented curriculum refers to how teachers perceive and apply the guidelines to classroom instruction, and the attained curriculum represents the learners’ experience, i.e. outcomes of the curriculum. Understanding the intellectual demands of an intended curriculum is critical as it defines the guidelines for the other dependent levels of curricula. For example, an intellectual demand of understanding a concept requests another kind of learning process than analysing a procedure. Thus, understanding the intellectual demands helps to align the teaching and learning processes with the intended curriculum's objectives (Lee et al., 2016). In this sense, the outcomes of this kind of study are significant for curriculum developers, ministries, textbook authors, teachers, and almost all stakeholders in the educational system. Curriculum developers may use the findings of this research when assessing the present and future national intended curricula against those published in other countries. Textbook authors and other learning material designers and teachers can use these findings as evidence to set the objectives of their materials and interventions, both for knowledge and cognitive processes. Additionally, teachers might benefit from the findings of this research when evaluating and redesigning their instruction and assessment to align with the intended curriculum. If they are aware of the level of the objectives’ cognitive process, they can specify and adapt their instructional methods. For instance, the objective “A student is able to prepare solutions at different concentrations” should require hands-on experience in the laboratory rather than expository teaching. Besides, this level of objective holds for authentic assessment (e.g., a lesson observation rubric) rather than conventional (paper–pencil) tests.There are few methodologically sound studies focusing on the intellectual demands of chemistry or science curricula (Lee et al., 2016; Lee et al., 2015; Wei and Ou, 2018; Zorluoglu et al., 2016). To consider intellectual demands, the researchers focused on the cognitive processes and knowledge demands. The fundamentals of determining the cognitive and knowledge demands lie in the curriculum objectives. The type of knowledge the objectives are comprised of, is referred to as the knowledge dimension. The level of cognitive process the objectives are in is referred to as the cognitive dimension.
In this study, the curricula of the three countries were analysed. They were selected based on different aspects, as identified by a study in which factors affecting the study of chemistry were analysed (Blonder and Mamlok-Naaman, 2019). According to the chemistry lessons (periods) per week at secondary school, Czechia and Serbia stand out as countries with the lowest number of lessons (p. 627). As far as integration of science and mathematical disciplines is concerned (p. 627), only in Portugal and Turkey are they reported as being taught in an integrating manner. In Finland and Turkey, only one year of chemistry is compulsory, whereas in Estonia, Czechia, Sweden, and Slovakia, it is three years. Another factor taken into account in the cited study was the chemistry teachers’ salary (p. 628). Whereas Czechia and Slovakia reported the lowest average salary, the Netherlands, Finland, and Sweden reported the highest teacher salaries for the compared countries. There are three countries which stand out in the observed categories: Czechia, Finland, and Turkey. Moreover, students from these countries perform differently in international exams such as PISA. Finland is ranked top in these exams in terms of scientific literacy, Czechia average, and Turkey below average depending on the exam years (Organization for Economic Cooperation and Development, 2019). The reasons that these trends affect 15-year-olds’ performance in the test might be the factors outside those mentioned above or these countries’ elementary science curricula (see Wei and Ou, 2018). Comparing the intellectual demands of these three curricula might provide us with interesting information about how they vary, how expectations from chemistry teachers differ, and if the variation correlates with the three countries’ PISA ranking. For example, the chemistry curriculum is supposed to be followed loyally in Turkey, and there is no flexibility on the subject matter because of the university entrance examination's coverage (Elmas et al., 2014). In Czechia, the upper-secondary chemistry curriculum at the national level represents a general framework presenting compulsory expected student learning outcomes as well as compulsory subject matter which needs to be included. This framework is then adopted by each school individually and stands as a model for a school curriculum document. It needs to respect the extent of the subject matter given by the national curriculum but can extend it in a flexible way. In Finland, similar to Czechia, there is a national core curriculum to strengthen educational equality in the country. The chemistry core curriculum defines the rationale, outcomes, content and principles of learning, and assessment. This core curriculum gives a framework to design more specific local school curricula and annual plans. These are to take into account local special features, such as timing, resources and traditions. Each student designs his or her personal study plan based on the local curriculum (Finnish National Agency of Education, 2016, p. 9). The Finnish National Agency of Education defines the local curricula as “living and flexible support for teaching and school activities.”
Analysing intellectual demands with the use of taxonomies
The aim of analysing the curriculum's intellectual demands is to map out what levels of learning are expected in a certain discipline and grade. For this purpose, several taxonomies can be used as a framework: The Bloom's (Bloom et al., 1956), Klopfer's (1971), the revised Bloom's taxonomy (Anderson et al., 2001), Marzano and Kendall's (2006), Smith's (based on Bloom's, adapted for mathematics) (Smith et al., 1996), the Structure of the Observed Learning Outcome (SOLO) taxonomy (Biggs, 1995) or the Porter's taxonomy (Porter, 2002). Quite a few studies use these taxonomies as a framework in several disciplines (DeMers, 2009; Domin, 1999; Hanna, 2007; Ritchotte and Zaghlawan, 2019; Toledo and Dubas, 2015). Their use can bring benefits to interpret a curriculum (Bloom, 1994; Krathwohl, 2002; Näsström, 2009). The taxonomies can be helpful for several reasons, such as:1. They display the panoramic view of the curriculum's intellectual capacity, which gives educators the advantage of making comparisons between different sets of standards/questions/activities/objectives.
2. They make the objectives more understandable for all stakeholders, providing them with a baseline to refer to educational problems with more precision.
3. Educators can have a chance to follow the curricular trends in one or more disciplines.
4. Taxonomies can help educators compare the alignment of standards/questions/activities/objectives with the mission and vision of the educational system.
Bloom's taxonomy and the revised Bloom's taxonomy (RBT) are the most well-known and cited (cf.Krathwohl, 2002; Näsström, 2009; Toledo and Dubas, 2015). This could be due to the pioneering role of B. S. Bloom in taxonomy development. Besides, the revised Bloom's taxonomy (RBT) has an extensive range of use in education and is generally understood. There are examples of research focusing on curriculum objectives (Wei and Ou, 2018; Zorluoglu et al., 2016; Lee et al., 2015; Näsström, 2009), higher-level questions with twice-exceptional children (Ritchotte and Zaghlawan, 2019), physics exam questions (Motlhabane, 2017) or alignment of standards and assessment (Näsström and Henriksson, 2008) with use of the RBT.
The RBT has several advantages over the original Bloom's taxonomy. These come from its two-dimensional structure. The RBT has two dimensions: knowledge and cognitive processes. The knowledge dimension is comprised of four major categories and 11 subcategories (Anderson et al., 2001). The primary knowledge dimension categories are factual, conceptual, procedural, and metacognitive knowledge. Factual knowledge is the essential element or piece of knowledge vocabulary in any discipline. Conceptual knowledge is about concepts and their larger relational web structure compared to others. Procedural knowledge stands for how to do something following a set of rules, and metacognitive knowledge is about self-realization and awareness of and reflection upon one's cognitive process. The cognitive process dimension has six primary categories and 19 subcategories (Anderson et al., 2001). The primary cognitive process dimension categories are remember, understand, apply, analyse, evaluate, and create. These major dimensional categories are directly related to their verb form meaning. For instance, the evaluate dimension is used to categorize the standards, questions, activities, or objectives based on their inclusion in any judgment based on a set of rules or criteria. The main structure of the cognitive process dimension is based on a hierarchical model (Bloom et al., 1956). For example, the understand category needs to be mastered as a prerequisite before one can apply.
The original purpose of Bloom's taxonomy was to classify test items for higher education (Bloom et al., 1956), however many educators, especially in lower grades, devalue basic skills based on the taxonomy (Booker, 2007). There is a risk that learning these reverts in the higher grades before the students can use them in a full capacity for higher-level categories. There are some critics of Bloom's taxonomy (e.g., Furst, 1981; Marzano and Kendall, 2006; Ormell, 1974) and the RBT (Booker, 2007; Marzano and Kendall, 2006) because of their similar structure. Some researchers developed new taxonomies encompassing the RBT (Marzano and Kendall, 2006). Despite the critics, some studies concluded that RBT is a beneficial tool to interpret standards, questions, activities, or objectives (Näsström, 2009). Amer (2006) reported the RBT's potential areas of use as analysing objectives, helping teachers gain more understanding between objectives and activities, supporting teachers to understand the importance of aligning the teaching/learning and assessment process, and examining curriculum alignment.
To analyse the intellectual demands of the chemistry curricula, the authors of this paper chose objectives as a unit of analysis because they are the most specific content parts valuable to all stakeholders (Amer, 2006; Porter, 2002). Also, any other part of the curriculum makes sense only when linked to the objectives. The RBT was chosen as a framework to categorize the objectives for several reasons (cf.Anderson et al., 2001; Bloom et al., 1956): first, there is a common understanding of the RBT and its categories (cognitive and knowledge dimensions); therefore, it is convenient to conduct a curriculum comparison study on its solid and established structure. Second, the RBT is generally used to categorize objectives, standards, questions, and such to align curriculum components. Third, the use of the RBT and action verbs allows researchers to interpret the intended objectives for teachers’ practice and students’ performance. Fourth, educational stakeholders can see the reciprocal perspectives between cognitive and knowledge dimensions embedded in objectives. Fifth, the RBT presents clear principles about the transitional nature of teaching from objectives to assessment. Finally, the RBT is helpful for all stakeholders to make better sense of many concepts and ideas in the curriculum and the learning process.
The highlights of chemistry curriculum development in Czechia
The upper-secondary chemistry curriculum in Czechia is connected with a long tradition and, similar to other parts of the learning content, was changed for the last time after the “Velvet Revolution“ in former Czechoslovakia in 1989, at a time of society transforming from a totalitarian regime to democracy (Čtrnáctová and Zajíček, 2010). In the 90s, a large number of new upper-secondary schools were established, not only 4-year (grade 10–13) as an example of upper-secondary school, but also 6-year (grade 8–13) and 8-year (grade 6–13) grammar schools affecting the whole secondary education level. Chemistry education at the upper-secondary level before 1989 was governed by a centrally valid and binding curriculum with a traditional structure: general chemistry–inorganic chemistry–organic chemistry–biochemistry. The school subject chemistry covered three years of study at 2–3 lessons each week. From the 90s, the upper-secondary school chemistry curriculum was only slightly modified, both content- and organization-wise; however, the concept runs unchanged. The current situation started in 2007, when the so-called two-level curricular system: Framework Educational Programme (FEP) was established as a guideline for schools and School Educational Programme (SEP) as its product for upper-secondary schools. In the FEP (Rámcový vzdělávací program pro gymnasia, 2007), chemistry is a part of the educational field including man and nature together with physics, biology, geography, and geology. In the educational field, the school can decide on teaching subjects and other activities as projects, integrated topics, etc.Čtrnáctová and Zajíček (2010) evaluated that the criteria for success in this system are based on only very variable recruitment procedures from universities. It means that teachers try to make students understand a large number of terms/facts mentioned in textbooks and which teachers consider a given standard, without giving enough time and space to learn the concepts, making connections between them and applying them. The concepts are mostly only theoretically introduced, and often their practical experimental verification is completely absent. It causes negative output in the form of low interest for further chemistry study. The Fundamentals of this state are, however, set by only minor changes in the national curricula since 1989 (Vojíř and Rusek, 2020).
The highlights of chemistry curriculum development in Finland
In Finland, the government decided the school curricula until the mid-’80s, but nowadays The Finnish National Agency of Education determines the national core curricula. The development process engages experts in different areas: school administration, research, teacher education, and teaching practice. To ensure equity and democracy, citizens can also make comments on the draft curriculum. The national upper-secondary core curriculum in Finland was last reformed in 2019, but this document will not be valid until 2021. The present National Core Curriculum for General Upper Secondary Schools 2015 (grade 10–12, Finnish National Agency of Education, 2016), which was analysed here, was put into effect on 1 August 2016. It includes the objectives and contents for cross-curricular themes and for separate school subjects. It also suggests teaching processes in general, such as active and meaningful inquiry-based learning in diverse environments, also including digital technology. The document addresses the mission and values of upper-secondary education, implementation, guidance, and assessment to foster equality and equity in education in Finland. At the local level, the national core curricula just frame the design of the local curricula, which direct teaching and schoolwork in more detail, aiming to consider the needs, resources, and perspectives at local setups.Chemistry has been a distinct school subject in Finland since 1918, but it was only qualified in the upper-secondary school curriculum in 1941. This curriculum was valid for almost 30 years, and it was only officially reformed in 1970. The wide learning objective in 1941 was to “understand key theories and laws” (Vaskuri, 2017). In the curriculum for upper-secondary school 1970, the objectives for learning chemistry already included attitudes (interest in phenomena and laws in chemistry), skills (apply concepts and laws of chemistry in discrete cases) and knowledge (i.e., knowing elements and their most important compounds) (Vaskuri, 2017). The first national core curriculum for upper-secondary high schools in 1985 already included a cross-cutting objective to “realize general principles of science by special nature of chemistry” and chemistry in society: “give a versatile picture of achievements of chemistry in different areas of life” (Vaskuri, 2017). In the following national upper secondary core curriculum 1994, the objectives were expressed very generally, which caused big problems for teachers to implement, and thus, the national core curriculum in 2003 included a clear list of the separate courses’ key contents (Vaskuri, 2017). The present Finnish National Core Curriculum for General Upper Secondary Schools 2015 (grade 10–12, Finnish National Agency of Education, 2016) describes learning objectives for five chemistry courses. Chemistry all around is the only “must” course for every student with 3 cognitive domain objectives. The four optional courses are the chemistry of man and of the living environment (4 objectives), reactions and energy (3 objectives), materials and technology (4 objectives) and reactions and equilibrium (3 objectives).
The highlights of chemistry curriculum development in Turkey
The upper-secondary curriculum is developed and disseminated by the Ministry of Education as are all other curricula. In 2013, the chemistry curriculum (9–12th grades) was updated by simplifying its objectives and content based on major criticism of content overload. The objectives and content of the chemistry curriculum were divided into two levels: the basic level (9–10th grades) and the advanced level (11–12th grades). The chemistry curriculum was revised and renewed in 2018 as well, again due to major criticism on its content load (Ministry of National Education, 2018). In this context, in line with the suggestions and demands from chemistry teachers and different stakeholders, some concepts and issues have been removed from the curriculum, and it has been simplified. In addition, the emphasis on using information and communication technologies in chemistry teaching and the association of objectives restructured to reflect higher-order thinking skills and daily life has been increased (Ministry of National Education, 2018). The chemistry curriculum from 2018 has been developed based on a thematic approach. Its content consists of five units (with 38 objectives) at the 9th grade level, four units (with 23 objectives) at the 10th grade level, six units (with 35 objectives) at the 11th grade level, and four (with 31 objectives) at the 12th grade level (Ministry of National Education, 2018).Significance and research questions
There are several programmes, which focus on output for assessing educational systems, i.e., students’ knowledge and skills (PISA, TIMSS, etc.). However, they do not provide enough information about the reasons behind the students’ performance. The role of institutionalized education is only one of the factors, yet it is reasonable to search for explanations between different groups of students’ results within the differences in instruction. Solving this problem requires analysing the implemented curriculum (i.e., lesson observations) and combining them with the attained curriculum in the form of the (PISA, TIMSS, etc.) test results. Under the premise that in-classroom practice follows the school curriculum, it can be analysed with a certain level of abstraction. Even more, the national (intended) curricula on top of the curricular document pyramid can be analysed, as they prescribe what the school subordinate curricula need to contain.The significance of this research comes from its multinational perspective and the use of a well-proven taxonomy – RBT – as a framework for chemistry curriculum objective analysis. There are two studies analysing different countries’ or regions’ science curricula. However, they focus on top-performing countries in multinational exams (Lee et al., 2016; Lee et al., 2015; Wei and Ou, 2018). The present study targets three countries from various score levels in these multinational exams. So far, a general comparison of Turkey and Finland has been made for their previous chemistry curricula (Er and Atici, 2016). Nevertheless, the present study aims to analyse the current curricula of three countries. Including the Czech context adds to the study's value for the reasons mentioned earlier. The intellectual demands documented in the intended curriculum define the teaching processes, and learning experiences perceived by learners. Actual, implemented, and attained curricula vary among schools, teachers, and individual learners. Comparing these needs would require a large pool of data from several different schools or alternatively comparing some more general national documents. This is beyond the scope of this study. In addition, this work cannot be done without prior knowledge of the intended curricular setting. The analyses of the intended curricula provide an overview of the similarities and differences in the visions and intentions of chemistry education in the three countries and, as such, inform us of the process of teaching chemistry. Therefore, the following research questions were formulated: (1) what are the general features of the analysed curricula in Czechia, Finland, and Turkey? (2) What are the intellectual demands of the intended secondary school chemistry curriculum in Czechia, Finland, and Turkey? (3) What are the differences in the intellectual demands of the intended secondary school chemistry curriculum in Czechia, Finland, and Turkey?
Methodology
The official versions of the Czech, Finnish, and Turkish upper-secondary school (ISCED 3) core curricula were subjected to routine documentary analysis. Both dimensions (cognitive processes and knowledge) of the RBT were used as the coding frame. Coders first located an action verb and noun in each objective then they classified the action verb into one of the six cognitive process dimension categories. Then they placed the noun of each objective into one of the four knowledge dimension categories. After determining both levels, coders put the relevant objective into the intersecting cell in the taxonomy matrix table.For example, in the objective “Student characterizes basic metabolic processes” (Czech curriculum), the verb characterizes suggests understanding. Characterization was then coded as a conceptual dimension. Some of the curriculum objectives contained two command verbs (e.g. “Student knows how to use and apply…” in the Finnish curriculum). In this case, this objective was coded with the higher-level verb, as advised by the revised Bloom's taxonomy (Anderson et al., 2001). For examples, see Table 1.
| Knowledge dimension | Cognitive dimension | |||||
|---|---|---|---|---|---|---|
| Remember | Understand | Apply | Analyse | Evaluate | Create | |
| Factual knowledge | Lists alternative energy sources (TR) | Classifies the elements according to their place in the periodic system (TR) | ||||
| Conceptual knowledge | Names ionic-bonded compounds systematically (TR) | Explains the process of chemistry becoming science (TR) | Uses scientific terminology to describe matter and describe chemical phenomena (CZ) | Explains the importance of sustainable life and development for society and environment by associating with chemistry (TR) | Characterizes basic metabolic processes and their significance (CZ) | |
| Procedural knowledge | Understands how chemical knowledge is built through experimentation and related modelling (FI) | Calculates the reaction enthalpy through standard formation enthalpy (TR) | Estimates properties of elements and their behaviour in chemical processes based on findings about periodic table of elements (CZ) | Is able to study different chemical phenomena experimentally and using different models as well as take into account occupational safety aspects (FI) | ||
| Metacognitive knowledge | Evaluates the developments in nanotechnology in terms of its effects on science, society, technology, environment, and economy (TR) | Is able to inquire phenomena relating to organic compounds, the amount of substance, and concentration through experimentation and by using different models (FI) | ||||
This paper has six authors, two from each country whose curriculum objectives were analysed. At the beginning, the first, second, third, and sixth authors met in person several times to plan the procedure and ensure they had a shared understanding of the RBT, and the consistency of the codes was uniform. Two researchers first independently analysed their national curriculum. A third coder then analysed approximately 20% of the objectives. The third coder's opinion on the disputable objectives served to support the original coders’ when they revisited their coding and managed to reach a conclusion by discussion. This was the case in six objectives in the Finnish curriculum, 14 in the Czech curriculum and 16 in the Turkish curriculum. For this purpose, the official English translation of the Finnish and Czech curriculum was used; the Turkish curriculum was translated by the members of the research team as there is no official English translation of the curricula. Special attention was paid to the verbs during the translations. After that, the original two researchers discussed the particular objectives again to reach a consensus. The Kohen's kappa values and percentage agreement for the original independent within-country inter-rater agreements are shown in Table 2.
| Within-country inter-rater kappa values | Within-country inter-rater agreement (%) | |||
|---|---|---|---|---|
| Cognitive process dimensions | Knowledge dimensions | Cognitive process dimensions | Knowledge dimensions | |
| Czechia | 0.68 (substantial agreement) | 0.78 (substantial agreement) | 80 | 86.67 |
| Finland | 0.58 (moderate agreement) | 0.77 (substantial agreement) | 68 | 85 |
| Turkey | 0.94 (almost perfect agreement) | 0.90 (almost perfect agreement) | 97.64 | 94.49 |
Findings
General features of upper-secondary school chemistry curricula in Czechia, Finland, and Turkey
The Turkish upper-secondary chemistry curriculum is more structured and detailed than the Czech and Finnish curricula. These two curricula contain more generally formulated objectives in the form of outcomes, which are further made more concrete within the subject matter. The Czech and Finnish chemistry curricula are organized into thematic units (e.g., general chemistry, inorganic chemistry – Czech or chemistry all around, the chemistry of man and the living environment – Finnish), whereas the Turkish chemistry curriculum is divided according to school years. Categorizing the curriculum by thematic units, rather than dividing it according to school grades gives teachers more freedom to schedule their teaching/lessons. In Finland, students do not have to participate in the thematic units along with their class, but they can choose one mandatory and four voluntary courses in chemistry at any time during their 2–4 years at upper-secondary school. On the contrary, the Turkish curriculum has less flexibility and a more concrete structure. In this respect, the Czech chemistry curriculum follows the chemical sub-disciplines as the titles for the thematic units. Compared to this, the Turkish and Finnish curricula contain more real-life oriented topics and are more structured and detailed. As far as the relevance of the subject-matter is concerned, the Czech curriculum, compared to the Finnish and Turkish curricula, does not take new trends in the field of chemistry into account. For instance, the Finnish and Turkish curricula contain explicitly mentioned topics such as sustainability, nanotechnology, alternative energy resources, etc. as the subject-matter organization.As far as the educational objectives are concerned, the Czech and Finnish upper-secondary school chemistry curricula contain a smaller number of more complexly formulated objectives (15 resp. 17). In contrast, the Turkish upper-secondary chemistry curriculum contains 127 objectives. Although objectives that are more complex imply higher intellectual demands, teachers may have difficulty in aligning the intended curriculum with their school curriculum (its implementation and assessment). The implemented (school) curriculum uses these complexly formulated objectives broken down into specific, measurable, attainable, relevant and time-specific objectives (cf.Skrbic and Burrows, 2014) for both implementation and assessment. Correspondingly, the alignment between the curriculum's learning objectives and both its implementation and assessment can be done easily via defining smart learning objectives rather than too broad learning goals (Chatterjee and Corral, 2017). For more information about the structure of the curricula, see Table 3 below.
| CZ | FI | TR | |
|---|---|---|---|
| General chemistry | Chemistry all around | 9th grade | 11th grade |
| Systems of substances and their composition | Significance of chemistry for the present time, further studies and the world of work | 1. Chemistry as a Science | 1. Modern atom theory |
| Quantities and calculations in chemistry | The main characteristics of the structure of the atom and the periodic system | Alchemy to chemistry | Quantum model of the atom |
| Atomic structure | Properties of substances and compounds | Chemical discipline and chemists' working areas | Periodic system and electron configurations |
| The periodic table of elements | Explaining the properties of substances based on the structure of matter, chemical bonds, and polarity | The symbolic language of chemistry | Periodic properties |
| Chemical bonds and properties of substances | Questions as the basis for information acquisition | Occupational health and safety in chemical applications | Get to know the elements |
| Temperature changes in chemical reactions | Working safely, methods for separating substances, examining, observing and making a conclusion on the properties of substances | 2. Atom and periodic system | Oxidation states |
| Rates of chemical reactions and chemical equilibrium | The chemistry of man and the living environment | Atom models | 2. Gases |
| Inorganic chemistry | The significance of chemistry for well-being and health | Structure of atom | Properties of gases and gas laws |
| Hydrogen and its compounds | Modelling and describing the structure of organic compounds, such as hydrocarbons, and oxygen and nitrogen compounds, with different models | Periodic system | Ideal gas law |
| s-Elements and their compounds | Molecular geometry and isometry | 3. Interactions between chemical species | Kinetic theory of gases |
| p-Elements and their compounds | Describing the properties of organic compounds with their structure | Chemical species | Gas mixtures |
| d- and f-elements and their compounds | Amount of substances and concentration | Classification of interactions between chemical species | Real gases |
| Organic chemistry | The use of tools and reagents and preparing solutions, | Strong interactions | 3. Liquid solutions and solubility |
| Hydrocarbons and their classification | The methods of analysing the structure of substances, such as spectroscopy | Weak interactions | Solvent–solute interactions |
| Hydrocarbon derivatives and their classification | Reaction and energy | Physical and chemical changes | Concentration units |
| Heterocyclic compounds | The significance of chemistry for energy solution and the environment | 4. States of matter | Colligative properties |
| Synthetic macromolecular substances | The symbolic representation and balancing of chemical reactions | The physical states of matter | Solubility |
| Drugs, pesticides, colouring agents and detergents | Reactions of inorganic and organic compounds and their applications | Solids | Factors affecting solubility |
| Biochemistry | The conservation of energy in a chemical reaction, binding energy, and Hess's law | Liquids | 4. The energy in chemical reactions |
| Lipids | The properties of gasses and the Ideal Gas Law | Gases | Heat exchange in reactions |
| Saccharides | Materials and technology | Plasma | Formation enthalpy |
| Proteins | The significance of chemistry in technology and society | 5. Nature and chemistry | Bond energy |
| Nucleic acids | The properties use and the life cycle of metals and polymers | Water and life | Summability of reaction heats |
| Enzymes, vitamins, and hormones | The valence electron structure of an atom and the periodic system in explaining the properties of elements | Environmental chemistry | 5. Speed in chemical reactions |
| Oxidation numbers and redox reactions | 10th grade | Reaction rates | |
| Key principles of electrochemistry: electrochemical series, standard electrode potentials, chemical cells and electrolysis | 1. The basic laws of chemistry and chemical calculations | Factors affecting reaction rate | |
| Designing and planning of an experiment or problem-solving | Basic laws of chemistry | 6. Equilibrium in chemical reactions | |
| The role of collaboration in producing chemical information | Mole concept | Chemical equilibrium | |
| Reactions and equilibrium | Chemical reactions and equations | Factors affecting equilibrium | |
| The significance of chemistry in building a sustainable future | Calculations in chemical reactions | Aqueous solution equilibrium | |
| Reaction rate and the factor that affects it | 2. Mixtures | 12th grade | |
| Homogenous and heterogeneous equilibrium and factors affecting the equilibrium | Homogeneous and heterogeneous mixtures | 1. Chemistry and electricity | |
| Acid–base equilibrium, strong and weak acids and bases, and buffer solutions | Separation and purification techniques | Electric current in reduction–oxidation reactions | |
| Graphical presentations related to equilibrium | 3. Acids, bases, and salts | Electrodes and electrochemical cells | |
| Computational processing of homogenous and acid–base equilibrium | Acids and bases | Electrode potentials | |
| Evaluation of research findings and process | Reactions of acids and bases | Electricity production from chemicals | |
| Acids and bases in our lives | Electrolysis | ||
| Salts | Corrosion | ||
| 4. Chemistry is everywhere | 2. Basics of carbon chemistry | ||
| Common daily life chemicals | Inorganic and organic compounds | ||
| Foods | Basic formula and molecular formula | ||
| Carbon in nature | |||
| Lewis formulas | |||
| Hybridization-molecular geometries | |||
| 3. Organic compounds | |||
| Hydrocarbons | |||
| Functional groups | |||
| Alcohols | |||
| Ethers | |||
| Carbonyl compounds | |||
| Carboxylic acids | |||
| Esters | |||
| 4. Energy sources and scientific developments | |||
| Fossil fuels | |||
| Alternative energy resources | |||
| Sustainability | |||
| Nanotechnology | |||
Intellectual demands from the intended national curricula based on RBT
Similar to other studies using the RBT, the results of the analysis were summed up and are displayed in taxonomy tables. The profiles from upper-secondary school chemistry intended learning objectives from the three compared countries are shown in Tables 4–6. The data were classified according to the two-dimensional RBT showing the number of objectives in the knowledge and cognitive process dimensions.| Remember | Understand | Apply | Analyse | Evaluate | Create | No. of knowledge items | |
|---|---|---|---|---|---|---|---|
| Percentages are shown in parentheses (%). | |||||||
| Factual | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Conceptual | 0 | 3 (20) | 2 (13.3) | 0 | 3 (20) | 0 | 8 (53.3) |
| Procedural | 0 | 0 | 6 (40) | 1 (6.7) | 0 | 0 | 7 (46.7) |
| Metacognitive | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of cognitive items | 0 | 3 (20) | 8 (40) | 1 (6.7) | 3 (20) | 0 | 15 |
| Remember | Understand | Apply | Analyse | Evaluate | Create | Number of knowledge items | |
|---|---|---|---|---|---|---|---|
| Percentages are shown in parentheses (%). | |||||||
| Factual | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Conceptual | 0 | 1 (5.9) | 6 (35.3) | 0 | 0 | 0 | 7 (41.2) |
| Procedural | 0 | 2 (11.8) | 2 (11.8) | 1 (5.9) | 1 (5.9) | 0 | 6 (35.3) |
| Metacognitive | 0 | 0 | 0 | 0 | 0 | 4 (23.5) | 4 (23.5) |
| Number of cognitive items | 0 | 3 (17.6) | 8 (47.1) | 1 (5.9) | 1 (5.9) | 4 (23.5) | 17 |
| Remember | Understand | Apply | Analyse | Evaluate | Create | Number of knowledge items | |
|---|---|---|---|---|---|---|---|
| Percentages are shown in parentheses (%). | |||||||
| Factual | 3 (2.4) | 6 (4.7) | 0 | 0 | 0 | 0 | 9 (7.1) |
| Conceptual | 3 (2.4) | 66 (52) | 1 (0.8) | 6 (4.8) | 0 | 0 | 76 (59.8) |
| Procedural | 0 | 26 (20.5) | 8 (6.3) | 3 (2.4) | 0 | 0 | 37 (29.1) |
| Metacognitive | 0 | 0 | 0 | 0 | 2 (1.6) | 3 (2.4) | 5 (3.9) |
| Number of cognitive items | 6 (4.7) | 98 (77.2) | 9 (7.1) | 9 (7.1) | 2 (1.6) | 3 (2.4) | 127 |
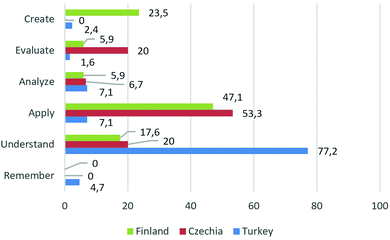
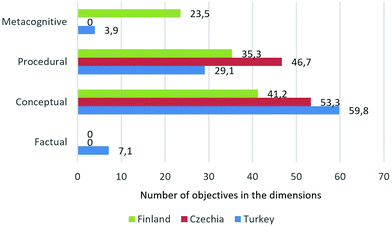
The cognitive demands in the Czech curriculum were skewed toward apply (40%) with understand and evaluate accordingly represented by 20%. No objectives in either remember or create were identified, neither factual nor metacognitive knowledge objectives. The conceptual items dominate with a 53.3% occurrence (Table 4).
In the Finnish chemistry curriculum, the cognitive demands were skewed toward apply (47.1%) with create (23.5%) and understand (17.6%). The dimension remember was not identified among the Finnish objectives. Also, no factual item was identified with conceptual (41.2%) and with procedural (35.3%) domains predominating, although metacognitive objectives represent a significant share (23.5%) – see Table 5.
In the Turkish curriculum, the domineering objectives in cognitive demands were represented by understand (77.2%) with analyse and apply (both 7.1%). Compared to the other two categories, the percentage of items in understand is more than tenfold. Conceptual items dominated (59.8%) with procedural items identified (29.1%). Also, there are five metacognitive items (3.9%) – see Table 6.
To compare the three countries’ upper-secondary chemistry curricula, Fig. 1 and 2 are shown. Compared to the Turkish curriculum, with 77% of the items in understand, the Czech and Finnish curricula are more balanced (Fig. 1). In the case of the Czech and Finnish curriculum objectives, a focus on apply was noticed. In addition, remember did not appear in the Finnish or Czech curriculum and only 4.7% in the Turkish curriculum. In the create category, no objectives appeared in the Czech curriculum, only 2.4% in the Turkish curriculum and 23.5% in the Finnish curriculum. Analyse, evaluate, and create are considered upper-level categories. The highest percentage of objectives from the three upper-level categories (35.3%) appeared in the Finnish, second (26.7%) in the Czech and third (11.1%) in the Turkish curriculum.
In the knowledge dimension, only the Turkish curriculum contains objectives in the factual knowledge dimension (7.1%), and neither Czech nor Finnish curriculum contains such objectives (Fig. 2). As far as the conceptual dimension is concerned, the highest percentage (59.8%) was identified in the Turkish curriculum, followed by the Czech (53.3) and the Finnish (41.2). Together with the procedural dimension, these dominate in the three analysed curricula. In the metacognitive category, 23.5% of the Finnish curriculum objectives appeared. Compared to this, the Turkish curriculum contains only 3.9% of the objectives; there are no objectives in this category in the Czech chemistry curriculum.
Conclusions and discussion
The analysis revealed several distinctions in the analysed national core curricula. To conclude the main findings, the Czech chemistry upper-secondary school curriculum was taken as the reference curriculum. As far as the nature of the objective is concerned, the Czech curriculum, in a way, represents a middle agent between the two other curricula (see Fig. 1 and 2). In its concreteness, it is comparably open like the Finnish curriculum. Both curricula offer more freedom with wide, only lightly specified topics translated into 15 resp. 17 objectives, whereas in the Turkish curriculum, the structure is much more extensive and the curriculum more specific (127 objectives). Even though the Turkish curriculum, having more objectives than the Czech and Finnish ones, covers similar content areas, many Turkish curriculum objectives can be nested under the more general objectives in the Czech and Finnish curriculum. We can argue that the explicit objectives direct novice, less experienced teachers more, making the setting and assessment of the lesson-specific outcomes easier. Another advantage might be related to assessment and evaluation. If the curriculum includes more detailed objectives, it is partially easier to implement, assess, and evaluate the learning outcomes and the overall effect. Also, a more strict state-level curriculum has a higher potential to be adopted in schools as the wider curricula are usually adjusted according to textbooks so teachers would understand what to teach (cf.Lepik et al., 2015; Mullis et al., 2012). Nevertheless, they can also be too constraining for innovative teachers with different teaching concepts. In this respect, the Czech and Finnish curricula offer more room for these teachers, forcing less experienced or innovative teachers to look for guidance either from their colleagues or in textbooks, books, etc. (Laws and Horsley, 1992; Loewenberg-Ball and Cohen, 1996). The openness also makes top-down innovation introduction more difficult as the system is not centralized. In this system, the key actors diffusing innovation are teachers themselves (cf.Rusek et al., 2017).In cognitive processes, the Czech curriculum is similar to the Finnish, with no objectives in the remember cognitive process dimension and a similar share of objectives in understand, apply, and analyse. However, the Finnish curriculum stands out with more than 23% of the objectives in the create dimension. The Czech chemistry curriculum, on the contrary, does not contain any objectives in this dimension. This may be caused by the year of its creation. The curriculum developers may not have been that familiar with the RBT, therefore being attached to the original Bloom's taxonomy with evaluate as the top dimension. Also, creativity has been emphasized in many science education policy documents only after developing the present Czech chemistry curriculum for secondary schools (Hazelkorn et al., 2015). Thus, by providing a framework for assessment, the RBT helps curriculum developers go beyond factual knowledge and comprehension and accent higher cognitive skills such as application, analysis, evaluation, and creation (Jideani and Jideani, 2012). The Turkish curriculum covers all the cognitive process dimensions, despite being in small proportions. The majority of its objectives feature in the understand dimension (77.2%) and, in comparison with the other two analysed curricula, several (4.7%) objectives in the remember dimension. This could be explained by the Czech and Finnish curricula having the lower cognitive processes hidden under the higher-order objectives implicitly, whereas they are explicit in the extensive Turkish curriculum. They may also already be introduced as objectives in the curricula for lower grades but analysing this is beyond the scope of this study. Similar to the findings in the Czech and Finnish curricula, Wei (2020) also found higher cognitive process levels in the Senior High School Chemistry Curriculum in China. On the contrary, the Turkish curriculum might not be representative of a shift from simple recollection to more complex skills in chemistry (cf.Edwards, 2010). Another reason behind the lower cognitive skills in the Turkish curriculum might be that assessing lower cognitive skills through multiple-choice items in nation-wide examinations (e.g., university admission examinations) is easier and more reliable than assessing higher cognitive skills. Curriculum developers in Turkey might state learning objectives at lower cognitive levels, explicitly thinking about the feasibility of assessing millions of upper-secondary school students taking university entrance examinations more easily and fairly. Again, with respect to the link between the state and school curricula, this step can also prevent the objectives from being altered by teachers (Son and Kim, 2015) and therefore translated into school practice differently than intended by the curriculum developers.
Similar to the cognitive processes, the Turkish chemistry curriculum covers all four knowledge dimensions, although it strongly focuses on conceptual and procedural knowledge (almost 90% of objectives in these two dimensions). The Czech curriculum is similar in its emphasis on these two dimensions with no objectives in the others. In the Finnish curriculum, these are also domineering dimensions; however, there are over 20% of the objectives in the metacognitive dimension too. The distribution of conceptual and procedural knowledge at higher percentages might be explained by the nature of the school subject/course (Ang, 2019). Correspondingly, Ang (2019) found that conceptual and procedural knowledge explained the majority of learning objectives, and procedural knowledge is particularly dominant in chemistry and physics curricula because using calculations, investigating, and applying in new contexts are characteristic domains in these two subjects. There was no significant difference found in these among the three analysed curricula. The Czech and Turkish curricula authors should consider having more metacognitive knowledge dimension objectives incorporated in further editions because the evidence related to its effectiveness comes from contemporary cognitive science research (Wei and Ou, 2018). An example of its functionality can be found, for example, in the newly emerging Senior High School Chemistry Curriculum in China, where it poses a challenging objective (Wei, 2020).
We can also argue that despite reforming schools by policymakers, changes in the intended curricula also consider the opinions and approaches of teachers. For example, the Finnish curriculum was developed with teachers significantly involved in the process. In the case of the Czech curriculum, teachers also commented on the draft of the curriculum. It is then reasonable to infer that teachers project their practice to shape the curriculum, and it, in a way, reflects their concept of teaching into the intended curriculum. Therefore, we can argue that most learning objectives in the Turkish written chemistry curriculum outline the skill of understanding, while those in the Czech and Finnish chemistry curricula use the skills of applying and analysing. This supposition could explain the results in PISA (Organization for Economic Cooperation and Development, 2019). The results suggest the students’ level of scientific literacy, which is mostly built on the application of science's conceptual and procedural knowledge (Janoušková et al., 2019; Organization for Economic Cooperation and Development, 2016). The emphasis on understanding may be the cause of Turkish students’ lower PISA results compared to Czech or Finnish students. The correlation of PISA results can be seen with both cognitive and knowledge results (Fig. 1 and 2) if we consider the levels as an ordinal scale. This might be noticed here, even though not analysed statistically in any sense.
Analysis such as that presented is being made to ensure the alignment of instruction and testing, i.e., support the implemented and attained curriculum. The evidence of the requirement to develop higher-order cognitive processes requires a completely different approach from generic frontal teaching (Raiyn and Tilchin, 2015). The intended chemistry curricula analysed in this study imply more than lecturing or direct instruction because the cognitive level of learning objectives require teachers to do more student-centred education which involves experiences, making them learn actively. Drawing teachers’ attention is one of the main appeals of this paper. Another target group is teacher trainers, (science) education experts, and also textbook authors who help to shape the methods teachers use in their practice. From the scientific literacy development point of view, the results could bring evidence for the need for Turkish curriculum change. Objectives focusing on understanding are not sufficient and do not give teachers the necessary impulse to foster their students’ scientific literacy. It is considered to be more effective when being more explicitly anchored in the national curriculum (Janoušková et al., 2019).
Implications
This study is intended to help educators and curriculum developers see where and what the intellectual demands of each country's curriculum are under scrutiny (Ang, 2019) and link it to another country's curriculum together with information about the educational system. Furthermore, the findings of this research might be used by teachers in designing lessons and developing test items to be aligned with the intended curriculum (Ang, 2019). Alignment studies might be conducted further to detect and elucidate differences between the intended curriculum and national exams or tests if there are any (Edwards, 2010). For instance, Firman (2013) concluded that the level of alignment between national high school chemistry curriculum content and national exam questions is moderate due to the overemphasis on lower cognitive skills in national exams and the use of items regardless of each curricular topic's relative proportions. Such types of study might be carried out on various subjects and across different grade levels. Analysing intended curricula is also valuable to verify what is to be changed in teaching and learning practice as a consequence of curricular reform.Limitations
Despite the fact the coders met in person and repeatedly discussed their understanding of the RBT, there were some discrepancies in their coding. This could be the main limitation of this study. This effect was, however, reduced by introducing a third coder to the process to code the objectives of each curriculum. This, we believe, brought more control to the consistency in coding.This study was focused on the upper-secondary chemistry curriculum, which could be seen as a limitation when no information on the lower-secondary education objectives was provided. Although lower-secondary education creates the base for future studies, chemistry is not taught as a separate school subject at the lower-secondary level in many countries. Besides, upper-secondary school chemistry education contains the full breadth of the field students are presented with. For this reason, the upper-secondary chemistry curriculum was chosen for the analysis.
Another limitation of this study could be seen as its focus on the intended curriculum. The findings of this study should be cautiously interpreted because of this intended versus implemented curriculum difference, not to mention the attained curriculum difference, since there are no data from in-class observations related to what is going on in a chemistry classroom in these three countries. It is appropriate to say that the intended chemistry curriculum in any of the countries is only one factor that can influence students’ chemistry learning outcomes and cannot adequately present the overall picture of chemistry education in the country. To be able to assess education's reality, the implemented curriculum needs to be observed and analysed. A combination of the data from lesson observations, curriculum objective analysis, and national exam results could enable triangulated, solid data for future evidence-based curriculum reform. For example, Yasar and Sozbilir (2019) concluded that there is no congruence between the intended, perceived and observed chemistry curriculum according to the data collected through interviews with teachers and observations of their chemistry lessons. In other words, the chemistry curriculum is not perceived and implemented as constructivist as it is intended (see Lepik et al., 2015; Mullis et al., 2012). In this case, policymakers’ control, as well as attempts for innovation, are diminished and the role of commercial curricula (cf.Hemmi et al., 2013), including textbooks, grows.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Charles University Research Centre Program No. UNCE/HUM/024 and the Grant PROGRES Q17 Program, Part 5 “Teachers Education and Teacher's Profession in Science and Research Context” at the Faculty of Education, Charles University. The authors are grateful for this support.Notes and references
- Amer A., (2006), Reflections on Bloom's revised taxonomy, Electr. J. Res. Educ. Psychol., 4(1), 213–230.
- Anderson L. W., Krathwohl D. R., Airasian P. W., Cruikshank K. A., Mayer R. E., Pintrich P. R., Raths J. and Wittrock M. C. (ed.), (2001), A taxonomy for learning, teaching, and assessing: A revision of Bloom's taxonomy of educational objectives (Abridged edn), New York, NY: Longman.
- Ang G. X., (2019), Comparing science learning outcomes from Singapore and New South Wales, Australia, using revised Bloom's taxonomy (Unpublished manuscript), National Institute of Education, Singapore: Nanyang Technological University.
- Biggs J., (1995), Assessing for learning: Some dimensions underlying new approaches to educational assessment, Alberta J. Educ. Res., 41(1), 1–17.
- Blonder R. and Mamlok-Naaman R., (2019), Factors affecting the study of chemistry in different countries around the world: Findings from an international survey, Isr. J. Chem., 59(6–7), 625–634.
- Bloom B. S., (1994), Reflections on the development and use of the taxonomy. in Anderson L. W. and Sosniak L. A. (ed.), Bloom's taxonomy: A forty-year retrospective, Chicago: University of Chicago Press, pp. 1–8.
- Bloom B. S., Engelhart M. D., Furst E. J., Hill W. H. and Krathwohl D. R., (1956), Taxonomy of educational objectives: The classification of educational goals. Handbook I: Cognitive domain, New York, NY: David McKay.
- Booker M. J., (2007), A roof without walls: Benjamin Bloom's taxonomy and the misdirection of American education, Acad. Quest., 20(4), 347–355.
- Bussey T. J., Orgill M. and Crippen K. J., (2013), Variation theory: A theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14(1), 9–22.
- Chatterjee D. and Corral J., (2017), How to write well-defined learning objectives, J. Educ. Perioper. Med., 19(4), 1–4.
- Čtrnáctová H. and Zajíček J., (2010), Současné školství a výuka chemie v České republice [The education and teaching of chemistry in the Czech Republic today], Chem. Listy, 104(8), 811–818.
- DeMers M. N., (2009), Using intended learning objectives to assess curriculum materials: The UCGIS body of knowledge. J. Geogr. Higher Educ., 33(Suppl 1), 70–77 DOI:10.1080/03098260903033980.
- Domin D. S., (1999), A content analysis of general chemistry laboratory manuals for evidence of higher-order cognitive tasks, J. Chem. Educ., 76(1), 109–111 DOI:10.1021/ed076p109.
- Edwards N., (2010), An analysis of the alignment of the grade 12 physical sciences examination and the core curriculum in South Africa, S. Afr. J. Educ., 30, 571–590.
- Elmas R., Ozturk N., Irmak M. and Cobern W. W., (2014), An investigation of teacher response to national science curriculum reforms in Turkey, Eur. J. Phys. Chem. Educ., 6(1), 2–33.
- Er K. O. and Atici S., (2016), A comparative investigation of the chemistry curricula of Finland and Turkey, Necatibey Faculty of Education Electronic Journal of Science and Mathematics Education, 10(1), 238–259.
- Finnish National Agency of Education, (2016), National core curriculum for general upper secondary schools in 2015. Retrieved February 12, 2020 from https://www.oph.fi/sites/default/files/documents/172124_lukion_opetussuunnitelman_perusteet_2015.pdf.
- Firman H., (2013), Alignment between national examination and national content standard of high school chemistry. Paper presented at the Mathematics, Science, Computer Education International Seminar, Indonesia University of Education, Bandung.
- Furst E. J., (1981), Bloom's taxonomy of educational objectives for the cognitive domain: Philosophical and educational issues, Rev. Educ. Res., 51(4), 441–453.
- Hanna W., (2007), The new Bloom's taxonomy: Implications for music education, Arts Educ. Policy Rev., 108(4), 7–16.
- Hazelkorn E., Ryan C., Beernaert Y., Constantinou C. P., Deca L., Grangeat M. and Welzel-Breuer M., (2015), Science education for responsible citizenship: Report to the European Commission of the expert group on science education, Luxembourg: Publications Office of the European Union.
- Hemmi K., Koljonen T., Hoelgaard L., Ahl L. and Ryve A., (2013), Analysing mathematics curriculum materials in Sweden and Finland: Developing an analytical tool, in Ubuz B., Haser C. and Mariotti M. A. (ed.), Proceedings of the eighth congress of the European Society for Research in Mathematics Education (CERME 8, February 6–10, 2013), Ankara, Turkey: Middle East Technical University and ERME.
- Janoušková S., Žák V. and Rusek M., (2019), Koncept přírodovědné gramotnosti v České republice – analýza a porovnání [The concept of scientific literacy in the Czech Republic – analysis and comparison], Studia Paedagogica, 24(3), 93–109.
- Jideani V. A. and Jideani I. A., (2012), Alignment of assessment objectives with instructional objectives using revised Bloom's taxonomy – the case for food science and technology education, J. Food Sci. Educ., 11(3), 34–42.
- Klopfer L. E., (1971), Evaluation of learning in science, in Bloom B. S., Hastings J. T. and Madaus G. F., (ed.), Handbook of formative and summative evaluation of students’ learning, New York: McGraw-Hill, pp. 559–642.
- Krathwohl D. R., (2002), A revision of Bloom's taxonomy: An overview, Theory Pract., 41(4), 212–218.
- Laws K. and Horsley M., (1992), Educational equity? Textbooks in NSW government and non-government schools, Curric. Perspect., 13(3), 7–17.
- Lee Y.-J., Kim M. and Yoon H.-G., (2015), The intellectual demands of the intended primary science curriculum in Korea and Singapore: An analysis based on revised Bloom's taxonomy, Int. J. Sci. Educ., 37(13), 2193–2213.
- Lee Y.-J., Kim M., Jin Q., Yoon H.-G. and Matsubara K., (2016), East-Asian primary science curricula: An overview using revised Bloom's taxonomy, Beach Road, Singapore: Springer, DOI:10.1007/978-981-10-2690-4.
- Lepik M., Grevholm B. and Viholainen A., (2015), Using textbooks in the mathematics classroom – the teachers’ view, Nordic Stud. Math. Educ., 20(3–4), 129–156.
- Loewenberg-Ball D. and Cohen D. K., (1996), Reform by the book: what is: or might be: the role of curriculum materials in teacher learning and instructional reform? Educ. Res., 25(9), 6–14 DOI:10.3102/0013189X025009006.
- Marzano R. J. and Kendall J. S., (2006), The new taxonomy of educational objectives, 2nd edn, Thousand Oaks, CA: Corwin Press.
- Ministry of National Education, (2018), Secondary education chemistry curriculum (9-12th grades), Ankara: Board of Education.
- Motlhabane A., (2017), Unpacking the South African physics-examination questions according to Blooms’ revised taxonomy, J. Balt. Sci. Educ., 16(6), 919–931.
- Mullis I. V., Martin M. O., Foy P. and Arora A., (2012), TIMSS 2011 international results in mathematics, Chestnut Hill, MA: TIMSS & PIRLS International Study Center.
- Näsström G., (2009), Interpretation of standards with Bloom's revised taxonomy: A comparison of teachers and assessment experts, Int. J. Res. Method Educ., 32(1), 39–51.
- Näsström G. and Henriksson W., (2008), Alignment of standards and assessment: A theoretical and empirical study of methods for alignment, Educ. J. Res. Educ. Psychol., 6(3), 667–690.
- Organization for Economic Cooperation and Development, (2016), PISA 2015 results (Volume I): Excellence and equity in education, Paris: PISA, OECD Publishing DOI:10.1787/9789264266490.
- Organization for Economic Cooperation and Development, (2019), PISA 2018 results (Volume I): What students know and can do, Paris: PISA, OECD Publishing DOI:10.1787/5f07c754.
- Ormell C. P., (1974), Bloom's taxonomy and the objectives of education, Educ. Res., 17(1), 3–18.
- Porter A. C., (2002), Measuring the content of instruction: Uses in research and practice, Educ. Res., 31(7), 3–14.
- Raiyn J. and Tilchin O., (2015), Higher-order thinking development through adaptive problem-based learning, J. Educ. Train. Stud., 3(4), 93–100.
- Rámcový vzdělávací program pro gymnasia, (2007), [The general educational programme for high schools], Praha: Výzkumný ústav pedagogický v Praze.
- Ritchotte J. A. and Zaghlawan H. Y., (2019), Coaching parents to use higher level questioning with their twice-exceptional children, Gift. Child Q., 63(2), 86–101.
- Rusek M., Stárková D., Chytrý V. and Bílek M., (2017), Adoption of ICT innovations by secondary school teachers and pre-service teachers within education, J. Balt. Sci. Educ., 16(4), 510–523.
- Schildkamp K. and Kuiper W., (2010), Data-informed curriculum reform: Which data, what purposes, and promoting and hindering factors, Teach. Teach. Educ., 26(3), 482–496.
- Skrbic N. and Burrows J., (2014), Specifying learning objectives, in Ashmore L. and Robinson D. (ed.), Learning, teaching and development: Strategies for action, London: Sage, pp. 39–69.
- Smith G., Wood L., Coupland M., Stephenson B., Crawford K. and Ball G., (1996), Constructing mathematical examinations to assess a range of knowledge and skills, Int. J. Math. Educ. Sci. Technol., 27(1), 65–77 DOI:10.1080/0020739960270109.
- Son J.-W. and Kim O.-K., (2015), Teachers’ selection and enactment of mathematical problems from textbooks, Math. Educ. Res. J., 27(4), 491–518.
- Thijs A. and van den Akker J., (2009), Curriculum in development, Enschede, the Netherlands: SLO.
- Toledo S. and Dubas J. M., (2015), Encouraging higher-order thinking in general chemistry by scaffolding student learning using Marzano's taxonomy, J. Chem. Educ., 93(1), 64–69.
- van den Akker J., (2013), Curricular development research as specimen of educational design research, in Plomp T. and Nieveen N. (ed.), Educational design research, Enschede: Netherlands Institute for Curriculum Development, pp. 52–71.
- Vaskuri J., (2017), Oppiennätyksistä opetussuunnitelman perusteisiin: Lukion kemian kansallisen opetussuunnitelman kehittyminen Suomessa vuosina 1918–2016 [The development of the national upper secondary school chemistry curriculum in Finland from 1918 to 2016] (Research Report No. 201). Jyväskylä: Department of Chemistry, University of Jyväskylä.
- Vojíř K. and Rusek M., (2020), Vývoj kurikula chemie pro základní vzdělávání v České republice po roce 1989 [Development of curriculum for chemical education in the Czech Republic after 1989], Chemické Listy, 114(5), in press.
- Wei B., (2020), The change in the intended senior high school chemistry curriculum in China: Focus on intellectual demands, Chem. Educ. Res. Pract., 21(1), 14–23.
- Wei B. and Ou Y., (2018), A comparative analysis of junior high school science curriculum standards in Mainland China, Taiwan, Hong Kong, and Macao: Based on revised Bloom's taxonomy, Int. J. Sci. Math. Educ., 17(8), 1459–1474.
- Yasar M. D. and Sozbilir M., (2019), Investigating teachers' fidelity to constructivist chemistry curriculum in Turkey: Congruence between intended, perceived and observed curriculum in Turkey, Int. J. Phys. Chem. Educ., 11(4), 93–104.
- Zorluoglu S. L., Kizilaslan A. and Sozbilir M., (2016), Analysis and evaluation of learning outcomes in high school chemistry curriculum according to revised Bloom taxonomy, Necatibey Faculty of Education Electronic Journal of Science and Mathematics Education, 10(1), 260–279.
Footnote |
| † This study is an extended version of the paper entitled, “A Comparative Analysis of the High School Chemistry Curriculum Objectives in Czechia and Turkey based on the Revised Bloom's Taxonomy” presented at the 8th International Conference New Perspectives in Science Education held on March 21–22, 2019 in Florence, Italy. |
| This journal is © The Royal Society of Chemistry 2020 |